Abstract
To break the decades-old yield barrier in pigeonpea [Cajanus cajan (L.) Millsp.] a hybrid breeding technology was successfully developed and the first two hybrids were recently released in India. In order to produce heterotic hybrid combinations, the first logical step is the identification and selection of genetically diverse parents with favorable alleles. In this context, the concept of classifying hybrid parents into different heterotic groups was developed and successfully used in maize and later adopted in other crops. Since hybrid technology in pigeonpea is new, the authors have made the first attempt to identify heterotic groups using SCA effects of 102 crosses generated from line × tester mating and evaluated them at four locations. Based on the performance of hybrids in terms of SCA effects, seven heterotic groups were constituted. Besides this, a scheme to use this information in breeding high yielding hybrids with specific or wide adaptation is also discussed herein. Genetic diversity between lines and tester showed positive association with the heterotic pools generated on the basis of SCA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigeonpea [Cajanus cajan (L.) Millsp.] is an integral crop component of subsistence agriculture in India and parts of Africa and South America. Globally, it is grown on 5.32 M Ha (FAO 2012) and besides being a high protein food, it provides additional benefits to the farmers such as fixing of atmospheric nitrogen, releasing of soil-bound phosphorus, improving soil structure, etc. (Saxena 2008). India (3.86 m ha) is the major pigeonpea growing country accounting for 72.5 % of the global area. Considering the importance of pigeonpea in Indian agriculture, the Indian Council of Agricultural Research (ICAR) launched an extensive crop improvement program in 1960 and over 100 pure line cultivars were released in the last 5–6 decades. This resulted in significant increases in the cropped area; but the crop productivity remained low at around 750 kg/ha (IIPR 2013). The hybrid technology developed recently in pigeonpea has provided an opportunity to break this decades-old yield barrier. The two released hybrids ICPH 2671 (Saxena et al. 2013a) and ICPH 2740 (Saxena et al. 2013b) have demonstrated >40 % yield advantage over existing cultivars in farmers’ fields; but even this gain may not to be enough to meet the needs of growing population of the country. Hence, there is a need to breed and popularize hybrids which have the potential to produce 75–100 % greater yields than the most present day cultivars. To achieve this goal, it is imperative to breed elite hybrid parents, which would be able to produce exceptionally high yielding hybrids. In this context, the formation and use of diverse heterotic groups of inbred parents can help in breeding high-yielding hybrids. The successful breeding and utilization of elite maize inbred lines in diverse heterotic groups has not only helped in increasing maize productivity by a big margin but also encouraged breeders to adopt this approach in other crops (Melchinger and Gumber 1998; Hallauer 1999). In pigeonpea, the hybrid technology has just been developed and the concept of heterotic groups has not been explored so far. This paper describes the results of the first ever attempt to constitute heterotic groups in pigeonpea by using the data generated from the evaluation of a set of crosses generated from a line × tester mating.
Materials and methods
Cytoplasmic nuclear male-sterility (CMS) in pigeonpea, representing the A4 system, was developed from an inter-specific cross by Saxena et al. (2005). This primary source of CMS was used to breed three diverse CMS lines through backcrossing. These A-lines, designated as ICPA 2043, ICPA 2047, and ICPA 2092 were crossed with 34 known fertility restorers in a line × tester mating scheme to produce 102 hybrids during 2008 at Marathwada Agricultural University, Parbhani, Maharashtra. All the F1s and their parents were grown with control BSMR 736 in an alpha-lattice design with two replications at Patancheru (17°53′N, 78°27′E, 545.0 m), Parbhani (19°16′N, 67°47′E, 409.0 m), Latur (18°24′N, 76°36′E, 633.8 m), and Badnapur (19°50′N, 47°53′E, 519.6 m) during 2009 rainy season. Each entry was sown in 4.2 m long single rows, and 14 plants were maintained after thinning. To provide uniform competition each plot was flanked on either side by a single row of cultivar BSMR 736. The inter- and intra-row spacing was kept at 75 and 30 cm, respectively. The recommended package of cultural practices (Saxena 2006) was followed to raise a healthy crop. In each plot five competitive plants were selected randomly for recording data on grain yield/plant (g). Standard analysis of data was performed to determine general (GCA) and specific combining ability (SCA) effects. To develop heterotic groups in germplasm, so far there is no single standard method and the procedures such as pedigree analysis, quantitative genetic analysis, diversity analysis or use of molecular marker data have been suggested (Yuan et al. 2000). In the present study the testers were classified into different heterotic groups on the basis of their SCA effects. The crosses of testers with male-sterile lines ICPA 2043, ICPA 2047, and ICPA 2092 were assigned to heterotic group ‘K’, ‘B’, or ‘S’, respectively. In addition, heterotic groups ‘KB’, ‘KS’, ‘BS’ or ‘KBS’ were established using the performance of testers in hybrid combination with two or three A-lines. Based on the standardized trait value cluster analysis was performed using the statistical package NTSYS-PC 2.0 (Rohlf 1992), producing a dendrogram depicting the relationship among the lines and testers used to construct heterotic pools relative to the morphological characteristics.
Results
Analysis of variance (Table 1) revealed highly significant variation for yield among the genotypes and locations. The variation due to lines and testers was also highly significant, suggesting an important role of additive genetic variation in determining yield. The variation due to crosses was also significant and it suggested the importance of non-additive genetic variation for yield. The GCA effects of A-lines across the testers were also highly significant. Significance of lines × testers and SCA effect of the line × testers crosses suggested the importance of both additive and non-additive genetic variation in the manifestation of seed yield. The magnitude of variance due to line × testers was lesser than that for lines or testers, suggesting thereby testers were highly divergent from lines which satisfies the choice of testers (Sharma 1988). Variance of SCA was higher than the GCA variance for yield plant−1 which indicated preponderance of non-additive gene action in the inheritance of the yield. This was further supported by low magnitude of MS σ2ΣXA/σ2ΓXA ratio. It suggested greater importance of non-additive gene action in its expression and indicated very good prospect for the exploitation of non-additive genetic variation for traits through hybrid breeding Ramalingam et al. (1997).
General combining ability
General combining ability (GCA) effects give an idea about the breeding behavior of the parental lines and help in selecting parental lines for variety improvement programs. The estimates of GCA effects (Table 2) of the female parents revealed that ICPA 2092 had significant and positive GCA effect at all the four locations and hence, it was adjudged the best male sterile line for breeding high yielding hybrids with predominance of additive genetic variance. Another female parent ICPA 2047 recorded significant positive GCA effect at Parbhani only; while ICPA 2043 was found to be a poor general combiner with significant negative GCA effects observed at all the four locations.
Among 34 testers evaluated, 13 were good general combiners for grain yield at Patancheru. Similarly, 10 testers at Parbhani, 11 at Latur, and 11 at Badnapur showed significant and positive GCA effects (Table 2). Eight testers BSMR 198, BDN 2001-6, ICP 10934, AKT 9913, ICP 11376, ICP 3514, ICP 3374, and ICPL 20106 had positive and significant GCA effects at all the four locations. In addition, three testers HPL 24, ICP 3407, and ICP 3475 at three locations; and ICP 12749 and ICP 10650 at two locations also exhibited significant GCA effects. These 13 testers demonstrated the presence of additive gene action in determining yield and it further suggested that the favorable alleles from the testers and CMS lines complemented each other in a positive manner to produce genotypes rich in seed yield.
A total of 19 testers exhibited significant negative GCA effects, suggesting that these testers and the A-lines had nuclear genomes that produced some deleterious effects/interactions when combined together and hence did not help in enhancing productivity of the hybrids. The utility of selecting parents on the basis of GCA effects has also been demonstrated in pigeonpea by Venkateswarlu and Singh (1982), Patel et al. (1991), Khapre et al. (1993), Narladkar and Khapre (1996), Srinivas et al. (1998), Pandey (1999), Vanniarajan et al.(1999), Jahagirdar (2003), Yadav et al. (2008), Phad et al. (2009), and Sameer Kumar et al. (2009).
Specific combining ability
Specific combining ability effects are considered to be the best indicator for selecting superior hybrid combinations. The SCA data generated in this study from 102 hybrids at four locations are not included in this paper for the sake of brevity. However, it was observed that at each location 30–32 % crosses exhibited significant positive SCA effects. At Parbhani 33 hybrids showed highly significant positive SCA effects, while each at Latur and Badnapur 36 hybrids exhibited significant positive SCA effects. At Patancheru, eight inbreds produced crosses with high SCA effects with ICPA 2043, four with ICPA 2047, and six with ICPA 2092. Only one inbred ICPL 20106 produced hybrids with high SCA with all the three A-lines. Of these, hybrids ICPA 2047 × HPL 24, ICPA 2092 × BSMR 164 and ICPA 2043 × ICP 3374 showed highly significant positive SCA effects at all the four locations. A close perusal of the hybrid data revealed that the hybrid combinations with significant positive SCA effects involved parents with low × high, high × low or low × high GCA effects; and thereby suggested involvement of non-allelic interactions. Vanniarajan et al. (1999) reported that some of the cross combinations having parents with high × low and low × high GCA effects also produced significant SCA effects. Jahagirdar (2003) reported that high × low and low × low general combiners were involved in promising specific cross combinations. Phad et al. (2009) reported that the hybrids with high SCA involved parents with high × low, low × high, low × low GCA effects. Baskaran and Muthiah (2006) observed that the hybrid CORG 94 × ICPL 83027 had high SCA effect and it involved parents with high × low GCA. These observations indicated the presence of both additive and non-additive gene effects and hence could be used in heterosis breeding. Yadav et al. (2008) observed that the hybrids expressed high SCA irrespective of the extent and direction of GCA effects of the parents, indicating involvement of both dominance and epistatic gene action in the inheritance of traits. Sameer Kumar et al. (2009) revealed that the crosses with high SCA involved both the parents with high GCA effects.
Formation of heterotic groups
A total of seven heterotic groups were established and these included heterotic group ‘K’ (ICPA 2043 crosses), heterotic group ‘B’ (ICPA 2047 crosses), heterotic group ‘S’ (ICPA 2092 crosses), heterotic group ‘KB’ (ICPA 2043 + ICPA 2047 crosses), heterotic group ‘KS’ (ICPA 2043 + ICPA 2092 crosses), heterotic group ‘BS’ (ICPA 2047 + ICPA 2092 crosses), and heterotic group ‘KBS’ (ICPA 2043 + ICPA 2047 + ICPA 2092 crosses).
At Patancheru out of 34 testers used, 32 demonstrated heterotic responses with one or more male-sterile lines (Table 3a). Eight inbreds AKT 9915, ICP 3475, BSMR 736, AKT 8811, Phule 25-1, Phule 6-2, AKT 222521, and ICP 3514 occupied place in heterotic group K,; while ICP 13991, HPL 24, ICP 10650, and AKT 6-4 were placed in heterotic group B. Six inbred lines BSMR 164, BSMR 203, ICP 3525, ICP 10934, ICP 3407, and ICP 11376 represented heterotic group S. In addition, three lines were grouped each in heterotic group KB and KS. Seven lines were included in heterotic group BS. Inbred ICPL 20106 expressed significant SCA effect with all the three CMS lines and hence it was classified in heterotic group KBS. At Parbhani, 25 testers constituted the three major heterotic groups. These included 10 lines in heterotic group K, 7 lines in heterotic group B, and 8 lines in heterotic group S (Table 3b). None of the testers could be classified in heterotic group BS and KBS. BSMR 2 (heterotic group KB) and BSMR 198, BSMR 864, Vipula, and ICP 11376 (heterotic group KS) were the other potential genotypes identified for exploiting hybrid vigor in pigeonpea at Parbhani. At Latur, eleven crosses with ICPA 2047 exhibited significant SCA effects and their male parents were classified in heterotic group B (Table 3c), while ICPA 2043 produced eight heterotic hybrids and these constituted heterotic group K. Similarly, seven inbreds had significant SCA effects when crossed with ICPA 2092 and their testers formed heterotic group S. Three testers (TV1, Vipula, and ICPL 20106) were assigned to heterotic group KB; cultivar BDN 2001-6 was placed in heterotic group KS and ICP 11376 in heterotic group BS. At Badnapur, eleven inbreds were classified each in heterotic groups K and S (Table 3d). Six testers (BSMR 175, ICP 12749, ICP 13991, HPL 24, ICP 10650, and AKT 6-4) were classified in heterotic group B. The heterotic group KS had three testers (BSMR 203, Vipula, ICP 11376) while Phule 4-1 was grouped in heterotic group KB. There was no tester in heterotic groups BS and KBS.
Considering overall performance of hybrids across the locations (Table 4) 12 testers occupied places in heterotic group K. Of these, ICP 3475, BMSR 736, AKT 8811, Phule 6-2, ICP 3514 and Phule 25-1 were most promising testers which yielded significant SCA with ICPA 2043 at all the four locations. ICP 3374 was found promising at Latur, Badnapur and Parbhani. Testers AKT 9915, AKT 222521, BDN 2001-6, ICPL 20106, and BSMR 2 were found promising at two locations. A total of 20 testers were included in heterotic group B. Of these, only ICP 13991, ICP 10650, HPL 24, and AKT 6-4, had significant SCA with ICPA 2047 at all the test sites. In heterotic group S, of the 34 testers evaluated, 22 exhibited heterotic effect with one or more A-lines. In this group only one tester ICP 11376 had significant SCA effects at all the four locations. Twelve testers were found promising at three locations, while nine testers were found good at two locations and nine testers were found good at only one location. Out of eight testers which exhibited significant and positive GCA effects at all the four locations, seven appeared in different heterotic groups. HPL 24, ICP 10650, ICP 3407, and ICP 3475 were the other testers with high GCA at three locations which produced heterotic hybrids. Therefore, it can be inferred that these 12 testers contributed to the expression of heterosis through their additive genetic variances.
Eighteen testers exhibited significant SCA with different A-lines but had non-significant GCA and these appeared to have contributed to heterosis through their non-additive genetic variance. ICP 10934 had highly significant and positive GCA but did not appear in any heterotic group. This may be due to its genetic similarity with the three A-lines. Among the 34 testers used, line ICPL 20106 was the best which not only had highly significant GCA but also produced highly heterotic hybrids with all the three A-lines. Hence, line ICPL 20106 appears to be a good candidate for incorporation of the male-sterility characteristics for hybrid pigeonpea breeding programs.
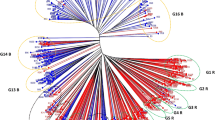
Based on the clustering pattern using six morphological traits of 34 testers and 3 lines, all three lines (ICPA 2043, ICPA 2047 and ICPA 2092) grouped separately in different clusters along with some of the testers (Fig. 1). On the basis of overall performance of the hybrids across the location, testers were classified into different groups (Table 4). Heterotic group K consisted of 6 testers and 4 of them grouped distantly from its line ICPA 2043, while 17 testers were associate with group K, KB, KS and KBS. Heterotic groups B and S consisted of 4 and 3 inbreds respectively, however, all the 7 inbred fell in different sub clusters in comparison to their lines (ICPA 2047 and ICPA 2092). Sixteen testers were associated (B, KB, BS and KBS) with group B which was grouped distantly. The heterotic group KB consisted of 5 testers and 4 of them grouped distantly except one namely ICP 12749, which falls together with ICPA 2043. All the five testers (ICP 3374, BSMR 198, BSMR 846, BSMR 203 and Phule 3-1) belonging to heterotic group KS were grouped distantly with both the lines (ICPA 2043 and ICPA 2092). Six testers were classified in heterotic group BS and except one (BSMR 571) all the five testers grouped distantly with ICPA 2047 and ICPA 2092. Interestingly, sole tester ICPL 20106 which showed heterotic pattern was grouped distantly with all the three lines (ICPA 2043, ICPA 2047 and ICPA 2092) and it was classified in heterotic group KBS. However, one tester namely ICP 10934, showed more dissimilarity than the other testers in comparison to the three lines, did not find place in any of the heterotic groups.
Discussion
The classical breeding experiments of Shull in the early part of the twentieth century provided an insight into the phenomenon of hybrid vigor and inbreeding depression in maize (Zea mays). Subsequently, Richey (1922) demonstrated the power of hybrid vigor for seed yield by crossing two diverse maize lines. Sprague and Tatum (1942) developed the concept of combining ability of the parental lines with respect to their potential in producing high yielding hybrids. These pieces of information on hybrid parents eventually evolved into the concept of “heterotic groups”. Melchinger and Gumber (1998) defined heterotic group as ‘a group of related or unrelated genotypes from the same or different populations which display similar combining ability and heterotic response when crossed with genotypes from other genetically distinct germplasm groups’. Reif et al. (2003) opined that for cost effective hybrid breeding it is desirable to classify the crop germplasm into different heterotic groups on the basis of their performance in F1 generation, origin, or genetic diversity. According to Fan et al. (2003) and Jelena et al. (2007) although various methods of classifying inbreds into heterotic groups are available, judicious use of pedigree information, combining ability analysis, and molecular markers together may be the most effective tools in formulating heterotic groups. In the present investigation seven heterotic groups were formed using estimates of SCA effects derived from a set of line × tester crosses. This is the first such attempt in pigeonpea. Use of this approach in hybrid pigeonpea breeding will not only lift the performance level of hybrids but also bring down the expenses involved in carrying forward the unproductive breeding materials. Specific hybrid × environment interaction generally influences the expression of hybrid plants, therefore to minimize g × e effects in the present study the F1s were evaluated at four locations.
In pigeonpea, unlike other crops, meager literature is available on genetic diversity and so far there is no conclusive published evidence to suggest the role of genetic diversity in the manifestation of heterosis. This may be due to limited genetic materials used in such studies. In addition in pigeonpea it is an established fact that some key traits such as maturity, growth habit, and photo-sensitivity influence the expression of genes governing major yield components (Saxena 2008). For example, in a plant with determinate growth habit the expression of number of pod-bearing branches and plant height is masked due to determinacy in plant growth. Similarly, genes responsible for photo-period sensitivity alter the expression of plant growth both under early (April) or late (September) sowings (Saxena 2008). In the former the plants will be tall and have long primary and secondary branches with huge biomass. Such plants take more time to flower due to non-inductive long photo-periods. On the contrary, the plants of the same variety in September sowing remain short with small branches and a few pods. Under this environment the flowering time of the plants is considerably reduced due forced floral induction caused by short photo-periods. Therefore, to minimize such interactions Byth et al. (1981) recommended that important genetic studies should be undertaken within the same maturity group with uniform agronomy. Hence, in pigeonpea the genetic improvement of parental lines should be undertaken within specific heterotic group(s) and for breeding heterotic hybrids the crosses between two diverse groups will be a more productive exercise. To overcome the problems associated with morphological data and high g × e interactions it is advisable to develop heterotic groups based on molecular diversity of the germplasm. In pigeonpea only a few studies on morphological (Manyasa et al. 2008) and genetic diversity using microsatellite molecular markers (Songok et al. 2010) have been reported and the information generated so far is insufficient for use in constituting heterotic groups. Aguiar et al. (2008) demonstrated that SSR markers eliminated environment and G × E effects. Among scientifically established heterotic groups, the crosses within a group are not expected to be highly heterotic; while the crosses between the groups are likely to yield heterotic hybrids.
In the present exercise seven heterotic groups were constituted and the problem associated with plant phenology may not be serious as all the lines and testers had similar maturity (medium) and phenology (non-determinate and semi-spreading) but the g × e interactions were significant. Therefore, confirmation of the observed diversity with support from genomics will be a useful step forward. The data from the present study also suggested that use of the inbreds such as BSMR 198, AKT 9913, ICP 11376, ICPL 20106 etc., which exhibited highly significant GCA effects and belonged to different heterotic groups, can potentially exploit both additive and non-additive genetic variation and it will be useful in breeding both pure line cultivars and hybrids. Crosses involving ICPA 2092 and the testers with significant GCA at all the locations can be used to breed potential inbred materials from which high yielding pure line cultivars and hybrids can be developed.
Classification of testers showed good association with the heterotic pools, developed in the present study. Out of 34 testers, 30 found places in different heterotic groups as previously mentioned. However on the basis of genetic distance it was evident that out of 30, 26 testers showed good association between presence of genetic diversity and heterosis. The association of diversity and heterosis was also revealed with the line ICPL 20106, which grouped distantly with the all three lines and showed the overall best heterotic performance. However, genotype ICP 10934, a field collection from Assam, grouped more distantly than ICPL 20106 but did not find any place in overall performance. Moreover, the same line ICPL 20106 was included in Group KBS of Patancheru, group KB of Latur and group K of Badanpur. To understand these differences in near future molecular markers can be used for assessing the presence of genetic diversity across the lines and its hybrid performances. The SCA effects of two inbred lines from different heterotic groups were greater than those from the same group and this confirmed the observations of Fan et al. (2003). This means that the inbreds representing different groups were genetically more diverse and it played an important role in producing heterotic hybrids.
References
Aguiar CG, Schuster I, Amaral AT Jr, Scapim CA, Viera ESN (2008) Heterotic groups in tropical maize germplasm by test crosses and simple sequence repeat markers. Genet Mol Res 7:1233–1244
Baskaran K, Muthiah AR (2006) Interpretation of hybrid vigour in different cross combinations of pigeonpea [Cajanus cajan (L.) Millsp.]. Res Crop 7:243–248
Byth DE, Wallis ES, Saxena KB (1981) Adaptation and breeding strategies for pigeonpea. In: Proceedings international workshop on pigeonpeas 1:15–19 December 1980. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, pp 450–465
Fan XM, Tan JY, Zhang MS, Li YX, Huang YX, Yang JY, Peng ZB, Li XH (2003) Heterotic grouping for 25 tropical maize inbreds and 4 temperate maize inbreds by SSR markers. Acta Sin 29:835–840
FAO (2012) Online agriculture statistics. http://www.faosat.org
Hallauer AR (1999) Temperate maize and heterosis. In: Coors JG, Pandey S (eds) The genetics and exploitation of heterosis in crops. American Society of Agronomy, Crop Science Society of America, pp 353–361
IIPR (2013) All India coordinated research project on pigeonpea, project coordinator’s report. Indian Institute of Pulses Research, Kanpur 13–15th May, 2013
Jahagirdar JE (2003) Line × tester analysis for combining ability in pigeonpea. Ind J Pulses Res 16:17–19
Jelena S, Snežana MD, Zorica P, Milomir F (2007) Characterization of maize inbred lines based on molecular markers, heterosis and pedigree data. Genetika 39:355–363
Khapre PR, Nerkar YS, Makne VG (1993) Combining ability analysis over cropping systems for grain yield and related characters in pigeonpea. Ind J Genet 53:147–152
Manyasa EO, Silim SN, Githiri SM, Christiansen JL (2008) Diversity in Tanzanian pigeonpea [Cajanus cajan (L.) Millsp.] landraces and their response to environments. Genet Res Crop Evol 55:379–387
Melchinger AE, Gumber RK (1998) Overview of heterosis and heterotic groups in agronomic crops. In: Lamkey KR, Stoub JE (eds) Concepts and breeding of heterosis in crop plants. CSSA Special Publication no. 25. CSSA, Madison, pp 29–44
Narladkar VW, Khapre PR (1996) Heterosis for yield and yield components in pigeonpea. Ind J Pulses Res 4:35–41
Pandey N (1999) Heterosis and combining ability in pigeonpea. Leg Res 22:147–151
Patel GV, Zaveri PP, Pathak AR (1991) Heterosis for morphological attributes in pigeonpea. Ann Agric Res 8:184–186
Phad DS, Madrap IA, Dalvi VA (2009) Heterosis in relation to combining ability effects and phenotypic stability in pigeonpea. J Food Leg 22:59–61
Ramalingam J, Nadarajan N, Vanniyarajan C, Rangasamy P (1997) Combining ability studies involving cms lines in rice. Oryza 34:4–7
Reif JC, Melchinger AE, Xia XC, Warburton ML, Hoishington DA, Vasal SK, Bohn M, Frisch M (2003) Use of SSRs for establishing heterotic groups in sub-tropical maize. Theor Appl Genet 107:947–957
Richey FD (1922) The experimental basis for the present status for corn breeding. J Am Agron 14:1–17
Rohlf FJ (1992) NTSYS-pc (Numerical Taxonomy and Multivariate Analysis System). Version 1.70. Exeter, Setauket
Sameer Kumar CV, Sreelakshmi Ch, Varma P (2009) Studies on combining ability and heterosis in pigeonpea (Cajanus cajan L.). Legume Res 32:92–97
Saxena KB (2006) Seed production systems in pigeonpea. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, 76 pp. ISBN 92-9066-490-8
Saxena KB (2008) Genetic improvement of pigeonpea—a review. Trop Plant Biol 1:159–178
Saxena KB, Kumar RV, Srivastava N, Shiying B (2005) A cytoplasmic-nuclear male-sterility system derived from a cross between Cajanus cajanifolius and Cajanus cajan. Euphytica 145:289–294
Saxena KB, Kumar RV, Tikle AN, Saxena MK, Gautam VS, Rao SK, Khare D, Chauhan YS, Gowda CLL, Sawargaonkar SL (2013a) ICPH2671—the world’s first commercial food legume hybrid. Plant Breed 132(5):479–485
Saxena KB, Kumar RV, Sameer Kumar CV, Saxena RK, Mula M, Pande S, Srivastava RK, Varshney RK, Khare D, Rao SK (2013b) ICPH 2740—a high yielding CMS based pigeonpea hybrid for central and south India. Ind J Genet (submitted, unpublished)
Sharma JR (1988) Statistical and biometrical techniques in plant breeding. New Age International Publishers, New Delhi, pp 17–24. ISBN: 81-224-0888-5
Songok S, Ferguson M, Muigai AW, Silim S (2010) Genetic diversity in pigeonpea [Cajanus cajan (L.) Millsp.] landraces as revealed by simple sequence repeat markers. Afr J Biotechnol 9:3231–3241
Sprague GJ, Tatum LA (1942) General vs. specific combining ability in single crosses of corn. J Am Agron 34:923–932
Srinivas TK, Jain C, Reddy MV (1998) Combining ability studies of sterility mosaic resistant pigeonpea [Cajanus cajan(L.) Millsp.]. Crop Res 15:99–103
Vanniarajan CP, Rangasamy P, Nadrajan N, Ramlingam J (1999) Genotypes grouping based on stability parameters in pigeonpea. J Mah Agric Univ 24:293–294
Venkateswarlu S, Singh RB (1982) Combining ability in pigeonpea. Ind J Genet 42:11–14
Yadav AS, Tank CJ, Acharya S, Patel JB (2008) Combining ability analysis involving Indo-African genotypes of pigeonpea. J Food Leg 21:95–98
Yuan LX, Fu JV, Liu XJ, Peng ZB, Zhang SH, Li XU, Li LC (2000) On significance of heterotic groups theory in hybrid rice breeding. Sci Agric Sin 33:1–9
Acknowledgments
The financial support received from Bill and Melinda Gates Foundation (TL II Project) is acknowledged. Authors acknowledge support of Dr. Vkas Singh for his contribution in improving this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saxena, K.B., Sawargaonkar, S.L. First information on heterotic groups in pigeonpea [Cajanus cajan (L.) Millsp.]. Euphytica 200, 187–196 (2014). https://doi.org/10.1007/s10681-014-1142-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-014-1142-0