Abstract
Telenomus remus (Nixon) is a promising egg parasitoid for the management of Spodoptera frugiperda (J. E. Smith). This species has been successfully reared on alternative hosts under laboratory conditions. However, the production of biocontrol agents is often out of sync with the demands in the field. Appropriate cold storage techniques can drastically prolong their shelf-life to synchronize the release schedule with field needs and reduce production costs. Past studies on the cold storage of T. remus only focused on certain developmental stages, but not all stages. Here, we comprehensively evaluated the impacts of storage temperature (8, 11, and 14 °C) and duration (7, 14, 21, 28, and 35 days) on the maternal emergence and offspring fitness of T. remus stored at different developmental stages (first instar larvae, second instar larvae, prepupae, and pupae) using Spodoptera litura (Fabricius) eggs as alternative hosts. For each developmental stage, emergence percentage and parasitism capacity of parents all decreased with increased storage duration and decreased storage temperature. Maternal female longevity, offspring emergence percentage and percentage of females were barely affected by cold storage. We concluded that storage of the first instar larvae at 14 °C for 21 days was the optimum storage scheme for T. remus. Our findings can be directly used as guidance in mass production and storage of this parasitoid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key messages
-
We comprehensively and systematically evaluated the impact of cold storage on Telenomus remus at different developmental stages.
-
We concluded that the ideal storage conditions for Telenomus remus in Spodoptera litura eggs were storing the first instar larvae at 14 °C for up to 21 days.
-
This information can allow Telenomus remus products to be efficiently stored for later release and to maintain the populations at relatively lower costs.
Introduction
Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), native to tropical and subtropical America, is a notorious, invasive agricultural pest worldwide (Sparks 1979). The pest presents a serious threat to agricultural production and food security due to its polyphagous behavior (Montezano et al. 2018), high reproductive level (CABI 2019), strong migratory ability (Njuguna et al. 2021), and powerful adaptability (CABI 2019). For example, since the invasion of China in January 2019 (Sun et al. 2021), it spreads to 27 provinces (autonomous regions and municipalities) by 2020 (Zhou et al. 2021). Synthetic chemical pesticides are currently considered the most effective emergency strategy for rapid control of this species; however, the frequent or inappropriate application of pesticides may cause pesticide resistance or other environmental and health issues (Day et al. 2017; Rioba and Stevenson 2020). Consequently, identifying a sustainable and eco-friendly method to control this pest is urgently needed.
To date, approximately 290 different natural enemies have been reported to control S. frugiperda worldwide (Chen et al. 2019a, b). Among them, Telenomus remus (Nixon) is one of the most promising biological control agents. This solitary egg parasitoid has been successfully applied in augmentative biological control in several countries or regions (Cave 2000; Salazar-Mendoza et al. 2020; Dong et al. 2021). Its success is mainly attributed to the ability of the female parasitoid to effectively locate and parasitize the inner layer of S. frugiperda egg masses (Cave 2000; Laminou et al. 2020; Salazar-Mendoza et al. 2020). In order to control S. frugiperda using natural enemy insects, millions biocontrol products need to be produced efficiently and cost-effectively. Therefore, the identification of alternative hosts and suitable mass-rearing conditions are necessary for the establishment of efficient rearing system to produce natural enemy products. At present, most of the research on T. remus were mainly based on the mass rearing system with Corcyra cephalonica (Stainton) eggs as alternative host, including studies on mass-rearing (Vieira et al. 2017; Naranjo-Guevara et al. 2020), cold storage (Queiroz et al. 2017a), quality control (Pomari-Fernandes et al. 2016), and practical application (Queiroz et al. 2017b). A study in China, however, found that the local populations of T. remus cannot parasitize and develop in C. cephalonica eggs. Meanwhile, this report also suggested that Spodoptera litura (Fabricius) eggs could serve as a potential alternative host (Huo et al. 2019). Based on this, our previous works have confirmed this (Chen et al. 2021a) and identified the optimal rearing conditions (Chen et al. 2021b).
Another problem hindering mass-rearing of parasitoids is their short storage period. Unlike pesticides, beneficial insects have a relatively short shelf-life and must therefore be produced as soon as possible before application (Glenister and Hoffmann 1998). If the productivity of the laboratory and the requirements in the field are out of sync, the natural enemy products will either be wasted or insufficient to meet demand. Suitable storage techniques of biological control agents to extend their shelf-life would be a valuable tool to reduce production costs, improve utilization efficiency, maintain sufficient product availability year-round, and facilitate long-distance transport (Colinet and Boivin 2011; Cagnotti et al. 2018). The two forms of dormancy that can be applied to extend the shelf-life of beneficial insects for the long- and short-term are diapause and quiescence (Ghosh and Ballal 2019; Lu et al. 2019). Diapause, a developmental phase combining developmental arrest and adaptive physiological changes, requires a series of rigorous induction, maintenance, and termination treatments. Once insects enter diapause, development takes time to resume even after favorable conditions are restored (Chapman 1998). Quiescence is a temporary slowdown or cessation of development, which will resume soon after favorable conditions restored, thus allowing flexibility to meet the unpredictable demands of the field (Ghosh and Ballal 2019). Temporary storage of parasitoids at low temperature induces quiescence, which is the subject of this study because of its superior flexibility compared to diapause induction.
The initial studies on cold storage of insect parasitoids began in the 1930s (King 1934; Hanna 1935). Since then, a large number of insect species were studied (Colinet and Boivin 2011). The adaptability of a parasitoid to cold storage may be related to their specific evolutionary history; thus, it is necessary to explore the effects of the main endogenous and exogenous factors on each species or strain in order to successfully identify the optimal conditions for their cold storage (Colinet and Boivin 2011). Several studies on the cold storage of other parasitoids have found negative effects on their quality and fitness. Parasitoids may show varying degrees of reduction in emergence percentage, longevity, fecundity, sex ratio, flight capacity, and morphological characteristics after cold storage. In addition, adverse impacts caused by cold storage may be transmitted to their offspring (Colinet and Boivin 2011; Zhang et al. 2020). Thus, evaluating the fitness of the offspring produced from parental generations treated with cold storage is necessary to determine the ideal storage conditions, so as to minimize the harm to the biocontrol potential of the parasitoids in the field.
Two significant exogenous factors affecting the fitness and survival of parasitoids during cold storage are temperature and storage duration (Rathee and Ram 2018). Parasitoids are usually stored at low temperatures ranging from 0 °C to 15 °C (van Lenteren and Tommasini 2002). The optimal storage temperatures should be based on the balance between chilling injuries and reduced metabolic rate (Rathee and Ram 2018). One of the endogenous factors that must be considered is developmental stage of the parasitoids being stored, as sensitivity to cold temperature varies with developmental stage and parasitoid species (Rathee and Ram 2018). Some cases have previously demonstrated that pupae have better cold resistance than eggs, larvae, or adults (Jalali and Singh 1992; Nakama and Foerster 2001; Kidane et al. 2015), and it is generally accepted that the pupae are the most appropriate stage for storage (van Lenteren and Tommasini 2002; Colinet and Boivin 2011; Rathe and Ram 2014). However, there is no shortage of studies arguing that the egg (Bowler and Terblanche 2008), larva (Mohamed and El-Heneidy 2020), or adult stages (Bayram et al. 2005; Foerster and Doetzer 2006) are more suitable. For example, Mohamed and EI-Heneidy (2020) found that Trichogrammatoidea bactrae (Nagaraja) larvae could be stored at 10 °C for 2.5 months without significant losses of fitness for them or their progeny.
Although some studies have evaluated the impacts of cold storage on certain developmental stages of T. remus reared on C. cephalonica eggs and other host eggs (Cave 2000; Queiroz et al. 2017a), no comprehensive research has ever been conducted on the effects of cold storage on all developmental stages of T. remus when reared on S. litura eggs. Therefore, to identify the optimal conditions for storing this promising parasitoid of S. frugiperda in areas where C. cephalonica eggs are not suitable, and to provide guidance for future large-scale production of parasitoid in a cost-effective manner, this study aimed to evaluate the effects of storing different developmental stages of T. remus reared on S. litura eggs at different temperatures for varying durations on parasitoid performance and fitness. We hypothesized that certain combinations of storage temperature, storage duration, and developmental stage during storage minimize the effects on parasitoid fitness and efficacy and would thus be ideal for efficient mass production of this biocontrol agent.
Materials and methods
Hosts
The S. frugiperda culture was initiated from larvae collected from maize fields in Kunming, Yun’nan Province, China, in 2019. The population was reared continuously at 28 ± 1 °C, 60 ± 5% relative humidity (RH), 16:8 Light (L): Dark (D) in a climatic incubator (RXZ-500, Ningbo Jiangnan Instrument Factory, Zhejiang Province, China). Caterpillars were maintained in plastic containers (34 cm length × 22 cm width × 4 cm height) with steel mesh-infused lids and fed by fresh maize leaves. To avoid cannibalism (He et al. 2022), the fourth instar larvae were reared separately in sauce boxes (3 cm height × 5 cm diameter) using an artificial diet as described by Greene et al. (1976), but replacing the pinto beans with soybean powder. Pupae were collected and placed in cages (24 cm diameter × 28 cm height) whose upper opening and the inner surface were covered with wet gauze and wax paper as egg-laying substrates for the emerged adults, and 20% honey solution was supplied as adult food. Substrates with egg masses were collected, and the honey solution was replaced daily.
S. litura egg masses were originally provided by the Institute of Plant Protection, Jilin Academy of Agricultural Sciences, Jilin Province, China. The population was reared on artificial diet (Chen et al. 2000) using the same methods and conditions as for S. frugiperda.
Parasitoid
T. remus were initially collected from parasitized S. frugiperda egg masses in a maize field in Jinhua, Zhejiang Province, China, in 2019. To maintain the experimental populations, the newly emerged wasps were released into transparent plastic tubes (10 cm height × 2.5 cm diameter) for mating for about one hour (h). Fresh S. frugiperda eggs (< 24 h) glued onto paper cards were put into these tubes and maintained in a climatic incubator (26 ± 1 °C, 70 ± 5% RH, 14L:10D). The parasitized eggs were removed to a new tube after 24 h and kept under the same conditions until the offspring emerged. Adult parasitoids were provided with a 20% honey solution as food.
Effects of storage temperature, storage duration, and developmental stage on the emergence parameters of T. remus
The trials consisted of four developmental stages of T. remus (first instar larvae, second instar larvae, prepupae, and pupae), three cold storage temperature levels (8, 11, and 14 ± 1 °C, all with 70 ± 5% RH and complete darkness), and five cold storage durations (7, 14, 21, 28, and 35 days), for a total of 60 different treatments. Parasitized eggs kept continuously under the rearing conditions (26 °C) without cold storage were served as the control group. As in previous similar studies on other parasitoids (Cagnotti et al. 2018; Lin et al. 2021), to obtain T. remus at different developmental stages, S. litura egg cards were exposed to mated female parasitoids for one hour under rearing conditions. As per a study on the identification of T. remus developmental stages (Gerling 1972; Chen et al. 2021a), parasitized eggs were maintained under rearing conditions for 48, 72, 96, and 120 h to develop into first instar larvae, second instar larvae, prepupae, and pupae, respectively. The egg cards (approximately 60 parasitized S. litura eggs/card) with corresponding developmental stages were individually placed into a plastic tube as mentioned above and sealed with a sponge plug, after which the tubes were transferred to different low temperature conditions and stored for 7–35 days. Each tube was observed daily to determine whether T. remus adults had emerged during cold storage. At the end of cold storage, the egg cards were transferred back to rearing conditions until emerged (F0). In order to access the emergence percentage and the percentage of females of the F0 generation, the number of female and male adults that emerged from parasitized eggs after cold storage was recorded. The females’ antennae have a four-segmented club, which is the main morphological feature that distinguishes them from the males (Cave 2000). Each treatment was performed with 10 replicates (namely, 10 egg cards).
Quality assessment of maternal and offspring parasitoids
To screen the optimal storage conditions, the treatments with no significant difference from the control group in emergence percentage and in female proportion of the F0 generation were selected for further evaluation. These treatments are shown in Table 1. In order to evaluate the emergence time from the end of cold storage to adult emergence for these 14 treatments, the number of emerged adults was observed daily in this experiment. Each treatment was repeated as described above with 10 replicates (10 egg cards).
To estimate the effects of cold storage on female longevity and parasitism capacity of the F0 generation, one recently mated female T. remus (< 24 h) from these egg cards was randomly selected and introduced into a plastic tube with about 160 fresh S. frugiperda eggs (< 24 h), and provided with a droplet of 20% honey solution as food in the inner surface of the tube. T. remus female adults developing under rearing conditions (< 24 h) were used as control group. These tests were also conducted under the conditions as described above (26 °C). At least 20 replicates were carried out per treatment. Our previous study indicated that the majority of the parasitism activity of T. remus occurs in the first five days of their life under these conditions (Chen et al. 2021a); thus, during that time, the S. frugiperda eggs were refreshed daily. Then, in order to calculate their longevity, the number of surviving female adults was recorded daily until they died. Parasitized eggs were transferred into a new tube for continued development under rearing conditions until the offspring emerged (F1). During this process, to prevent S. frugiperda larvae from attacking parasitized eggs, any larvae that emerged from unparasitized eggs were promptly cleared away using a brush. The numbers of parasitized eggs and offspring of both sexes were recorded to determine the parasitism capacity of the F0 generation, emergence percentage and percentage of females in the F1 generation.
Data analysis
As in previous studies on other parasitoids (Lu et al. 2019; Lin et al. 2021), three-way analysis of variance (ANOVA) was employed to estimate the effects of storage temperature, storage duration, developmental stage and their interaction on the emergence percentage, percentage of females, number of parasitized eggs of the F0 generation and the offspring fitness. The means were separated by Dunnett’s test at a 0.05 level to identify the differences between all treatments and controls, and the treatments with no significant differences from the control were screened for further evaluation. Tukey’s honest significant difference (HSD) test at a 0.05 level was adopted to access the differences among the eligible treatments. The Cox proportional hazards model was applied to assess emergence time and female longevity (P < 0.05) (Costa-Lima et al. 2019; Bertanha et al. 2021; Dong et al., 2021). All the percentage data were arcsine square-root-transformed to homogenize the variances prior to analysis, and the data for the number of parasitized eggs were log 10-transformed to fit a normal distribution. Data were analyzed with SPSS version 19.0 (IBM Corp., Chicago, IL, USA) and figures created with GraphPad Prism version 8.0 (GraphPad Software, Inc., San Diego, CA, USA) for Windows.
Results
Emergence percentage and percentage of females of the F 0 generation after cold storage
Emergence percentage of T. remus after cold storage was significantly affected by the interactions among storage temperature, developmental stage, and storage duration (Table 2). Generally, for all developmental stages, emergence percentage decreased with increasing storage duration and decreasing storage temperature. However, the prepupae stored at 14 °C for 28 days and pupae stored at 14 °C for 21 days emerged before the ends of their cold storage. In comparison with the control group, no significant differences were observed for the first instar larvae stored at 11 °C for 7–14 days and 14 °C for 7–21 days; the second instar larvae stored at 8 °C for 7 days and 14 °C for 7–14 days; prepupae stored at 11 and 14 °C for 7–14 days; and pupae stored at 11 °C for 14 days and 14 °C for 7–14 days (F60, 569 = 461.893, P < 0.0001) (Table 3). Percentage of females was influenced by storage temperature and storage duration but not by developmental stage. The interactions among these three factors also significantly influenced this parameter, except for the storage temperature-by-storage duration interaction (Table 2). Most treatments had a similar percentage of females compared with the control, except that the second instar larvae stored at 8 °C for 7 days had a significantly lower ratio, and the second instar larvae stored at 11 and 14 °C for 35 days had a higher ratio (F43, 416 = 4.586, P < 0.0001) (Table 4).
Emergence time, female longevity and parasitism capacity of the 14 F 0 generation treatments after cold storage
According to the Cox analysis, no difference in emergence time among each treatment was observed after cold storage of the second instar larvae and pupae. However, for the first instar larvae and prepupae, the emergence time was significantly influenced by storage temperature and storage duration. Generally, at specific developmental stage, the emergence time decreased with increasing storage temperature and/or increasing storage duration (Fig. 1). The analysis showed that storage temperature and storage duration had no significant effect on female longevity for a given developmental stage (Fig. 2).
The interactions among storage temperature, storage duration, and developmental stage had significant effects on the number of parasitized eggs (Table 2). In general, with the exception of the treatments in which the second instar larvae were stored, longer storage duration and lower storage temperature resulted in fewer numbers of parasitized eggs (first instar larvae: F5, 124 = 2.889, P = 0.017; second instar larvae: F2, 67 = 0.273, P = 0.762; prepupae: F4, 105 = 13.038, P < 0.0001; pupae: F3, 86 = 13.836, P < 0.0001) (Fig. 3).
Fitness of the F 1 generation
The emergence percentage of offspring was affected by the storage duration and the interactions among the three factors (Table 2). Significant differences in emergence percentage of the F1 generation were observed among different treatments after storage of the first instar larvae, second instar larvae, and pupae, but not prepupae, at 11 °C and 14 °C (first instar larvae: F5, 124 = 2.972, P = 0.014; second instar larvae: F2, 67 = 4.586, P = 0.014; prepupae: F4, 105 = 1.993, P = 0.101; pupae: F3, 86 = 3.713, P = 0.015). However, the emergence percentages recorded in this test were higher than 85% for all treatments (Fig. 4a). The percentage of females of the F1 generation was significantly influenced by the storage duration and developmental stage but not by the storage temperature and the interactions among the three factors (Table 2). Significantly lower percentages of females were observed for the first instar larvae stored at 14 °C for 21 days and pupae stored at 11 °C for 14 days (first instar larvae: F5, 124 = 6.091, P < 0.0001; second instar larvae: F2, 67 = 2.174, P = 0.122; prepupae: F4, 105 = 1.359, P = 0.253; pupae: F3, 86 = 5.228, P = 0.002). Despite this difference, in all treatments the percentage of females of the F1 generation was always higher than 60% (Fig. 4b).
Discussion
The present study, to the best of our knowledge, is the first to comprehensively and systematically evaluate the effects of cold storage on different developmental stages of T. remus reared on S. litura eggs. Parasitoids are expected to suffer major fitness costs if they are stored at suboptimal temperatures (Colinet and Boivin 2011; Yan et al. 2017; Lu et al. 2019). For each species, design of an appropriate cold storage scheme often involves the determination of the developmental threshold temperature (T0) (Leopold 2007). Insects will not suffer harm when they are stored at T0, but the storage temperature should be lower than T0 (Colinet and Boivin 2011). Among the tested temperatures in the current study, the lowest temperature of 8 °C was the least suitable, which corroborates the results of Rathee and Ram (2016) and Lu et al. (2019). Majority mortality occurred when the first instar larvae, prepupae, and pupae were stored at 8 °C for 14 days. Based on our previous study, the T0 of T. remus for the first instar larvae, prepupae, and pupae reared on S. litura eggs were 13.3, 15.5 and 13.8 °C, respectively (Chen et al. 2021a), so the storage temperature of 8 °C was probably too low for these developmental stages. However, the high emergence percentage of the second instar larvae after storing at 8 °C for 14 days might be due to their lower T0 (8.9 °C) (Chen et al. 2021a). By contrast, too high storage temperature allows parasitoids to continue to develop and results in emergence during storage (Colinet and Boivin 2011; Lu et al. 2019). In the current study, the prepupae stored at 14 °C for 28 days and pupae stored for 21 days emerged before reaching the desired storage duration. Studies evaluating the influence of cold storage on other parasitoids also recorded this situation (Kidane et al. 2020; Zhang et al. 2020). For example, Trichogramma dendrolimi (Matsumura) pupae stored at 13 °C for four weeks and late pupae stored for two to four weeks emerged before the end of the storage (Lu et al. 2019). As recorded in similar studies (Colinet and Boivin 2011; Lu et al. 2019), a decrease in emergence percentage was observed with prolonged storage duration, especially at 8 °C. The interaction between temperature and storage duration can be defined as ‘dose of cold exposure,’ which will increase with the decrease in the temperature and/or increase in the storage duration (Colinet and Boivin 2011). At higher doses, chilling injuries are progressively accumulated, eventually affecting the survival rate (Colinet and Boivin 2011). A prior study indicated that parasitoids require a lot of energy to support their muscle contractions during emergence (Yocum et al. 1994); thus, energy consumption during cold storage might deplete the energy stores for muscle function and negatively impact emergence (Zhang et al. 2020; Lin et al. 2021).
A similar study reported that T. remus pupae reared on C. cephalonica eggs survived more than 86% after 7 days of stored at 10 °C, whereas pupae stored at 10 °C for 14 days or at 5 °C for 7 days had poor performance (Queiroz et al. 2017a). These differences might correspond to the differences in host egg species. Parasitoids require massive amounts of energy to endure cold stress during storage, so they may only have a limited amount of energy to support their development after cold storage (Colinet and Hance 2010). Therefore, the nutritional resources available in the host eggs would be a major factor affecting the survival of the parasitoid during cold storage. The C. cephalonica eggs are 0.35 mm in width and 0.57 mm in length (Consoli et al. 1999), whereas S. litura eggs are 0.6 mm in diameter (CABI 2014). The bigger host egg might provide more nutrition for T. remus, helping it tolerate lower temperatures or longer storage durations. In addition, different changes in the osmotic pressure, consistency, and other physicochemical properties of the different host eggs with storage might affect the development of the parasitoid to different degrees (Lu et al. 2019). For example, changes in triglyceride levels in eggs had significant effects on the fecundity and lifespans of Trichogramma brassicae (Westwood) adults (Kishani et al. 2016).
In the present study, the first instar larvae were the ideal developmental stage for longer duration cold storage of T. remus, which differs from previous reports. Gautum (1986) demonstrated that the T. remus pupae reared on S. litura eggs could be stored at 10 °C for 7 days. This difference may be due to the different geographical origins of the tested parasitoid populations. For example, variability in cold tolerance among geographical populations was observed in four Trichogramma chilonis (Ishii) populations collected from the Ludhiana, Sangrur, and Muktsar districts in India (Khosa and Brar 2000), and among six T. dendrolimi populations in China (Jilin, Beijing, Shandong, Zhejiang, Yunnan, Guangxi) (Shi et al. 1993). Therefore, the ideal storage stage for one species or strain cannot be universally extended to another. Instead, the most suitable stage for cold storage should be determined independently through research.
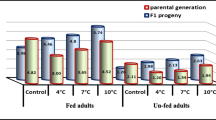
The percentage of females is one of the great concerns in the storage program, as it directly affects successive mass-rearing efficiency in the laboratory and control efficiency in the field (Tezze and Botto 2004; Queiroz et al. 2017a). In this study, there was no significant decrease in percentage of females after storage, except for the second instar larvae stored at 8 °C for 7 days. Queiroz et al. (2017a) observed that cold storage of T. remus at 5 °C and 10 °C produced fewer females. A variety of reasons can explain these changes in sex ratio, such as differential mortality rates between females and males because of low temperature stress (Cagnotti et al. 2018), epigenetic changes in female reproductive strategy caused by cold stress (Colinet and Hance 2009), or changes in sex ratio due to immune responses to low temperature (Zhang et al. 2020).
In order to synchronize production schedules with the requirements in the field, it is essential to know the timing of adult emergence after storage. Cold storage can affect the post-storage development of parasitoids, particularly their emergence time and the distribution pattern of emergence, to varying degrees (Colinet and Boivin 2011). Studies have found that although parasitoids can resume normal development after cold storage, the development may be delayed due to the physiological changes caused by cooler temperatures (Colinet and Hance 2010; Kidane et al. 2015; Cagnotti et al. 2018; Zhang et al. 2020). We found that as the storage temperature increased and/or the storage duration prolonged, T. remus at different developmental stages subjected to cold exposure took less time to complete their development, suggesting that the storage temperatures tested in the current study allowed them to continue development. The present results are consistent with the reports from other researchers in other genera (Colinet et al. 2006; Kidane et al. 2020). In terms of the distribution pattern of emergence, this study recorded only one, single emergence peak after T. remus were brought back to rearing conditions, while in Gonatocerus ashmeadi (Girault), cold storage resulted in two emergence peaks (Chen et al. 2008). As a typical feature of quiescence is that an organism immediately returns to normal development after cold storage (Chen et al. 2008; Cagnotti et al. 2018), we thus believe that T. remus in this study were quiescent during cold storage, rather than in diapause.
As documented in other studies, cold exposure has a detrimental effect on the fecundity of the parental parasitoids (Bernardo et al. 2008; Lessard and Boivin 2013; Cagnotti et al. 2018; Lu et al. 2019; Zhang et al. 2020). In this study, both longer storage duration and lower storage temperature reduced the parasitism ability of T. remus, and the same was also reported in a study using C. cephalonica eggs as an alternative host (Queiroz et al. 2017a), while they observed a greater decline in parasitism ability. In that research, the number of parasitized eggs for T. remus stored at 5 and 10 °C for 14 days was 8.4 and 14.1 eggs, respectively, which significantly differed from the control (49.8 eggs) (Queiroz et al. 2017a). The differences between the two studies may be caused by the use of different temperatures. Low temperature may delay or retard spermatogenesis (Colinet and Boivin 2011) and oocyte maturation rate (Foerster et al. 2004; Colinet and Boivin 2011). If the storage temperature is too low, the development of ovaries and testes may be seriously affected, even leading to infertility (Colinet and Boivin 2011). Differences in the energy reserves provided by different alternative host species may be another reason for this result. Specifically, the energy reserves of host eggs available to larvae must be divided between somatic maintenance and reproductive function (Colinet et al. 2007), which means that the parasitoids stored at lower temperatures must consume more resources to complete their somatic development, and therefore have fewer resources left for reproduction after emergence (Colinet and Boivin 2011).
It is widely accepted that although parasitoids can develop and emerge successfully after cold storage, their longevity is significantly reduced (Colinet and Boivin 2011). This decline has been reported in numerous species, such as Psyttalia incisi (Silvestri) (Lin et al. 2021), Tamarixia radiata (Waterston) (Zhang et al. 2020), and Eretmocerus hayati (Zolnerowich & Rose) (Kidane et al. 2020). However, the parasitoid longevity in all storage treatments in this study was not significantly affected by the cold storage. One explanation is that the honey solution provided after emergence offered the parasitoids energy to survive, and another is that female adults use host-feeding to obtain additional nutrients during parasitism (Lessard and Boivin 2013). A previous study revealed that T. remus pupae reared on C. cephalonica eggs had a significantly shorter longevity after being stored at 5 or 10 °C for more than 7 days (Queiroz et al. 2017a), which differs from the results of the present study. Differences in host egg species and/or geographic strains of parasitoids may have contributed to this result.
A recent report indicated that the effects obtained from cold storage can be transmitted from parent to offspring and influence their fitness. These intergenerational effects have been observed in some insects, but their physiological mechanisms remain ambiguous (Colinet and Boivin 2011). In this study, although the emergence percentage and percentage of females of offspring in some treatments differed from the control, they were higher than 85 and 60%, respectively, indicating that the cold storage had no significant negative effects on the fitness of unstressed offspring.
From a practical point of view, we concluded that the ideal storage conditions for T. remus in S. litura eggs were storing the first instar larvae at 14 °C for up to 21 days. Such storage conditions may allow large-scale production and safe storage of parasitoid products for later release, and help maintain populations in the laboratory at relatively lower costs. Although appropriate short-term storage conditions have been screened in this study, further research needs to be carried out on the actual performance of stored parasitoids against target pests in the field, such as their search, dispersal and parasitism abilities. In addition, in order to explore ways to realize long-term storage, the overwintering strategy of T. remus under natural conditions should be further investigated to reveal whether they are capable of diapause.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bayram A, Ozcan H, Kornosor S (2005) Effect of cold storage on the performance of Telenomus busseolae Gahan (Hymenoptera: Scelionidae), an egg parasitoid of Sesamia nonagrioides (Lefebvre) (Lepidoptera: Noctuidae). Biol Control 35:68–77. https://doi.org/10.1016/j.biocontrol.2005.06.007
Bernardo U, Iodice L, Sasso R, Pedata PA (2008) Effects of cold storage on Thripobius javae (=T. Semiluteus) (Hymenoptera: Eulophidae). Biocontrol Sci Technol 18:921–933. https://doi.org/10.1080/09583150802412311
Bertanha LA, Diniz AJF, Garcia AG, Parra JRP (2021) Determining the minimum temperature for storage of Tamarixia radiata (Hymenoptera: Eulophidae) adults for biological control of Asian Citrus Psyllid. Neotrop Entomol 50:114–120. https://doi.org/10.1007/s13744-020-00832-4
Bowler K, Terblanche JS (2008) Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biol Rev 83:339–355. https://doi.org/10.1111/j.1469-185X.2008.00046.x
CABI (2014) Spodoptera litura (taro caterpillar). https://www.cabi.org/isc/datasheet/44520. Accessed 12 July 2021
CABI (2019) Spodoptera frugiperda (fall armyworm). https://www.cabi.org/isc/datasheet/29810#tonaturalEnemies. Accessed 12 July 2021
Cagnotti CL, Lois M, Lopez SN, Botto EN, Viscarret MM (2018) Cold storage of Trichogramma nerudai using an acclimation period. Biocontrol 63:565–573. https://doi.org/10.1007/s10526-018-9885-5
Cave RD (2000) Biology, ecology and use in pest management of Telenomus remus. Biocontrol News Inf 21:21–26
Chapman RF (1998) The insects: structure and function. Cambridge University Press, Cambridge
Chen QJ, Li GH, Pang Y (2000) A simple artificial diet for mass rearing of some noctuid species. Entomol Knowl 37:325–327
Chen WB, Li YY, Wang MQ, Liu CX, Mao JJ, Chen HY, Zhang LS (2019) Natural enemy insect resources of the fall armyworm Spodoptera frugiperda, their application status, and existing problems and suggestions. Chin J Biol Control 35:658–673. https://doi.org/10.16409/j.cnki.2095-039x.2019.05.013
Chen WB, Li YY, Wang MQ, Liu CX, Mao JJ, Chen HY, Zhang LS (2019) Entomopathogen resources of the fall armyworm Spodoptera frugiperda, and their application status. Plant Prot 45:1–9. https://doi.org/10.16688/j.zwbh.2019453
Chen WB, Li YY, Wang MQ, Mao JJ, Zhang LS (2021a) Evaluating the potential of using Spodoptera litura eggs for mass-rearing Telenomus remus, a promising egg parasitoid of Spodoptera frugiperda. Insects 12:384. https://doi.org/10.3390/insects12050384
Chen WB, Weng QF, Nie R, Zhang HZ, Jing XY, Wang MQ, Li YY, Mao JJ, Zhang LS (2021b) Optimizing photoperiod, exposure time, and host-to-parasitoid ratio for mass-rearing of Telenomus remus, an egg parasitoid of Spodoptera frugiperda, on Spodoptera litura eggs. Insects 12:1050. https://doi.org/10.3390/insects12121050
Chen WL, Leopold RA, Harris MO (2008) Cold storage effects on maternal and progeny quality of Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae). Biol Control 46:122–132. https://doi.org/10.1016/j.biocontrol.2008.04.007
Colinet H, Boivin G (2011) Insect parasitoids cold storage: a comprehensive review of factors of variability and consequences. Biol Control 58:83–95. https://doi.org/10.1016/j.biocontrol.2011.04.014
Colinet H, Boivin G, Hance T (2007) Manipulation of parasitoid size using the temperature-size rule: fitness consequences. Oecologia 152:425–433. https://doi.org/10.1007/s00442-007-0674-6
Colinet H, Hance T (2009) Male reproductive potential of Aphidius colemani (Hymenoptera: Aphidiinae) exposed to constant or fluctuating thermal regimens. Environ Entomol 38:242–249. https://doi.org/10.1603/022.038.0130
Colinet H, Hance T (2010) Interspecific variation in the response to low temperature storage in different aphid parasitoids. Ann Appl Biol 156:147–156. https://doi.org/10.1111/j.1744-7348.2009.00374.x
Colinet H, Renault D, Hance T, Vernon P (2006) The impact of fluctuating thermal regimes on the survival of a cold-exposed parasitic wasp, Aphidius colemani. Physiol Entomol 31:234–240. https://doi.org/10.1111/j.1365-3032.2006.00511.x
Consoli FL, Kitajima EW, Parra JRP (1999) Ultrastructure of the natural and factitious host eggs of Trichogramma galloi Zucchi and Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Int J Insect Morphol 28:211–231. https://doi.org/10.1016/S0020-7322(99)00026-4
Costa-Lima TC, Chagas MCM, Parra JRP (2019) Comparing potential as biocontrol agents of two neotropical parasitoids of Liriomyza sativae. Neotrop Entomol 48:660–667. https://doi.org/10.1007/s13744-018-00667-0
Day R, Abrahams P, Bateman M, Beale T, Clottey V, Cock M, Colmenarez Y, Corniani N, Early R, Godwin J, Gomez J, Moreno PG, Murphy ST, Oppong-Mensah B, Phiri N, Pratt C, Silvestri S, Witt A (2017) Fall armyworm: impacts and implications for Africa. Outlooks Pest Manag 28:196–201. https://doi.org/10.1564/v28_oct_02
Dong H, Zhu KH, Zhao Q, Bai XP, Zhou JC, Zhang LS (2021) Morphological defense of the egg mass of Spodoptera frugiperda (Lepidoptera: Noctuidae) affects parasitic capacity and alters behaviors of egg parasitoid wasps. J Asia-Pac Entomol 24:671–678. https://doi.org/10.1016/j.aspen.2021.05.015
Foerster LA, Doetzer AK, Castro LCF (2004) Emergence, longevity and fecundity of Trissolcus basalis and Telenomus podisi after cold storage in the pupal stage. Pesqui Agropecu Bras 39:841–845. https://doi.org/10.1590/S0100-204X2004000900002
Foerster LA, Doetzer AK (2006) Cold storage of the egg parasitoids Trissolcus basalis (Wollaston) and Telenomus podisi Ashmead (Hymenoptera: Scelionidae). Biol Control 36:232–237. https://doi.org/10.1016/j.biocontrol.2005.10.004
Gautum RD (1986) Effect of cold storage on the adult parasitoid Telenomus remus Nixon (Scelionidae: Hymenoptera) and the parasitized eggs of Spodoptera litura (Fabr.) (Noctuidae). J Entomol Res 10:125–131
Gerling D (1972) The developmental biology of Telenomus remus Nixon (Hym., Scelionidae). B Entomol Res 61:385–388. https://doi.org/10.1017/S0007485300047283
Ghosh E, Ballal CR (2019) Effect of host egg storage on the storage amenability of Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae). Phytoparasitica 47:523–529. https://doi.org/10.1007/s12600-019-00749-8
Glenister CS, Hoffmann MP (1998) Mass-reared natural enemies: scientific, technological, and informational needs and considerations. In: Ridgway R, Hoffmann MP, Inscoe MN, Glenister CS (eds) Mass-reared natural enemies: application, regulation, and needs. Thomas Say Publications in Entomology, Entomological Society of America, Maryland, pp 242–247
Greene GL, Leppla NC, Dickerson WA (1976) Velvetbean caterpillar: a rearing procedure and artificial medium. J Econ Entomol 69:487–488. https://doi.org/10.1093/jee/69.4.487
Hanna AD (1935) Fertility and toleration of low temperature in Euchalcidia caryobori, Hanna (Hymenoptera, Chalcidinae). B Entomol Res 26:315–322. https://doi.org/10.1017/S0007485300036622
He HL, Zhou AL, He L, Qiu L, Ding WB, Li YZ (2022) The frequency of cannibalism by Spodoptera frugiperda larvae determines their probability of surviving food deprivation. J Pest Sci 95:145–157. https://doi.org/10.1007/s10340-021-01371-6
Huo LX, Zhou JC, Ning SF, Zhao Q, Zhang LX, Zhang ZT, Zhang LS, Dong H (2019) Biological characteristics of Telenomus remus against Spodoptera frugiperda and Spodoptera litura eggs. Plant Prot 45:60–64. https://doi.org/10.16688/j.zwbh.2019406
Jalali SK, Singh SP (1992) Differential response of four Trichogramma species to low temperatures for short term storage. Entomophaga 37:159–165. https://doi.org/10.1007/BF02372984
Khosa SS, Brar KS (2000) Effect of storage on the emergence and parasitization efficiency of laboratory reared and field collected populations of Trichogramma chilonis Ishii. J Biol Control 14:71–74. https://doi.org/10.18311/jbc/2000/4166
Kidane D, Ferrante M, Man XM, Liu WX, Wan FH, Yang NW (2020) Cold storage effects on fitness of the whitefly parasitoids Encarsia sophia and Eretmocerus hayati. Insects 11:428. https://doi.org/10.3390/insects11070428
Kidane D, Yang NW, Wan FH (2015) Effect of cold storage on the biological fitness of Encarsia sophia (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Hemiptera: Aleyrodidae). Eur J Entomol 112:460–469. https://doi.org/10.14411/eje.2015.066
King CBR (1934) Cold storage effect on Trichogramma and on eggs of Ephestia kuehniella. Tea Q 1:19–27
Kishani FH, Ashouri A, Zibaee A, Abroon P, Alford L (2016) The effect of host nutritional quality on multiple components of Trichogramma brassicae fitness. B Entomol Res 106:633–641. https://doi.org/10.1017/S000748531600033X
Laminou SA, Ba MN, Karimoune L, Doumma A, Muniappan R (2020) Parasitism of locally recruited egg parasitoids of the fall armyworm in Africa. Insects 11:430. https://doi.org/10.3390/insects11070430
Leopold RA (2007) Colony maintenance and mass-rearing: using cold storage technology for extending the shelf-life of insects. In: Vreysen MJB, Robinson AS, Hendrichs J (eds) Area-wide control of insect pests. Springer, Dordrecht, pp 149–162
Lessard E, Boivin G (2013) Effect of low temperature on emergence, fecundity, longevity and host-feeding by Trichogramma brassicae. Biocontrol 58:319–329. https://doi.org/10.1007/s10526-012-9493-8
Lin J, Yang DQ, Hao XX, Cai PM, Guo YQ, Shi S, Liu CM, Ji QG (2021) Effect of cold storage on the quality of Psyttalia incisi (Hymenoptera: Braconidae), a larval parasitoid of Bactrocera dorsalis (Diptera: Tephritidae). Insects 12:558. https://doi.org/10.3390/insects12060558
Lu X, Han SC, Li J, Liu JS, Li ZG (2019) Effects of cold storage on the quality of Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) reared on artificial medium. Pest Manag Sci 75:1328–1338. https://doi.org/10.1002/ps.5247
Mohamed HO, El-Heneidy AH (2020) Effect of cold storage temperature on quality of the parasitoid, Trichogrammatoidea bactrae Nagaraja (Hymenoptera: Trichogrammatidae). Egyp J Biol Pest Control 30:87. https://doi.org/10.1186/s41938-020-00288-z
Montezano DG, Specht A, Sosa-Gómez DR, Roque-Specht VF, Sousa-Silva JC, Paula-Moraes SV, Peterson JA, Hunt TE (2018) Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr Entomol 26:286–300. https://doi.org/10.4001/003.026.0286
Nakama PA, Foerster LA (2001) Efeito da alternância de temperaturas no desenvolvimento e emergência de Trissolcus basalis (Wollaston) e Telenomus podisi Ashmead (Hymenoptera: Scelionidae). Neotrop Entomol 30:269–275. https://doi.org/10.1590/S1519-566X2001000200010
Naranjo-Guevara N, Santos LAO, Barbosa NCCP, Castro ACMC, Fernandes OA (2020) Long-term mass rearing impacts performance of the egg parasitoid Telenomus remus (Hymenoptera: Platygastridae). J Entomol Sci 55:69–86. https://doi.org/10.18474/0749-8004-55.1.69
Njuguna E, Nethononda P, Maredia K, Mbabazi R, Kachapulula P, Rowe A, Ndolo D (2021) Experiences and perspectives on Spodoptera frugiperda (Lepidoptera: Noctuidae) management in Sub-Saharan Africa. J Integr Pest Manag 12:1–9. https://doi.org/10.1093/jipm/pmab002
Pomari-Fernandes A, Bueno AF, Bortoli SA (2016) Size and flight ability of Telenomus remus parasitoids reared on eggs of the factitious host Corcyra cephalonica. Rev Bras Entomol 60:177–181. https://doi.org/10.1016/j.rbe.2016.02.004
Queiroz AP, Bueno AF, Pomari-Fernandes A, Grande MLM, Bortolotto OC, Silva DM (2017a) Low temperature storage of Telenomus remus (Nixon) (Hymenoptera: Platygastridae) and its factitious host Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae). Neotrop Entomol 46:182–192. https://doi.org/10.1007/s13744-016-0442-6
Queiroz AP, Bueno AF, Pomari-Fernande A, Bortolotto OC, Mikami AY, Olive L (2017b) Influence of host preference, mating, and release density on the parasitism of Telenomus remus (Nixon) (Hymenoptera, Platygastridae). Rev Bras Entomol 61:86–90. https://doi.org/10.1016/j.rbe.2016.12.004
Rathe M, Ram P (2014) Effect of cold storage of Aenasius bambawalei Hayat (Hymenoptera: Encyrtidae) during pupal stage on its key biological characteristics. J Biol Control 28:11–17
Rathee M, Ram P (2016) Mortality of Aenasius bambawalei Hayat following cold storage in mummies of Phenacoccus solenopsis Tinsley. Res Environ Life Sci 9:1215–1217
Rathee M, Ram P (2018) Impact of cold storage on the performance of entomophagous insects: an overview. Phytoparasitica 46:421–449. https://doi.org/10.1007/s12600-018-0683-5
Rioba NB, Stevenson PC (2020) Opportunities and scope for botanical extracts and products for the management of fall armyworm (Spodoptera frugiperda) for smallholders in Africa. Plants 9:207. https://doi.org/10.3390/plants9020207
Salazar-Mendoza P, Rodriguez-Saona C, Fernandes OA (2020) Release density, dispersal capacity, and optimal rearing conditions for Telenomus remus, an egg parasitoid of Spodoptera frugiperda, in maize. Biocontrol Sci Technol 30:1040–1059. https://doi.org/10.1080/09583157.2020.1776841
Shi ZH, Liu SS, Wu WL, He JH (1993) Comparative studies on the biological characteristics of geographical/host populatiopons of Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) in China III. Response to temperature and humidity. Chin J Biol Control 9:97–101. https://doi.org/10.1016/j.biocontrol.2011.04.014
Sparks AN (1979) A review of the biology of the fall armyworm. Fla Entomol 62:82–87. https://doi.org/10.2307/3494083
Sun XX, Hu CX, Jia HR, Shen XJ, Zhan SY, Jiang YY, Wu KM (2021) Case study on the first immigration of fall armyworm Spodoptera frugiperda invading into China. J Integr Agr 20:664–672. https://doi.org/10.1016/S2095-3119(19)62839-X
Tezze AA, Botto EN (2004) Effect of cold storage on the quality of Trichogramma nerudai (Hymenoptera: Trichogrammatidae). Biol Control 30:11–16. https://doi.org/10.1016/j.biocontrol.2003.09.008
van Lenteren JC, Tommasini MG (2002) Mass production, storage, shipment and quality control of natural enemies. In: Albajes R, Gullino ML, van Lenteren JC, Elad Y (eds) Integrated pest and disease management in greenhouse crops. Springer, Dordrecht, pp 276–294
Vieira NF, Pomari-Fernandes A, Lemes AAF, Vacari AM, de Bortoli SA, Bueno AF (2017) Cost of production of Telenomus remus (Hymenoptera: Platygastridae) grown in natural and alternative hosts. J Econ Entomol 110:2724–2726. https://doi.org/10.1093/jee/tox271
Yan Z, Yue JJ, Bai C, Peng ZQ, Zhang CH (2017) Effects of cold storage on the biological characteristics of Microplitis prodeniae (Hymenoptera: Braconidae). B Entomol Res 107:506–512. https://doi.org/10.1017/s0007485317000037
Yocum GD, Zdarek J, Joplin KH, Lee RE Jr, Smith DC, Manter KD, Denlinger DL (1994) Alteration of the eclosion rhythm and eclosion behavior in the flesh fly, Sarcophaga crassipalpis, by low and high temperature stress. J Insect Physiol 40:13–21. https://doi.org/10.1016/0022-1910(94)90107-4
Zhang LH, Lu ZT, Guo CF, Shen ZL, Wang ZQ, Sang W, Qiu BL (2020) Effects of cold storage on the fitness of Tamarixia radiata, a dominant parasitoid of Asian citrus psyllid Diaphorina citri. Crop Prot 128:104988. https://doi.org/10.1016/j.cropro.2019.104988
Zhou Y, Wu QL, Zhang HW, Wu KM (2021) Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J Integr Agr 20:637–645. https://doi.org/10.1016/S2095-3119(21)63621-3
Acknowledgements
We thank EditSprings (https://www.editsprings.com/) for providing expert linguistic services. This research was funded by the Major Projects of China National Tobacco Corporation (110202001032 (LS-01)), the Projects of Guizhou Tobacco Corporation (201936, 201937, and 201941), and the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202108). We would like to thank Prof. Zhenying Wang, Institute of Plant Protection, Chinese Academy of Agricultural Sciences; and Prof. Zhuhong Wang, College of Plant Protection, Fujian Agriculture and Forestry University, for the help provided with the experimental materials.
Author information
Authors and Affiliations
Contributions
LSZ, YYL, JJM and MQW designed the study. WBC conducted the experiments. WBC, CHZ, MSZ and FZJ performed data analyses. WBC wrote the manuscript. LSZ edited it. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Communicated by Jay Rosenheim.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, W., Li, Y., Zhang, C. et al. Cold storage effects on biological parameters of Telenomus remus, a promising egg parasitoid of Spodoptera frugiperda, reared on Spodoptera litura eggs. J Pest Sci 96, 1365–1378 (2023). https://doi.org/10.1007/s10340-022-01515-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-022-01515-2