Abstract
The occurrence of sub-optimal temperatures during development of immature parasitoids can have important consequences on adult fitness. We investigated the impact of different regimes of low temperature on emergence, differential mortality, longevity and fecundity in Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae). The host-feeding behaviour of adult females was also measured as an indicator of energy reserve at emergence. Acclimation of 30 days at 10 °C or 24 days at 13 °C allowed T. brassicae immatures to develop with a lower mortality than those exposed directly at 5 °C. Longevity and fecundity of females decreased at a lower rate with acclimation at 10 °C suggesting that acclimation at 13 °C may have depleted the energy reserves of individuals more than acclimation at 10 °C. Short photoperiod exposure during the maternal generation had no effect on progeny’s fitness. We found no difference among the treatments in females’ host-feeding behaviours, in differential mortality at emergence, in female’s mobility and in F1 sex ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development under sub-optimal temperature can have important consequences on the fitness of the resulting adult insect. These impacts are of interest in parasitoid insects, as their efficiency when used in biological control programs can be seriously affected. Low temperature can cause physiological dysfunctions (Colinet and Boivin 2011), depletion of energy reserves (Chen et al. 2008) and morphological alterations such as malformations of reproductive organs (Denlinger and Lee 1998), a reduction in body size (Rundle et al. 2004), wings deformity (Dutton and Bigler 1995; Tezze and Botto 2004) and alteration of antennal structure (Pintureau and Daumal 1995). Authors have reported a reduction in percentage of emergence (Uçkan and Gülel 2001; Bayram et al. 2005; Özder and Saglam 2005a), a decrease in longevity (Jalali and Singh 1992; Pandey and Johnson 2005) and in fecundity (Levie et al. 2005; Pandey and Johnson 2005), a drift in emerging sex ratio towards more males (Bayram et al. 2005; Chen et al. 2008) and a reduction in parasitoid’s mobility (Tezze and Botto 2004; Ayvaz et al. 2008). For most of these parameters, the intensity of the response varies between species and some parameters may not even be affected.
At low temperature, even if metabolism slows, development of immatures takes longer and more resources have to be allocated for metabolic maintenance at the detriment of other traits such as reproduction (Boivin 2010). In the case of idiobiont parasitoids, the resources available to the developing immature are fixed as the host is killed or paralysed at the time of oviposition (Godfray 1994). Thus, idiobiont parasitoids such as egg parasitoids are more constrained when subjected to low temperature as immatures than koinobiont, where the parasitoid allows hosts to continue to grow in size after parasitism (Godfray 1994).
The negative consequences of low temperature during immature development may also affect the behaviours associated with foraging for hosts. For instance, low temperature of 4 °C during prepupal stage affects the locomotor activity in Trichogramma nerudai Pintureau and Gerding, resulting in a lower ability to parasitize (Tezze and Botto 2004). Behaviours associated with host location and evaluation can also be modified by reducing the parasitoid’s learning and discrimination capacities (Hance et al. 2007). When the immatures of the mymarid Anaphes victus Huber (Hymenoptera: Mymaridae) are exposed to 4 °C for 3–12 weeks, the resulting adults have reduced capacities of learning external oviposition marks and also to optimize their patch time allocation (van Baaren et al. 2005). Low temperature can affect negatively the fat reserves of the emerging adult (Chen et al. 2008) and individuals with a lower energy reserve may have to search for food sources instead of concentrating their effort on reproduction (Lewis et al. 1998). If the energy reserves and budget of adults are modified by the conditions experienced during their immature development, behaviours such as host-feeding could reflect these changes.
Host-feeding, defined as the consumption of host hemolymph and tissues by the adult females (Jervis and Kidd 1986), could increase with an increase in the costs associated with low temperature exposure. The existing models for host-feeding predict that females host-feed more frequently when their egg load is low and when their nutrients reserve is low (Heimpel and Collier 1996). Observation of the host-feeding behaviour could allow us to measure indirectly the impact of low temperature on the energy reserve of the adult. The negative consequences on fitness are generally proportional to both the temperature and the duration of exposure (Colinet and Boivin 2011) and we expect that host-feeding frequency or intensity should also be proportional.
In nature, gradual changes in seasons allow the parasitoid insect to prepare for the cold season. We refer to “acclimation” when gradual physiological response to altered environmental conditions occurs in laboratory and “acclimatization” when it occurs in nature (Withers 1992; Levie et al. 2005). In addition to the decreasing temperature, the reduced photoperiod is another cue used to prepare for the incoming cold period in nature, but its effect is often only slight over one generation in Trichogramma species (Boivin 1994; Laing and Corrigan 1995; Ivanov and Reznik 2008). The immatures of several Trichogramma species can enter diapause or become quiescent within their host eggs, allowing them to tolerate long periods of suboptimal temperature (Pitcher et al. 2002).
In this paper, we measured the impact of low temperature during immature development on Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae) fitness (emergence, fecundity, longevity, F1 sex ratio and time taken to parasitize ten different eggs (used as an indicator of adult’s mobility)) with different acclimation regimes and with short photoperiod experienced by the maternal generation in laboratory conditions. We also determined if acclimation modified the females’ host feeding behaviour compared to direct low temperature exposure without acclimation.
Materials and methods
Insect rearing
The two T. brassicae strains (named B5 and B6) used in the experiments are commercially available in Canada (Anatis Bioprotection Inc.). Eggs of the Mediterranean flour moth, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) were from a laboratory culture. T. brassicae species originated from Moldavia (Black Sea region) and has been introduced into several countries in Central Europe and in North America, mostly to control the European corn borer (Ostrinia nubilalis Hübner (Lepidoptera: Crambidae)). The parasitoids were reared on cold-killed E. kuehniella eggs at 25 ± 2 °C, 50 ± 5 % r.h. and photoperiod of L16:D8. The developmental threshold for this species is approximatively 10 °C (Babendreier et al. 2003; Rundle and Hoffmann 2003; Rundle et al. 2004) and no development occurs at 5 °C (Rundle and Hoffmann 2003) in Trichogramma species.
Experiment 1
60 ± 30 newly emerged and mated females of each T. brassicae strain were allowed to parasitize 0.1 ± 0.02 g of E. kuehniella eggs (around 4,000 eggs) for 24 h in a petri dish. This ratio females/host eggs ensured a good percentage of parasitism and low superparasitism (trials were done previously). Parasitized eggs were then placed on small strips of 3 M Post-it® (200 ± 50 eggs per strip) (Leatemia et al. 1995; Martel et al. 2011) that were placed in glass vials (3.7 ml) with a muslin cap for aeration. The glass vials were then subjected to five different treatments (including control) that were repeated five times.
Control
For each replicate, one vial of each strain was placed at 25 ± 2 °C, 50 ± 5 % r.h. and photoperiod of L16:D8 until emergence of adults.
No acclimation
To assess the impact of a direct transfer to low temperature without acclimation, for each replicate, four vials per strain were placed directly at 5 ± 1 °C, 30 ± 10 % r.h., in complete darkness for 4, 8, 12 and 16 weeks.
Acclimation at 13 °C
To evaluate the impact of acclimation at low temperature, for each replicate, five vials per strain were placed at 13 ± 1 °C, 55 ± 10 % r.h and L12:D12 for 24 days prior to being transferred at 5 ± 1 °C, 30 ± 10 % r.h., in complete darkness for 0, 4, 8, 12 and 16 weeks.
Acclimation at 10 °C
To assess the impact of acclimation to a temperature closer to the development threshold of 10 °C for T. brassicae (Babendreier et al. 2003; Rundle and Hoffmann 2003; Rundle et al. 2004), for each replicate, five vials per strain were placed in an incubator maintained at 10 ± 1 °C, 60 ± 15 % r.h and L12:D12 for 30 days prior to being transferred at 5 ± 1 °C, 30 ± 10 % r.h., in complete darkness for 0, 4, 8, 12 and 16 weeks.
Acclimation at 13 °C and short photoperiod
To evaluate the impact of short photoperiod during the maternal generation, for each replicate, one vial per strain was placed in an incubator at 25 ± 2 °C, 50 ± 5 % r.h. and L10:D14 until emergence of adults. These females were allowed access to a petri dish containing 0.1 ± 0.02 g of E. kuehniella eggs during 24 h. The contact period was also held under the short photoperiod conditions. We used 60 ± 30 newly emerged and mated females for the contact. Parasitized eggs were placed on small strips made of 3 M Post-it® (200 ± 50 eggs per strip) and placed in vials that experienced the same conditions described in “Acclimation at 13 °C” treatment.
After each treatment, vials were brought back to 25 °C, 50 ± 5 % r.h., L16:D8 until emergence that was checked daily. On the emergence day, five females per vial were isolated to assess 48 h-fecundity and longevity. To assess 48 h-fecundity, each isolated female received cold-killed eggs of E. kuehniella glued on a Post-it® strip (200 ± 50 eggs per strip). After 48 h, the strip was removed and incubated for six days after which parasitized eggs that had turned black were counted to determine fecundity. To assess longevity, the isolated females were observed daily until death. Death was assumed to occur at mid-point between the last two observations. Neither water nor food was offered to the females but a new strip with cold-killed eggs of E. kuehniella was provided to the females until death.
In order to assess the percentages of emergence, the total number of adults emerged was divided by the total number of hosts that turned black during the experiment. The differential mortality between sexes was also measured by comparing the weekly sex ratio within a treatment. Vials were verified for emergence until no emergence occurred for eight weeks.
Experiment 2
The impact of low temperature during immature development on the frequency and intensity of host-feeding by adult females was evaluated through behavioural observations. Parasitized eggs of strain B6 were subjected to three treatments. The parasitized eggs were glued on Post-it® paper strips (200 ± 50 eggs per strip) after contacts and then placed in vials as in experiment 1. In the first and second treatments, the vials were kept for 30 days at 10 ± 1 °C, 60 ± 15 % r.h and L12:D12 and, for second treatment only, the vials were in addition subjected to four weeks at 5 ± 1 °C, 30 ± 10 % r.h. and in complete darkness. All the vials were then brought back to 25 ± 2 °C, 50 ± 5 % r.h., L16:D8 until emergence. For the last treatment (control), the vials were kept at 25 ± 2 °C, 50 ± 5 % r.h. and photoperiod of L16:D8 until emergence. A total of 66 females (22 per treatment) were observed in the 10 h following their emergence. Neither water nor food was offered to the females prior to the observations.
Trichogramma brassicae females were offered a square patch of 16 fresh (less than 24 h) E. kuehniella eggs on a filter paper of 12.5 mm under laboratory conditions (25 ± 2 °C, 30 ± 5 % r.h.). The distance between each egg was 3.0 mm. The behaviours observed were oviposition, walking, resting and host-feeding. The behaviours were recorded using The Observer XT software, a camera and a binocular microscope. Observations started when the female parasitized its first host and ended after ten different hosts were parasitized. Time taken to parasitize ten different hosts was used as an indicator of females’ mobility. The few females (Control: 2, “Acclimation at 10 °C”: 3, “Acclimation at 10 °C + four weeks at 5 °C”: 4) that did not reach ten eggs were excluded from statistics.
At the end of each observation, parasitized eggs were transferred into Beem® polyethylene capsules and incubated at 25 ± 2 °C, 50 ± 5 % r.h. and photoperiod of L16:D8 until emergence. Progeny wasps were then sexed and sex ratio was calculated.
Statistical analyses
Statistical analyses were done using R 2.11.1 software. Data did not confirm normal distribution (Shapiro–Wilk normality test) and non-parametric statistics were chosen. In experiment 1, comparisons between weeks within a treatment and within a strain were done using Kruskal–Wallis tests and, if significant at P < 0.05, followed by a non-parametric multiple comparison test. Mann–Whitney U tests have been used to compare “Acclimation at 13 °C” and “Acclimation at 13 °C and short photoperiod” treatments for the maternal effect and to compare both strains. In experiment 2, comparisons between the three treatments were done using a Kruskal–Wallis test.
Results
Experiment 1
In general, strains B5 and B6 responded the same way to the treatments and only few significant differences were observed between those strains (eight significant results out of 53 tests). The few significant results showed no pattern. For that reason, both strains were combined for analysis. The median percentage of emergence for the control was 88.4 % (first quartile = 84.0 %, third quartile = 97.1 %). We observed emergence up to 16 weeks at 5 ± 1 °C in “Acclimation at 13 °C”, “Acclimation at 10 °C” and “Acclimation at 13 °C and short photoperiod” treatments (Fig. 1b, c, d) while no emergence was observed after 12 weeks in the “No acclimation” treatment (Fig. 1a). Since 16 weeks is the longest duration at low temperature tested in this experiment and there was still emergence after this period with acclimation, we kept 16 weeks as a reference to compare acclimation treatments between them (Table 1). In Figs. 1, 2 and 3, all treatments share the same control that consisted of parasitized eggs placed directly at 25 °C or parasitized eggs that were not stored at 5 °C following the acclimation. The Kruskal–Wallis tests between weeks within a treatment including control showed few differences in differential mortality between sexes (No acclimation: χ2 = 7.289, df = 2, P = 0.026; Acclimation at 13 °C: χ2 = 8.845, df = 5, P = 0.115; Acclimation at 10 °C: χ2 = 11.227, df = 5, P = 0.047; Acclimation at 13 °C and short photoperiod: χ2 = 2.749, df = 5, P = 0.739).
Median longevity and quartiles (in days) of T. brassicae under four treatments. Different letters in a same graph indicate significant differences at P < 0.05 between weeks (after a multiple comparison test following a Kruskal–Wallis test). The symbol “–” indicates that two data or less were available
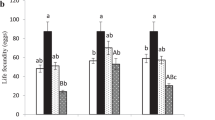
Median fecundity and quartiles (in number of hosts parasitized) of T. brassicae under four treatments. Different letters in a same graph indicate significant differences at P < 0.05 between weeks (after a multiple comparison test following a Kruskal–Wallis test). The symbol “–” indicates that two data or less were available
Longevity decreased with duration of exposure at 5 °C with the acclimation temperature of 13 °C (Fig. 2b), but stayed constant when the acclimation temperature was 10 °C (Fig. 2c). Only two females emerged in the “No acclimation” treatment after eight weeks (Fig. 2a) and one of these females did not survive the first 48 h. Surprisingly, the maximum longevity was observed in the “No acclimation” treatment after four weeks spent at 5 ± 1 °C (Median = 4.5 days, first quartile = 2.5 days, third quartile = 4.5 days) and not in the control (Median = 2.5 days, first quartile = 1.5 days, third quartile = 3.5 days). Longevity was also higher in the “Acclimation at 10 °C” treatment after 16 weeks compared with “Acclimation at 13 °C” and “Acclimation at 13 °C and short photoperiod” (Table 1).
Fecundity decreased gradually with duration of exposure at 5 °C but acclimation at 10 °C and 13 °C maintained it higher for a longer period (Fig. 3b, c, d). Fecundity after 16 weeks in the “Acclimation at 10 °C” treatment was superior to fecundity after 16 weeks in the “Acclimation at 13 °C and short photoperiod” treatment (Table 1). Comparison after 16 weeks with the other treatments was not possible since females did not survive long enough to ensure good fecundity tests. Females of the control treatment successfully parasitized 56 eggs (first quartile = 52 eggs, third quartile = 60 eggs) in 48 h (Fig. 3).
The comparisons between “Acclimation at 13 °C” (without maternal effect) and “Acclimation at 13 °C and short photoperiod” (with maternal effect) showed that placing the previous generation at short photoperiod had little effect on the parameters measured (Table 2). Only the median fecundity after four weeks (P = 0.019) was significantly higher for “Acclimation at 13 °C and short photoperiod”.
The host eggs turned black after 24 days at 13 °C and 30 days at 10 °C which is a sign that development occurred at 13 and 10 °C and that the prepupal stage was reached after both acclimation treatments.
In all treatments, when the vials were brought back to 25 ± 2 °C, 50 ± 5 % r.h. and photoperiod of L16:D8, a single peak of emergence was observed and no emergence was observed later, even after 8 weeks, suggesting that these insects were in a quiescent state during the treatment rather than in diapause.
Experiment 2
The time taken to parasitize ten hosts (the female’s mobility), the frequency (number of host-feeding bouts during the observations) and duration (time spent doing host-feeding during the observations) of host-feeding were not different between “Acclimation at 10 °C” treatment, “Acclimation at 10 °C treatment + four weeks at 5 °C” and the control (Table 3). The number of eggs used for host-feeding during the observations and the sex ratio obtained in F1 generation were also similar between the three treatments. Only data from females performing host-feeding were kept for comparisons (zeros were excluded of the calculation after a χ2 test (5/22, 3/22, 6/22), df = 2, χ2 = 1.269, P value = 0.530).
Discussion
Even at non-freezing temperature, immature parasitoids that develop at sub-optimal temperature usually suffer major fitness costs (van Baaren et al. 2005; Hance et al. 2007; Colinet and Boivin 2011). However, preconditioning individuals by exposing them to low temperature close to their developmental threshold can improve parasitoid survival (Pandey and Johnson 2005). Our results show that T. brassicae can survive and emerge after up to 16 weeks at 5 °C with an acclimation period of 24 days at 13 °C or 30 days at 10 °C. Without acclimation, the majority of individuals did not emerge after only four weeks at 5 °C and 100 % did not emerge after 12 weeks. Özder and Saglam (2005a) observed similar results with T. brassicae and a significant decline in emergence was observed after three weeks at 4 °C. A gradual decrease in longevity and in fecundity is generally observed in egg parasitoids as the duration of low temperature exposure increases (Colinet and Boivin 2011). Acclimations at 10 and 13 °C both improved the parasitoids’ fitness, but 30 days at 10 °C was the best treatment to acclimate T. brassicae. When we compare the acclimation treatments after 16 weeks spent at 5 °C, females subjected to an acclimation treatment at 10 °C obtained higher longevity and fecundity. The relative humidity during storage (30 % r.h.) was probably too low for optimal storage of T. brassicae and could account for some of the mortality observed. However, acclimation at higher temperature decreased the level of mortality suggesting that low relative humidity was not the major mortality factor.
The durations of exposure and acclimation temperature are both very important. During the acclimation of 30 days at 10 °C, and even 24 days at 13 °C, the immature continued to develop and reached the prepupal stage by the end of this period since the host eggs had turned black. The prepupal stage is less sensitive to low temperature than the larvae in Trichogramma spp. (Garcia et al. 2002). The better results obtained after acclimation at 10 °C rather than 13 °C or rather than 13 °C and short photoperiod could be explained by the fact that the metabolic rate was lower at 10 °C and therefore that individuals acclimated at 10 °C entered storage at 5 °C with higher energy reserve than individuals acclimated at 13 °C. It could also explain the higher longevity and fecundity obtained after 16 weeks when acclimation at 10 °C was used. The fact that 10 °C appear to be the approximate development threshold for T. brassicae (Babendreier et al. 2003; Rundle and Hoffmann 2003; Rundle et al. 2004) support the hypothesis of a low metabolic rate at 10 °C.
Acclimation can also have a cost and its global effect on fitness will depend on the overall balance between costs and benefits (Hoffmann and Hewa-Kapuge 2000; Rako and Hoffmann 2006). Here, the cost of being at suboptimal temperature included a decrease in percentage of emergence, longevity and fecundity, but the cost did not overcome the benefits of acclimation. Our results are similar to what has been described in other Trichogramma species. T. ostriniae Pang and Chen can be stored at 9 °C or 12 °C for four weeks without a decline in percentage of emergence, longevity and fecundity (Pitcher et al. 2002) and the same range of temperatures is acceptable to store T. evanescens Westwood (Iacob and Iacob 1972 cited in Pitcher et al. 2002 and Jalali and Singh 1992). T. achaeae Nagaraja and Nagarkatti, T. eldanae Viggiani, T. chilonis Ishii and T. japonicum Ashmead see their fecundity and longevity decline drastically after 21 days at 10 °C (Jalali and Singh 1992) and are thus a little more sensitive than our species. Our results showed that T. brassicae can be exposed to an additional four weeks at 5 °C after the acclimation period without much decrease in fitness. Acclimation can therefore protect Trichogramma’s fitness and quality and should be considered in storage protocols.
When exposed to low temperatures, insects may enter two types of dormancy in order to survive adverse conditions: quiescence and diapause (Danks 1987). Quiescence is an immediate response to a limiting factor but resumption of development occurs as soon as the conditions improve while diapause is a more profound interruption of development, a programmed response usually preceding the adverse conditions and that lasts longer than the adverse conditions (Danks 1987). Trichogramma spp. can use either against low temperature (Boivin 1994). The fact that a single peak of emergence was observed shortly after the vials were brought back to 25 °C, no matter how many weeks they have spent at 5 °C, suggests that T. brassicae was in a quiescent state in all treatments. An immediate resumption of development when adverse conditions end is typical to quiescence (Chen et al. 2008). Diapause of T. brassicae in natural conditions can last 132 days (Özder and Saglam 2005b) but we exceeded that period (acclimation period + X weeks at 5 °C + eight weeks of watching at 25 °C) without seeing other emergences from the black host eggs remaining after the first peak. In many cases, the remaining black eggs were in bad conditions and dry. The two main factors known to induce Trichogramma spp. dormancy are the photoperiod, especially on the maternal generation, and the temperature (degree and time of exposure) (Zaslavski and Umarova 1990; Boivin 1994; Ivanov and Reznik 2008; Pizzol and Pintureau 2008). Conditions similar to the ones we used (30 days at 10 °C and 24 days at 13 °C) induce diapause in other species of Trichogramma (Pintureau and Daumal 1995; Garcia et al. 2002; Ma and Chen 2006), but our two strains only entered quiescence.
Although a short photoperiod during the maternal generation is known to influence positively diapause induction in T. evanescens, T. embryophagum Hartig, T. aurosum Sugonjaev and Sorokina, T. pintoi Voegelé, and T. cacoeciae Marchal (Zaslasvki and Umarova 1990; Smith 1996; Ivanov and Reznik 2008; Pizzol and Pintureau 2008; Reznik et al. 2008), it did not induce it in T. brassicae. The short photoperiod experienced by the mother could have however prepared its progeny to low temperature exposure by reducing the fitness cost associated with it but our results showed that there is no positive maternal effect of photoperiod in quiescent T. brassicae either.
Not all fitness parameters measured were affected by exposure at low temperature. Both sexes had similar survival with the temperatures and duration of exposure tested here. Neutral effect on differential mortality was also observed in T. pretiosym Riley, T. atopovirilia Oatman and Platner, T. acacioi Brun, Moraes and Soares, T. rojasi Nagaraja and Nagakatti, T. lasallei Pinto and T. evanescens. (Ayvaz et al. 2008; Foerster and Foerster 2009).
Female parasitoids use host-feeding to obtain resources and the intensity of host-feeding should reflect the status of their reserves (Heimpel and Collier 1996). The frequency of host-feeding bouts and total duration of them could thus be used as an indicator of the energy reserve of a female. We hypothesized that females that experienced longer duration of sub-optimal temperature during their immature development could have lower energy reserve as they emerge and therefore express more and longer host-feeding bouts. Surprisingly, T. brassicae females did not increase their host-feeding behaviour after storage at 10 °C and exposure at 5 °C during four weeks compared with control treatment. During development, the accumulated reserves are allocated between somatic and reproduction functions (Colinet et al. 2007). Immature females placed below 10 °C (below developmental threshold) must still use some resources for somatic maintenance (Rundle et al. 2004) and burn their fat body reserves (Colinet et al. 2007; Chen et al. 2008) in order to survive adverse conditions. This results in fewer resources for reproduction (Colinet et al. 2007). Particularly for small parasitoids, fat reserves are a non-replaceable resource and potentially a limiting factor (Rivero and West 2002). Trichogramma females can gain proteins, amino acids and lipids by host-feeding on Lepidoptera eggs and as they are moderately synovigenic (i.e. they emerge with part of their egg complement but they are able to produce more during their life (Jervis et al. 2001)), those nutrients are used both for sustaining egg production and for somatic maintenance (Ferracini et al. 2006). The fact that females did not increase their host-feeding behaviour suggests that the fitness cost associated with low temperature exposure was not important enough to modify the frequency or intensity of host-feeding.
The sex ratio of the F1 generation in the second experiment was not different from the control and time taken to parasitize ten different eggs was the same among treatments. This indicates that low temperature did not affect the fertility of male or the capacity of females to move and localize hosts. In T. evanescens, a decrease in the percentage of females in F1 generation after low temperature exposure of parental lines has already been observed (Abd El-Gawad et al. 2010). Some studies have reported alteration in parasitoid’s mobility caused by low temperature injuries when no acclimation was used. For instance, when T. evanescens immatures are exposed to 4 °C for 28 days, the resulting adults see their walking speed decrease (Ayvaz et al. 2008) and when T. nerudai immatures are exposed at the same temperature for 50 days, the flying ability of the resulting adults is affected negatively (Tezze and Botto 2004).
The fitness cost associated with low temperature exposure is reduced in T. brassicae when acclimation is used and 10 °C during 30 days gave better results than 24 days at 13 °C. Both treatments allowed T. brassicae to reach the prepupal stage but acclimation at 13 °C may have depleted the energy reserves of individuals more than acclimation at 10 °C. Acclimation should therefore be used when Trichogramma immatures are stored for a long period in biological control programs.
References
Abd El-Gawad HAS, Sayed AMM, Ahmed SA (2010) Impact of cold storage temperature and period on performance of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). J Appl Sci Res 6:2188–2195
Ayvaz A, Karasu E, Karabörklü S, Tunçbilek A (2008) Effects of cold storage, rearing temperature, parasitoid age and irradiation on the performance of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). J Stored Prod Res 44:232–240
Babendreier D, Kuske S, Bigler F (2003) Overwintering of the egg parasitoid Trichogramma brassicae in northern Switzerland. BioControl 48:261–273
Bayram A, Ozcan H, Kornosor S (2005) Effect of cold storage on the performance of Telenomus busseolae Gahan (Hymenoptera: Scelionidae), an egg parasitoid of Sesamia nonagrioides (Lefebvre) (Lepidoptera: Noctuidae). Biol Control 35:68–77
Boivin G (1994) Overwintering strategies of egg parasitoids. In: Wajnberg E, Hassan SA (eds) Biological control with egg parasitoids. CAB International, Wallingford, UK, pp 219–244
Boivin G (2010) Phenotypic plasticity and fitness in egg parasitoids. Neotrop Entomol 39:457–463
Chen W, Leopold RA, Harris MO (2008) Cold storage effect on maternal and progeny quality of Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae). Biol Control 46:122–132
Colinet H, Boivin G (2011) Insect parasitoids cold storage: a comprehensive review of factors of variability and consequences. Biol Control 58:83–95
Colinet H, Boivin G, Hance T (2007) Manipulation of parasitoid size using the temperature-size rule: fitness consequences. Oecologia 152:425–433
Danks HV (1987) Insect dormancy: an ecological perspective. Biological Survey of Canada, Ottawa, Canada, p 439
Denlinger DL, Lee RE Jr (1998) Physiology of cold sensitivity. In: Hallman GJ, Denlinger DL (eds) Temperature sensitivity in insects and application in integrated pest management. Westview, Boulder, USA, pp 55–96
Dutton A, Bigler F (1995) Flight activity assessment of the egg parasitoid Trichogramma brassicae (Hym.: Trichogrammatidae) in laboratory and field conditions. Entomophaga 40:223–233
Ferracini C, Boivin G, Alma A (2006) Costs and benefits of host feeding in the parasitoid wasp Trichogramma turkestanica. Entomol Exp Appl 121:229–234
Foerster MR, Foerster LA (2009) Effects of temperature on the immature development and emergence of five species of Trichogramma. BioControl 54:445–450
Garcia VP, Wajnberg E, Pizzol J, Oliveira MLM (2002) Diapause in the egg parasitoid Trichogramma cordubensis: role of temperature. J Insect Physiol 48:349–355
Godfray HCJ (1994) Parasitoids, behavioral and evolutionary ecology. Princeton University, Princeton, USA, p 473
Hance T, van Baaren J, Vernon P, Boivin G (2007) Impact of extreme temperatures on parasitoids in a climate change perspective. Annu Rev Entomol 52:107–126
Heimpel GE, Collier TR (1996) The evolution of host-feeding behaviour in insect parasitoids. Biol Rev Camb Philos Soc 71:373–400
Hoffmann AA, Hewa-Kapuge S (2000) Acclimation for heat resistance in Trichogramma nr. brassicae: can it occur without costs? Funct Ecol 14:55–60
Iacob M, Iacob N (1972) Influence of temperature variations on the resistance of the wasp Trichogramma evanescens westwood to storage with a view to field releases. Inst Cerce Prot Plant 8:191–199
Ivanov MF, Reznik SY (2008) Photoperiodic regulation of the diapause of the progeny in Trichogramma embryophagum htg. (Hymenoptera, Trichogrammatidae): dynamics of sensitivity to photoperiod at the immature stages of maternal females. Entomol Rev 88:261–268
Jalali SK, Singh SP (1992) Differential response of four Trichogramma species to low temperatures for short term storage. Entomophaga 37:159–165
Jervis MA, Kidd NAC (1986) Host-feeding strategies in hymenopteran parasitoids. Biol Rev 61:395–434
Jervis MA, Heimpel GE, Ferns PN, Harvey JA, Kidd NAC (2001) Life-history strategies in parasitoid wasps: a comparative analysis of ‘ovigeny’. J Anim Ecol 70:442–458
Laing JE, Corrigan JE (1995) Diapause induction and post-diapause emergence in Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae): the role of host species, temperature, and photoperiod. Can Entomol 127:103–110
Leatemia JA, Laing JE, Corrigan JE (1995) Effects of adult nutrition on longevity, fecundity, and offspring sex ratio of Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae). Can Entomol 127:245–254
Levie A, Vernon P, Hance T (2005) Consequences of acclimation on survival and reproductive capacities of cold-stored mummies of Aphidius rhopalosiphi (Hymenoptera: Aphidiinae). J Econ Entomol 98:704–708
Lewis WJ, Stapel JO, Cortesero AM, Takasu K (1998) Understanding how parasitoids balance food and host needs: importance to biological control. Biol Control 11:175–183
Ma C, Chen Y (2006) Effects of constant temperature, exposure period, and age on diapause induction in Trichogramma dendrolimi. Biol Control 36:267–273
Martel V, Darrouzet T, Boivin G (2011) Phenotypic plasticity in the reproductive traits of a parasitoid. J Insect Physiol 57:682–687
Özder N, Saglam Ö (2005a) Effect of short term cold storage on the quality of Trichogramma brassicae, T. cacoeciae, and T. evanescens (Hymenoptera: Trichogrammatidae). Gt Lakes Entomol 37(3-4):183–187
Özder N, Saglam Ö (2005b) Overwintering of the egg parasitoids Trichogramma brassicae and T. cacoeciae (Hymenoptera: Trichogrammatidae) in the Thrace region of Turkey. J Pest Sci 78:129–132
Pandey RR, Johnson MW (2005) Effects of cool storage on Anagyrus ananatis Gahan (Hymenoptera: Encyrtidae). Biol Control 35:9–16
Pintureau B, Daumal J (1995) Effects of diapause and host species on some morphometric characters in Trichogramma (Hym.: Trichogrammatidae). Experientia 51:67–72
Pitcher SA, Hoffmann MP, Gardner J, Wright MG, Kuhar TP (2002) Cold storage of Trichogramma ostriniae reared on Sitotroga cerealella eggs. BioControl 47:525–535
Pizzol J, Pintureau B (2008) Effect of photoperiod experienced by parents on diapause induction in Trichogramma cacoeciae. Entomol Exp Appl 127:72–77
Rako L, Hoffmann AA (2006) Complexity of the cold acclimation response in Drosophila melanogaster. J Insect Physiol 52:94–104
Reznik SY, Vaghina NP, Voinovich ND (2008) Diapause induction in Trichogramma embryophagum htg. (Hym.: Trichogrammatidae): the dynamics of thermosensitivity. J Appl Entomol 132:502–509
Rivero A, West SA (2002) The physiological costs of being small in a parasitic wasp. Evol Ecol Res 4:407–420
Rundle BJ, Hoffmann AA (2003) Overwintering of Trichogramma funiculatum Carver (Hymenoptera: Trichogrammatidae) under semi-natural conditions. Environ Entomol 32:290–298
Rundle BJ, Thomson LJ, Hoffmann AA (2004) Effects of cold storage on field and laboratory performance of Trichogramma carverae (Hymenoptera: Trichogrammatidae) and the response of three Trichogramma spp. (T. carverae, T. nr. brassicae, and T. funiculatum) to cold. J Econ Entomol 97:213–221
Smith SM (1996) Biological control with Trichogramma: advances, successes, and potential of their use. Annu Rev Entomol 41:375–406
Tezze AA, Botto EN (2004) Effect of cold storage on the quality of Trichogramma nerudai (Hymenoptera: Trichogrammatidae). Biol Control 30:11–16
Uçkan F, Gülel A (2001) The effects of cold storage on the adult longevity, fecundity and sex ratio of Apanteles galleriae Wilkinson (Hym.: Braconidae). Turk J Zool 25:187–191
van Baaren J, Outreman Y, Boivin G (2005) Effect of low temperature exposure on oviposition behaviour and patch exploitation strategy in parasitic wasps. Anim Behav 70:153–163
Withers PC (1992) Comparative animal physiology. Harcourt Brace Jovanovich College, Fort Worth, USA
Zaslavski VA, Umarova TY (1990) Environmental and endogenous control of diapause in Trichogramma species. Entomophaga 35:23–29
Acknowledgments
We thank Josiane Vaillancourt, Danielle Thibodeau and Julie Frenette for supplying insect material and for technical assistance. This research was supported in part by a scholarship from Anatis Bioprotection Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Torsten Meiners
This research is part of the MSc project of Emilie Lessart devoted to the study of the impact of biotic and abiotic factors on the fitness and host feeding behavior in Trichogrammatidae. Dr Guy Boivin is studying the behaviorial and evolutionary ecology of egg parasitoids.
Rights and permissions
About this article
Cite this article
Lessard, E., Boivin, G. Effect of low temperature on emergence, fecundity, longevity and host-feeding by Trichogramma brassicae . BioControl 58, 319–329 (2013). https://doi.org/10.1007/s10526-012-9493-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-012-9493-8