Abstract
Increasing the ability to store mass-reared natural enemies during periods or seasons of low demand is a critical need of the biocontrol industry. We tested the hypothesis that chemicals can enhance long-term cold storage of a predatory mite Phytoseiulus persimilis Athias-Henriot. The research objective was to determine the effect of cryoprotectant and carbohydrate chemicals on in-storage survival of predators. In-storage survival at 8°C was greater for predators sprayed with glycerol (5%, v/v) or glucose (10% and 20%, v/v) than with water spray controls. After 74 days in the cryoprotectant experiment, predator survival declined to 11.5% in the 5% glycerol treatment and 7.8% in the water spray control. After 88 days in the carbohydrate experiment, predator survival declined to 22% in the 20% glucose treatment and 2% in the water spray control. Although many individuals expired within 50 days in both experiments, a few females survived more than 200 days. This research suggests that select cryoprotectants and carbohydrates have a limited capacity to facilitate long-term storage of P. persimilis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased expansion of the biological control industry may depend, in part, on the development of efficient techniques for storing natural enemies (Leopold 2007). Commercial producers often suffer economic losses when there is low demand for a natural enemy during periods of inclement weather or between growing seasons. Long-term storage of natural enemies is desirable because it (1) may allow economic production of a limited quantity year-round rather than a large quantity seasonally, (2) permit stockpiling during periods of low production or unanticipated periods of high demand, assuming that storage facilities are available, and (3) allow rearing to occur at a time of the year when conditions are best for growing host plants (van Lenteren and Tommasini 2003). More research is necessary to demonstrate that long-term cold storage is feasible and does not affect the reproductive potential of natural enemies (see Coudron et al. 2007).
Phytoseiuluspersimilis Athias-Henriot (Mesostigmata: Phytoseiidae) is currently under commercial production throughout the world. It is an effective specialized predator of Tetranychus (Prostigmata: Tetranychidae) spider mites and suppresses populations of Tetranychusurticae Koch on vegetables, small fruits, and other plants grown in greenhouses and in the field (van Lenteren 2003). P. persimilis was first discovered on various plants in nature in the Republic of Algeria (Galazzi and Nicoli 1996). Descendents from a population originating in Chile were first used in biological control of spider mites in greenhouses in several European countries (Zhang 2003). It presently occurs naturally in multiple localities that have a Mediterranean climate.
Short-term cold storage of P. persimilis adults is possible. For example, Morewood (1992) stored P. persimilis adults of unknown age (from mass-rearing cultures) for six weeks at 7.5°C, with a survival rate of 80%, when storage containers held water-moistened filter paper and spider mite eggs. Nicoli and Galazzi (1998) and Luczynski et al. (2008) suggest that young rather than old P. persimilis females have a greater capacity to survive up to 2.5 weeks in cold storage. Long-term cold storage of P. persimilis for several months presents more of a challenge. Although, inducing a natural enemy to transition into a state of diapause can lead to long-term storage (Chang et al. 1996), P. persimilis does not undergo diapause (Morewood 1993). Therefore, diapause induction is not suitable for increasing long-term cold storage of this predator.
Cryoprotectants, produced endogenously, permit survival of insects at freezing temperatures (Storey and Storey 1991; Leather et al. 1993). The possibility that ingestion of cryoprotectants could enhance long-term cold storage of predators, in a non-diapause state, at temperatures above freezing has not been determined previously. Similarly, ingestion of carbohydrates to provide additional energy for predators, even when prey is available, may enhance long-term cold storage. The hypothesis that chemicals can enhance long-term cold storage of P. persimilis was tested. The objective of this study was to measure the effects of chemicals on the survival of P. persimilis adults. This research should determine the feasibility of storing P. persimilis adults for several months at cool temperatures.
Materials and methods
The source of P. persimilis adults used in our experiments was Syngenta Bioline Inc. (Oxnard, California, USA). Predators were removed from shipping enclosures and placed in plastic shoe boxes with spider mite-infested Lima bean Phaseolus lunatus L. (Henderson variety) leaves for one day to allow feeding and mating. Spider mites were reared for multiple generations on Lima bean in a greenhouse at USDA-Agricultural Research Service, in Stoneville, Mississippi, USA. The original source of spider mites was Syngenta Bioline Inc.
Distilled water-based solutions (5, 10, 20% v/v) of the following chemicals were prepared and used in our experiments. In experiment A, three concentrations of glycerol and ethylene glycol along with a water spray control resulted in seven treatment groups. In experiment B, three concentrations of glucose and sucrose along with a water spray control resulted in seven treatment groups. Glycerol and ethylene glycol were from Anachemia Canada Inc. (Montreal, Quebec, Canada), sucrose from Fisher Scientific (Fair Lawn, New Jersey, USA), and glucose from Sigma–Aldrich (St. Louis, Missouri, USA). In both experiments, distilled water was the solvent in test solutions and the water spray control.
Using an Airbrush™ (Paasche Airbrush Co., Chicago, Illinois, USA), solutions were sprayed onto groups of predators (adults received from Syngenta Bioline Inc. the previous day) within a Petri dish arena (15 × 60 mm, diam) containing one young bean leaf infested with spider mite eggs at the base, on the same day in a randomized design. Lids were quickly removed (in 20–30 s) before spraying and returned to cover the contents (at the base) within 30 s after spraying had ceased. The distance between the Airbrush™ nozzle and the edge of the arena was approximately 15 cm each time spraying occurred. Predators often drank the test and control solutions, which formed microscopic droplets on their legs and body. Micro-droplet diameter averaged 17.4 μm (n = 82 droplets), as determined by using computer-based imaging software (Image Pro® Plus, Media Cybernetics, Inc, Maryland, USA) with camera attached to a stereo zoom microscope. Gaede (1992) indicated that partially dehydrated P. persimilis females drink liquid water when it is available. Ingestion occurred when predators “cleaned” themselves of the droplets, by threading the first legs (leg I) between the pedipalps and chelicerae. Droplets were wiped off the dorsal body surface by using the hind legs (leg IV); then the first legs cleaned the hind legs of any droplets. As before, the first legs were threaded through the pedipalps and chelicerae. Adding a dye (i.e., food coloring used to provide color to human food) to water prior to spraying and spraying predators confirmed that molecules were in fact ingested during this process. Within 1 h after spraying, no droplets were present on the mites or on the arena interior.
In experiments A and B, the average number of predators per arena was 19 (range = 7–50) and 44 (range = 17–75) adults at the onset, respectively, because some predators managed to escape from arenas while spraying. Twenty-one arenas (three replicate arenas in each of the seven treatment groups) were used in both experiments. Predators were held in the same arenas that they were sprayed in and placed in a growth chamber (60% RH, 16 h photophase) at 23°C until 24 h later (day 1), then 15°C until 72 h later (day 3), then 8°C for all subsequent days of inspection. A water-soaked cotton ball was placed at the base of each arena to maintain high humidity. Although not measured, the relative humidity inside each arena was probably 70–90%. On inspection dates, the number of living and dead predators in each arena was determined. Dead predators were removed from arenas. Old bean leaf pieces were replaced with fresh pieces infested with spider mite eggs. Inspection to determine predator survival required less than 10 min per arena and occurred at room temperature (23°C) in the laboratory. All arenas were encased with Parafilm® as another measure to maintain high humidity. Each arena was returned to the growth chamber immediately after inspection.
Experiments A and B began on 9 August and 1 September 2006, respectively. In experiment A, the percentage of predators alive in each replicate arena was determined on 1, 3, 8, 17, 29, 44, 74, 106, 147, 163, 179, 200, 218 days after spraying. In experiment B, the percentage of predators alive in each replicate arena was determined on 1, 3, 12, 27, 88, 103, 126, 142, 157, 172, 187, 204, and 225 days after spraying. Ninety-one observations (repeated measures) of predator survival were made in both experiments.
The female predators capable of surviving until the end of the storage experiments were transferred to clean arenas containing fresh bean leaf pieces infested with an excess of spider mites and held at room temperature (23°C) in the laboratory. The oviposition rates of these few predators were determined.
In-storage survival data were analyzed following a completely randomized design. Data were arcsine transformed prior to analysis. A Kaplan–Meier survival analysis (KMSA), followed by a Log-Rank test of equality, determined if there was a difference between survival curves in the treatment groups. Repeated measures analysis of variance (RM-ANOVA) tested the significance of cryoprotectants or carbohydrates (and concentration) on the percentage of predators alive at each observation date during the study. Means were considered significantly different if P ≤ 0.05. The Holm–Sidak multiple comparison method separated means after the RM-ANOVA. SigmaStat 3.0.1 (Systat Software Inc., Richmond, California, USA) and SAS 9.1.3 (SAS Institute Inc., Cary NC, USA) software assisted with analysis of data. All data presented in this manuscript represent non-transformed values. Data on post-storage fecundity of the few remaining predators did not necessitate statistical analysis.
Results
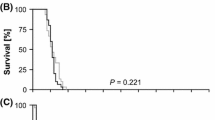
In experiment A, survival curves differed significantly between some treatments when considering all chemicals and concentrations in the same analysis (KMSA; Log-Rank test, χ2 = 30.7, df = 10, P < 0.001). Cryoprotectants affected the in-storage survival of P. persimilis (RM-ANOVA, F = 21.2; df = 6, 72; P < 0.001). Predators had a slightly greater ability to survive long-term storage in the 5% glycerol treatment than any other treatments, with the exception of the water spray control (Fig. 1A, Table 1). Survival was least for predators in the 20% ethylene glycol treatment group. The percentage of predators still alive 29 days after the beginning of the experiment was 46% in the 5% glycerol treatment and 27% in the water spray control. At 74 days, predator survival had dropped to 11.5% in the 5% glycerol treatment and 7.8% in the water spray control. On the last day of cold storage in experiment A (day 218), one female was alive in one arena of the 5% glycerol treatment and the water spray control. Predators in all other treatment groups had expired.
Mean ± SE percentage of P. persimilis alive in cold storage after treatment with 5, 10, and 20% concentrations of select cryoprotectants (A) or carbohydrates (B) in separate experiments. Sample size was 91 observations (repeated measures). GLY, ETH, GLU, and SUC represent glycerol, ethylene glycol, glucose, and sucrose, respectively
In experiment B, survival curves differed significantly between treatments (KMSA; Log-Rank test, χ2 = 31.02, df = 9, P < 0.001). Carbohydrates affected the in-storage survival of P. persimilis (RM-ANOVA; F = 20.7; df = 6, 72 P < 0.001). Predators had a slightly greater ability to survive long-term storage in the 10% and 20% glucose treatments (Fig. 1B, Table 1). The percentage of predators alive at 27 days from the beginning of the experiment was 60–72% in the 5–20% glucose treatment groups, and 59% survived the water spray control. At 88 days, predator survival had declined to 12% and 22% in the 10% and 20% glucose treatment groups, respectively. Only 5% were alive in the 5% glucose treatment and 2% were alive in the water spray control. On the last day of cold storage in experiment B (day 225), one female was still alive in an arena which had been treated with 5% glucose. Predators in all other treatment groups had expired.
The surviving female in the water spray control in experiment A produced 56 eggs (95% hatched) over an oviposition period of 21 days. The surviving female in the 5% glycerol treatment produced only one egg (that did not hatch) over an oviposition period of one day. The surviving female in the 5% glucose treatment in experiment B produced six eggs (100% hatched) over an oviposition period of two days.
Discussion
Several chemicals slightly enhanced the long-term storage survival of P. persimilis adults in comparison to controls. The observation that 46% of predators treated with 5% glycerol were alive at approximately one month after treatment, in comparison to 27% alive in the water spray control, suggests a limited role of low concentrations of glycerol on survival. Glycerol might have been stored in the cells of P. persimilis and provided some protection from extended exposure to cold. The observation that 72% of predators treated with 20% glucose were alive at approximately one month after treatment, in comparison to 59% alive in the water spray control, suggests that moderate concentrations of glucose have a limited role in survival. In addition, glucose may have served as an additional source of energy that circumvented starvation. A 1:1 glucose to fructose solution was a food supplement that increased the lifespan and fecundity of P. persimilis in comparison to cohorts presented with water only at room temperature (Rojas and Morales-Ramos 2008). P. persimilis obtain glucose and fructose from extrafloral nectaries on Lima bean infested with spider mites (Choh et al. 2006).
The quality of food that predators are given, prior to cold storage, may influence survival rate during storage (Coudron et al. 2009). In addition, storage of predators in enclosures provisioned with food (live prey, artificial food, or plant products) can have beneficial effects on in-storage survival (Morewood 1992; Tauber et al. 1997; Thorpe and Aldrich 2004).
The observation that 50% of P. persimilis adults (of unknown age) treated with water only survived for approximately one month at 8°C was lower than anticipated. Morewood (1992) showed that 80% of untreated P. persimilis adults of unknown age, but from a mass-rearing culture, survived 1.5 months at 7.5°C. Reasons for the differences in survival rate between this study and the Morewood (1992) study are unclear. Presumably, progeny rather than original adults from a commercial producer were tested in the Morewood (1992) study. This could explain, in part, the differences in predator survival between the studies.
Other factors might have affected the results of the cold storage experiments in this study, including the age and health of predators upon arrival in our laboratory. The age of females in our experiments was unknown, although we assume that they represented young adults of the same generation. Luczynski et al. (2008) showed that young rather than old P. persimilis females (obtained directly from a commercial producer) have a better capacity to survive storage, for up to 2.5 weeks at 5°C or 10°C. Shipping of predators over two days in crowded enclosures, inside Styrofoam™ boxes, under variable temperature and humidity conditions might have also affected the health of the predators. Bjornson and Raworth (2005) showed that internal temperature within Styrofoam™ boxes varies in direct relationship to ambient temperature. Finally, manipulating predators during transfer to arenas, monitoring mortality and removing dead individuals from arenas, during this study, could have affected their health.
Gaede (1992) found that P. persimilis females lived up to a maximum of 120 days, if they consumed at least one spider mite every three days at 20°C under very humid conditions. In light of the Gaede (1992) study, the observation that a few P. persimilis females survived over 200 days at 8°C in this study is believable. The cooler temperatures would reduce metabolic rate and therefore decrease the expenditure of energy reserves. According to Morewood (1993), P. persimilis does not undergo diapause under any conditions. Therefore, the few adults surviving for so long in the cold were probably not in a state of diapause.
The ability of a predator in the water spray control to produce more than 50 progeny after more than 200 days in cold storage is interesting. Note that a non-sprayed P. persimilis female produced 50 eggs (94% hatched) after more than 200 days at 8°C and non-sprayed, non-stored females produced an average of 69 eggs (100% hatched) within an average lifespan of 32 days at 23°C (EWR and ZW, unpublished data).
In conclusion, our hypothesis that cryoprotectant and carbohydrate chemicals can enhance the long-term storage of P. persimilis was partially true. This research suggests that select cryoprotectants and carbohydrates might have limited positive effects on P. persimilis survival. Future research on cold storage of this predator should consider the effect of shipping conditions on the health of adults prior to experimentation. Although testing predators (directly from a commercial producer) of unknown age, gender, and mating status gives a measure of reality, it introduces a number of complications that can confound the interpretation of results of laboratory tests. Lab-culturing then testing first or second-generation adult progeny should facilitate the obtainment of concise, clear-cut results in future tests on long-term storage of predatory mites. In future studies, P. persimilis females should be removed at monthly intervals, during long-term storage, to assess fecundity. This procedure would provide a clear picture of the interaction between storage time and fecundity of P. persimilis.
References
Bjornson S, Raworth DA (2005) Styrofoam boxes used for shipping mass-reared beneficial arthropods: internal temperature responds rapidly to ambient temperature. Biocontrol Sci Technol 15:755–760
Chang Y-F, Tauber MJ, Tauber CA (1996) Reproduction and quality of F1 offspring in Chrysoperla carnea: differential influence of quiescence, artificially induced diapause, and natural diapause. J Insect Physiol 42:521–528
Choh Y, Kugimiya S, Takabayashi J (2006) Induced production of extrafloral nectar in intact lima bean plants in response to volatiles from spider mite-infested conspecific plants as a possible indirect defense against spider mites. Oecologia 147:455–460
Coudron TA, Ellersieck MR, Shelby KS (2007) Influence of diet on long-term cold storage of the predator Podisus maculiventris (Say) (Heteroptera: Pentatomidae). Biol Control 42:186–195
Coudron TA, Popham HJR, Ellersieck MR (2009) Influence of diet on cold storage of the predator Perillus bioculatus (F.). BioControl 54:773–783
Gaede K (1992) On the water balance of Phytoseiulus persimilis A–H and its ecological significance. Exp Appl Acarol 15:181–198
Galazzi D, Nicoli G (1996) Comparative study of strains of Phytoseiulus persimilis Athias-Henriot (Acarina Phytoseiidae). I. Development and adult life. Boll Ist Ent “G Grandi” Univ Bologna 50:215–231
Leather SR, Walters KFA, Bale JS (1993) The ecology of insect overwintering. Cambridge, Great Britain
Leopold RA (2007) Colony maintenance and mass-rearing: using cold storage technology for extending the shelf-life of insects. In: Vreysen MJB, Robinson AS, Hendrichs J (eds) Area-wide control of insect pests: from research to field implementation. Springer, Dordrecht, pp 149–162
Luczynski A, Nyrop JP, Shi A (2008) Pattern of female reproductive age classes in mass-reared populations of Phytoseiulus persimilis (Acari: Phytoseiidae) and its influence on population characteristics and quality of predators following cold storage. Biol Control 47:159–166
Morewood WD (1992) Cold storage of Phytoseiulus persimilis (Phytoseiidae). Exp Appl Acarol 13:231–236
Morewood WD (1993) Diapause and cold hardiness of phytoseiid mites (Acarina: Phytoseiidae). Eur J Entomol 90:3–10
Nicoli G, Galazzi D (1998) Quality control of cold stored Phytoseiulus persimilis Athias-Henriot (Acarina: Phytoseiidae). Boll Ist Ent “G Grandi” Univ Bologna 52:61–73
Rojas MG, Morales-Ramos JA (2008) Phytoseiulus persimilis (Mesostigmata: Phytoseiidae) feeding on extrafloral nectar: reproductive impact of sugar sources in presence of prey. Biopestic Int 4:1–5
Storey KB, Storey JM (1991) Biochemistry of cryoprotectants. In: Lee RE, Denlinger DL (eds) Insects at low temperature. Chapman & Hall, New York, pp 64–93
Tauber MJ, Albuquerque GS, Tauber CA (1997) Storage of nondiapausing Chrysoperla externa adults: influence on survival and reproduction. Biol Control 10:69–72
Thorpe KW, Aldrich JR (2004) Conditions for short-term storage of field-collected spined soldier bug, Podisus maculiventris (Say) (Heteroptera: Pentatomidae), adults prior to augmentative release. J Entomol Sci 39:483–489
van Lenteren JC (2003) Commercial availability of biological control agents. In: van Lenteren JC (ed) Quality control and production of biological control agents: theory and testing procedures. CAB International, Oxon, UK, pp 167–179
van Lenteren JC, Tommasini MG (2003) Mass production, storage, shipment and release of natural enemies. In: van Lenteren JC (ed) Quality control and production of biological control agents: theory and testing procedures. CAB International, Oxon, UK, pp 181–189
Zhang Z-Q (2003) Mites of greenhouses: identification, biology and control. CAB International, Oxon, UK
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10526-010-9305-y
Rights and permissions
About this article
Cite this article
Riddick, E.W., Wu, Z. Potential long-term storage of the predatory mite Phytoseiulus persimilis . BioControl 55, 639–644 (2010). https://doi.org/10.1007/s10526-010-9297-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-010-9297-7