Abstract
Probiotic administration has the ability to increase broodstock performance and aquaculture productivity. The effect of dietary probiotics Bacillus cereus NP5 on the reproductive performance of female Clarias gariepinus and the survival of the resulting larvae was investigated in this study. Female fish with an initial weight of 553 ± 27.41 g, were placed in 12 hapas and fed for 12 weeks on three experimental diets supplemented with different concentrations of probiotics: 0 (control), 106 (B6), and 108 CFU g-1 feed (B8). This study found that B8 fish had higher protein retention (p<0.05), but body weight was comparable among treatments (p>0.05). Furthermore, probiotic diets increased digestive enzyme activity (p<0.05) such as amylase, protease, and lipase. Different doses of B. cereus NP5 probiotic significantly (p<0.05) affected intestinal villi height, but it did not increase villi length or intestinal diameter. Throughout the rearing period, the probiotic groups showed higher estradiol levels, and total cholesterol levels (p<0.05), stimulated early ovarian development, leading to higher GSI, HSI, and percentage of developed eggs (p<0.05). When compared to B6 and the control, the B8 treatment resulted in significantly higher fecundity, hatching rate, and larval survival (p<0.05). The results obtained support the hypothesis that probiotics could improve the reproductive performance and larval survival of female C. gariepinus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are now being studied extensively for both growth promotion and disease control in many aquatic species (Gobi et al. 2018; Meidong et al. 2018). The presence and metabolic action of various probiotics enhance the absorption of nutrients by stimulating enzyme activities, which has a positive impact on fish performance (Liu et al. 2017). Probiotics are also widely known to modulate immune responses by producing antimicrobial substances (Amin et al. 2017), and maintain gut health by competition with pathogens (Banerjee et al. 2017). The variety of types and ease of application also make it more straightforward to deliver probiotics in bulk orally via feed, which is particularly advantageous for aquaculture practices (Wuertz et al. 2021). The capacity of probiotics to enhance fish nutrition-related and digestive factors has piqued the interest of a number of studies in recent years, which are investigating the impact of probiotics on the reproductive factors of aquaculture species. The research in sea bream Sparus aurata (Ghoname et al. 2020), goldfish Carassius auratus (Mehdinejad et al. 2018), European eel Anguilla anguilla (Vílchez et al. 2015), and Nile tilapia Oreochromis niloticus (Mehrim et al. 2015) showed that the dietary administration of probiotics could improve the reproductive performance of the fish. These studies highlight the potential of probiotic treatments in improving broodstock performance and sustaining aquaculture productivity.
Clarias gariepinus, known as African catfish or African sharptooth catfish, plays a role in aquaculture across the world as a source of protein (Nelson et al. 2016; Muchlisin et al. 2014). Because of its adaptability (Hildebrand et al. 2023), robustness (Clols-Fuentes et al. 2023), and rapid growth (Hastuti and Subandiyono 2018), this freshwater species has acquired attention for aquaculture. C. gariepinus is now cultured throughout Africa, Asia, and Southeast Asian countries including Indonesia for consumption (FAO 2020). Controlling the reproductive activities of the fish is one of the most severe challenges in the development of commercial aquaculture, especially for female broodstock (Le François et al. 2021). The amount of time required to achieve gonad maturation, as well as the quality of eggs and larvae, are critical elements in C. gariepinus aquaculture for ensuring its productivity. Hormonal induction (Nainggolan et al. 2014) and manipulating the nutrient composition of broodfish diets (Nandi et al. 2023; Sezu et al. 2024) improves spawning response, lowers the number of broodfish required to achieve production goals, and enhances egg production and quality. Another alternative approach used to overcome problems in aquaculture is through the application of probiotics.
The study of probiotic administration in female C. gariepinus to evaluate reproductive performance in terms of gonad maturation, egg quality, and larval fitness is still lacking. In our preliminary work, the administration of probiotic B. cereus strain NP5 obtained from tilapia gut (Putra and Widanarni 2015) might modify the transcription of reproductive-related genes in male C. gariepinus and improve its sperm quality (Enzeline et al. 2022). Compared to other probiotics like Lactobacillus spp., Bacillus species have been shown to have superior probiotic properties due to their ability to produce antimicrobial substances, non-toxic when fed to fish, advantage in terms of survival (heat tolerance and longer shelf life) in a variety of environments because of their capacity for sporulation (Buruiană et al. 2014).
This previous study showed a promising way to manage the female C. gariepinus reproductive performance using the same treatment. Thus, the purpose of this study is to determine the effect of dietary probiotics B. cereus NP5 on the reproductive performance of female African catfish and the fitness of the ensuing larvae in terms of their survival. To offer an overview of the mechanism of action of the probiotics, the digestive, enzymatic, and associated hormonal assessment as a possible mechanism for improving reproductive function is also evaluated.
Materials and methodology
Bacteria and feed preparation
The experimental probiotic supplemented diet was prepared according to (Enzeline et al. 2022). Briefly, B. cereus NP5 was cultured on the sterile tryptic soy broth (TSB) media for 18 hours at 140 rpm and 29-30 °C for routine use. The bacterial cell was harvested by centrifugation at 5000 ×rpm and 4 °C and then rinsed twice with sterile phosphate-buffered saline (PBS; pH 7). The bacteria pellet was re-suspended and adjusted to 108 CFU mL-1 and 1010 CFU mL-1 by adding sterile PBS (pH 7). For experimental feed, 106 CFU g-1 feed, 108 CFU g-1 feed, and 0 CFU g-1 feed of NP5 were mixed with 3% v/w of egg white as a binder and then sprayed uniformly on commercial feed (HI-PRO-VITE 781-2, IDN) 1% (v/w) (Table 1). Feed was air-dried and stored in a dry, closed plastic bag at 4 °C. The proximate composition (AOAC 2012) of the experimental diets are shown in Table 2.
Fish and rearing
The fish used were female C. gariepinus from the same spawning batch obtained from the National Center for Freshwater Aquaculture (BBPBAT), Sukabumi, West Java with an average size of 553 ± 27.41 g . To ensure that the fish were all at the same stage of gonad maturity, gonads were taken at random from the population (n=5) and analyzed histologically. Following that, fish were put in 1 m3 hapas at a density of 12 fish/hapa (total of 12 hapas). The hapas were placed outdoors, inside a concrete pond with a transparent canopy that blocked direct sunlight and rain. Fish were acclimated for one week and fed commercial feed (29-30% protein) to satiation twice a day. After acclimatization, fish were fed experimental diets twice a day (6 a.m. and 6 p.m.) until they were satisfied for 12 weeks. Throughout the experiment, the water flow and change were kept constant. The photoperiod matched the natural photoperiod that occurred throughout the experiment. The water temperature ranged from 25 to 29.1 °C, the dissolved oxygen level was 3.5-6.2 mg L-1, the pH was 6.4-7.1, and the ammonia level was 0.01-0.03 mg L-1. No bacterial disease was observed during the rearing period.
The measurement of parameters
Growth parameter
The Average body weight of the fish was measured at the initial and at the end of the experiment by electrical balance. The fish feed conversion ratio (FCR) was counted using the following formula:
At the end of the experiment protein,fat retention, and body analysis were calculated by proximate analysis following the AOAC method (2012).
Intestine histology and digestive enzymes activity
Intestinal tissues were isolated randomly at the end of the experiment (n=3). The midgut was cut and stored in Bouin’s solution (5% acetic acid, 9% formaldehyde, and 1.5% picric acid) for 24 h. These samples were then dehydrated in progressively higher degrees of alcohol before being cleaned in xylene. The fixed tissues were immersed in paraffin wax before being sliced into small sections (4-6 μm) with a microtome (Thermo Scientific, USA). Three slices were fixed on glass slide and stained with hematoxylin-eosin for examination under a light microscope (Olympus, Japan) to measure the intestine diameter, basal villi width, and height. The digestive tract of catfish was weighed around 0.5 g, then was added with Tris buffer (20 mM Tris HCl, 1 mM EDTA, and 10 mM CaCl2 pH 7.5) with a ratio of 10% (b/v), afterward, it was put into microtube and was centrifuged for 10 minutes (12000 rpm, 4 °C). The supernatant was used as a crude enzyme extract (CEE) stored at -20 °C for enzyme activity assay. The activity of the intestine’s amylase and protease assay was conducted according to (Bergmeyer 1983) and lipase was analyzed following methods by (Tietz and Fiereck in Borlongan 1990). The enzyme activity was measured as the change in absorbance using a spectrophotometer (Thermo Scientific, USA) and expressed as specific activity (IU mL-1 protein).
Fish reproductive parameters
Fish were randomly selected from each dietary group (n=3) every three weeks. Blood was collected from the caudal vein using a sterile syringe that was previously rinsed with 3.8% sodium citrate (Enzeline et al. 2022). Blood was placed into a sterile microtube and centrifuged at 5000 ×rpm, 10 minutes, and 4 °C. Blood plasma (supernatant) was separated and stored at −20 °C before analysis. The plasma estradiol was then evaluated spectrophotometrically by ELISA technique on the 450 nm optical density using a commercial kit (EIA 2693 DRG®, USA) and an ELISA reader equipment (Elx808TM, Lonza). Total cholesterol was measured using enzymatic colorimetric test kits using HUMAN Cholesterol liquicolor complete kit (catalogue no. 10017, Germany). To evaluate the gonad maturation, GSI (%) and HSI (%) were calculated by the following formulae according to (Sulistyo et al. 2000):
The ovary was also collected for histological analysis. The ovary (n=3) was cut and stored in Bouin’s solution for 24 hours. These samples were then dehydrated, cleaned, fixed, cut, and stained as previously described. To assess the egg size distribution and the quantity of mature eggs, eggs were randomly selected from the fresh ovarian samples and the diameter was measured (n= 100) from each group. The number of mature eggs was determined using a calibrated micrometer placed on a microscope and the formula given by (Jusmaldi et al. 2020):
At the end of the experiment, the fecundity was calculated after stripping, calculated as the number of eggs per kg of total body weight (Ayuningtyas et al. 2020). Relative fecundity values are calculated using the gravimetric method on the samples taken, namely by counting the number of eggs from some of the gonads taken (samples). The number of eggs obtained was multiplied by the ratio between total gonad weight and gonad sample weight.
Fertilization, hatching rate, and larva survival
In this experiment, semen was obtained from 4 male fish from our lab stock. Semen was extracted from the testis after dissection and then collected by sterile syringe, then pooled into one stock. Eggs were collected by stripping from each feeding treatment randomly (n=3) and then divided into 1-gram portions each. The dry fertilization was conducted by combining the semen and the fertilized egg (1 g : 1 mL) (Nuraini et al. 2017), and the eggs were housed on the 60 L hatching tanks completed with the water heaters set at 25 °C and constant aeration. The fertilization rate value was determined after 8 hours of eggs and sperms mixing by counting the fertilized eggs (transparent color), and the hatching rate was counted 48 hours after incubation. Dead eggs were removed by siphon on day 3 after incubation. The hatched larvae were reared for 14 days inside the same tanks. From the third day of hatching, the larvae were provided with Artemia live feed and reared for 14 days. Percent survival of larvae was measured at day 14 of incubation:
Data analysis
The histology and gonad pictures data were provided descriptively in the figures. Growth and reproductive-related data were collated in MS. Excel 2007 (Microsoft, USA) and statistically evaluated using one-way ANOVA in SPSS ver. 25 (IBM, USA). The standard deviation (SD) was determined to define the range of means, and the P value was set to 0.05 to test for significant results, and the mean values were using Duncan's post hoc statistic.
Results
Growth performance and digestive enzyme activity
The growth performance parameters of the fish fed with probiotic B. cereus NP5 were presented in Table 3. The results demonstrated that 12 weeks of feeding with B. cereus NP5 at various doses did not result in substantial body weight gain, feed conversion ratio, and lipid retention in female C. gariepinus broodstock (p>0.05). However, the fish supplemented with probiotics had higher protein retention compared to control (p<0.05). The proximate body composition of the fish after the supplementation of dietary probiotic is shown in Table 4. There were significant differences between the groups in protein, lipid, and moisture (p<0.05). The protein levels and moisture in the fish body increased significantly with higher probiotic group compared to control. Interestingly, the probiotic group had significantly (p<0.05) lower lipid in their body.
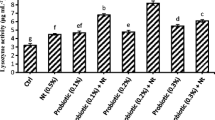
The villi height, width, and intestine diameter of fish after 12 weeks fed with probiotics are presented in Table 5. The results demonstrated that the administration of probiotics did not result in a significant difference in the intestine diameter and villi width of the fish (p>0.05). However, the villi height in the probiotic group was significantly larger compared to the control group. B8 had a larger villi height than B6 (p<0.05). Fig. 1 shows the activity of digestive enzymes in the fish gut after 12 weeks of probiotic administration. In general, fish fed with probiotics exhibited increased enzyme activity than controls (p<0.05). When compared to B6 and the control group, B8 had higher lipase and amylase activity. Protease levels of B8 and B6 were comparable and higher than controls (p<0.05).
Estradiol hormone and total cholesterol concentration
Fig. 2 shows the effects of probiotic administration on the plasma estradiol level of female C. gariepinus. After 3 weeks of feeding, the estradiol level in B6 and B8 was considerably higher than in the control (p<0.05), with B8 having the highest level followed by B6 and the control. This tendency continued until week 9 of the studies when the estradiol level might have peaked. Compared to week 6, all groups demonstrated a substantial drop in plasma estradiol at week 12. The levels of B8 and B6 estradiol were similar (p>0.05) but remained higher than in the control. At week 3, blood cholesterol in all treatments showed an increase and reached the highest peak at week 12 compared to the previous week (Fig. 3). Treatment B8 showed the highest value, followed by B6 and control (p<0.05). Although not significantly different in week 9, blood cholesterol levels were also found to be consistently higher in probiotic treatment.
Evaluation of gonadal development
Fig. 4 depicts the distribution of egg sizes and gonad histology (Fig. 5) in female C. gariepinus following the administration of probiotic B. cereus NP5. During the first sampling (week 0), more than 70% of the egg diameters varied from 0.189 to 0.353 mm. After three weeks of probiotic treatment, more than half of the eggs in the B8 treatment had a diameter of more than 1.173 mm, which was substantially larger than the other groups. Histological analysis demonstrated that the egg nucleus and egg size in the B8 treatment appeared to be larger than in the other treatments. However, with the control treatment, more than 30% of the egg is still 1 mm in diameter (Fig. 4).
The gonad histology of female C. gariepinus after 12 weeks of dietary B. cereus NP5 supplementation. N= nucleus; PG= primary growth oocyte; POF= postovulatory follicle complex; OW= ovarian wall; VTG1 = primary vitellogenic oocytes, VTG2 = secondary vitellogenic oocytes, VTG3 = tertiary vitellogenic oocytes; GVM= germinal vesicle migration; GVBD= germinal vesicle breakdown. The scale bar represents 200 μm, 40× magnification. The histomorphological category of the eggs refers to (Tyor and Pahwa 2017)
At week 6, the egg size distribution in all treatments was most likely similar, but very small eggs (0.8 mm) were still found in the control treatment (Fig. 4). A large number of eggs this week were more than 1 mm in diameter. All treatments showed the presence of lipid droplets and vitellogenic oocytes. Secondary vitellogenic oocytes were found in probiotic groups, but primary vitellogenic oocytes were found in the control group. Tertiary vitellogenic oocytes were observed in all treatments by week 9 of the experiment. In all treatments, the majority of the eggs were developing to be larger than 1 mm in size.
However, as compared to the control, the probiotics treatment had a larger egg size. By week 12, all treatments eggs had completed germinal vesicle migration and breakdown suggesting that the eggs were mature and ready to be ovulated. The control group still yielded comparatively small eggs (0.8 mm), and some eggs were still in VTG1 and VTG2, showing that egg maturation was unequal in comparison to the probiotic group. Consistent with these results, the GSI and number of mature eggs were presented in Table 6.
Reproductive performance
Table 6 shows the reproductive performance of female C. gariepinus following 12 weeks of dietary B. cereus NP5 supplementation. The results indicated that the dietary probiotic supplementation might alter the early gonad maturation processes in female C. gariepinus. After three weeks of treatment, the probiotic administration significantly increased gonad development, GSI, HSI, and the proportion of mature eggs (p<0.05). The ovaries in control were observed to be immature (stage 1), B6 treatment was in stage 2 (not ripe), and B8 was in stage 3 (nearly ripe). Moreover, B8 had more than 60% of eggs that were considered mature, while B6 had around 46% and 34% in the control group. The fish ovary maturation index in the control group had progressed to stage 2 by the sixth week of dietary probiotic treatment, whereas the probiotics treatment had progressed to stage 3. In the ninth week of the experiment, B8 produced around 82% of developed eggs, which was substantially higher (p<0.05) than B6 (72%), and control (63%). The GSI of B6 and B8 were similar but higher than the control (p<0.05). In the sixth week, the probiotic treatment fish ovaries were ripe (stage 4), whereas the control fish was nearly ripe (stage 3). At the end of the experiment, ovary maturation in all groups reached stage V (Running ripe). However, when compared to the control, the probiotics treatment exhibited greater GSI, HSI, and percentage of developed eggs (p<0.05). B8 had around 89% mature eggs, B6 had 87.67%, and 74.33% in the control group. The female ovaries of C. gariepinus at the end of the experiment are presented in Fig. 6.
The ovary membrane appeared quite thin after 12 weeks, and red-mat green eggs were visible to the naked eye (Fig. 6). The blood vessels in the wall were clearly visible. The eggs in the probiotic groups appeared darker than those in the control group, and their abdominal cavity was filled with ovaries, suggesting that the eggs were ready to be released by stripping or rough handling. The egg quality and larval survival were evaluated by fertilizing the eggs from the treatment group with the male’s sperm. Table 7 shows the results of an evaluation of fecundity, FR, HR, and larval survival. Female C. gariepinus fed a high concentration of probiotic (B8) exhibited increased fecundity than the control and the lower concentration groups (B6) (Table 7). The fertilization rate is comparable across treatments (p>0.05); however, the probiotics treatment significantly enhanced the percentage of hatching compared to the control (p<0.05). The B8 treatment had around 82% of HR, which was greater than B6 (69.76%) and control (58.61%). Moreover, the probiotic administration on the female broodstock was shown to considerably increase larval fitness (p<0.05). The B8 treatment resulted in considerably better larvae survival (62.51%) than the control (35.94%), whereas the B6 treatment resulted in equivalent survival with other treatments (p>0.05).
Discussion
Numerous studies have demonstrated that probiotics influence immunological and growth performance by influencing multiple aspects of digestion, absorption, and metabolism, as well as influencing various physiological responses (Kuebutornye et al. 2020; Mehdinejad et al. 2018; Dias et al. 2018). Additionally, the use of probiotic in fish diets including promoting economic development, stimulating digestion, and sustainable production in order to meet the global food demand. Thus, the current study observed the feed utilization indices, growth performance, estradiol level, cholesterol level, digestive enzyme activity, intestinal morphology, and reproduction performance to offer an overview of the mechanism of action of the probiotics for improving reproductive peformance.
The current findings revealed that the dietary administration of probiotic in female C. gariepinus diets had remarkable impacts on the growth parameters in term of protein retention. The probiotics group had higher protein retention compared to the control, which means that fish fed with probiotics might had more efficient sparring energy so the protein is more available for maintenance and reproduction (Radhakrishnan et al. 2020). This was because of those higher digestive enzyme activities (Fig. 1). Protease will break down protein into simpler compounds so that they are easier to be absorbed and the amount of protein stored in the body will be higher.
Probiotic administration also significantly affected the fish body composition (Table 4), which produces higher body protein and triggers an increase in protein retention. After maturity, protein mobilization occurs within the fish body from oocytes, liver, and intestine to muscle, thereby increasing muscle protein content compared to other tissues. In addition, increased protein in muscle can reduce fat level (Sezu et al. 2024). A significant positive outcome was also found when tilapia O. niloticus was fed with probiotic and prebiotic (synbiotic) (Putra et al. 2015). However in that study, probiotic administration also improved the fish growth and body weight which are not seen in the current study.
In all treatments, the average BW of the fish is not significantly different before and after 12 weeks of feeding (Table 3). The results might be attributed to the usage of relatively large fish (>500 g) that are nearly categorized as broodstock instead of fast-growing fry/juvenile. Protein and energy are mostly needed for development and maintenance in the early stages, and after the gonad maturation has occurred, it mainly is used for reproduction, contributing to a lesser growth rate (García-Fernández et al. 2022).
After 12 weeks fish rearing, probiotic treatments showed the higher of digestive enzyme activities compared to control (Fig. 1) and increasing the villi height for more optimal nutrient absorption (Fernandes et al. 2017) (Table 5). B. cereus NP5 are proven to produce digestive enzyme that support the nutrient absorption (Putra and Widanarni 2015). The results indicated that probiotic could improve enzyme activities in catfish intestine. Correspondingly, dietary probiotic increases villi height and length and digestive enzyme activity have been extensively documented to correlate with probiotic administration, including in our earlier investigation using B. cereus NP5 in Nile tilapia (Putra et al. 2015), common carp (Djauhari et al. 2016), and African catfish (Putra et al. 2020); as well as other species of Bacillus probiotics in rainbow trout (Sahraei et al. 2019), sea bream (Zaineldin et al. 2018), and Nile tilapia (Gobi et al. 2018).
The gonadal development of the fish given probiotics is substantial when compared to the control (Table 6; Fig. 5). The gonads of fish fed with probiotic B. cereus NP5 were developed earlier than controls, as evidenced by histological examination (Fig. 5), GSI, HSI, the number of mature eggs (Table 6), and the size distribution of the eggs during the course of the trial (Fig. 4). Similar to the current study in female C. gariepinus, a substantial increase in GSI, gonad development, and overall reproductive performance was also observed in rainbow trout Oncorhynchus mykiss female broodstock (Nargesi et al. 2019). For 8 weeks, the fish were administered 109 CFU kg-1 of commercial probiotics containing eight probiotic bacteria and exhibited an increase in mature eggs, egg diameter, fecundity, FR, HR, eyed eggs, and alevins survival that was greater than the control.
This study implied that the improvement was due to the nutritional benefits of probiotics, which were represented in the protein efficiency ratio, FCR, and blood total protein. In female and male goldfish C. auratus, the administration of a single probiotic Pediococcus acidilactici or combined with nucleotide also resulted in better reproductive performances for both sexes (Mehdinejad et al. 2018). Nutrition and reproduction are vital in ensuring that the reproductive event works in tandem with the availability of nutrients to ensure the availability of reproductive energy (Abduh et al. 2021), the reproductive process including oogenesis and vitellogenesis (Hara et al. 2016), and the survival of new offspring (Torsabo et al. 2022).
The results of the present study showed that showed the highest fecundity, hatching rate, and larvae survival rate, indicating that better quality of eggs. The probiotics B. cereus NP5 might alter the reproductive performance by inducing the circulating estradiol and also the gene expression that is related to reproduction. In the current study, the plasma estradiol of the fish fed with the probiotics was induced to be higher than the control from the 3 weeks of treatments until the end of the experiments (Fig. 2). Estradiol stimulates the production of vitellogenin in the liver, which is then released into the bloodstream and taken up by the developing oocytes to perform vitellogenesis and induce gonad development (Hara et al. 2016). Estradiol also influences the liver function and metabolism to perform vitellogenesis (Nelson and Habibi 2013). It might also explain why the probiotic treatments had greater HSI than the control (Table 6). Higher estradiol levels may also be associated with improved female C. gariepinus early gonad development, gonad index, egg diameters, and quantity of developed eggs in the probiotics therapy compared to the control. The ability of probiotics to influence vitellogenesis, faster oosit development, increased expression of neuropeptide hormones and metabolic signals in the central nervous system is well proven (Gioacchini et al. 2010; Lombardo et al. 2011). Accordingly, the cause of the estradiol level increase in broodstock in our study might be attributed to the positive effects of probiotics.
According to studies, dietary supplementation with probiotics can reduce cholesterol, triglycerides, and low density lipoproteins (Dawood et al. 2018). In contrast, this study showed an upward cholesterol level in the probiotic group (Fig. 3) was similar to the study result of Ekasari et al. (2013), which showed that the biofloc technology (BFT) treatment on Nile Tilapia had higher blood cholesterol levels than the control treatment. It is known that cholesterol serves as a precursor for the manufacture of steroid hormones, including progestogens, estrogens, and testosterone all of which are involved in reproduction (Lubzens et al. 2010). Moreover, Chen et al. (2003) explained that the lower total cholesterol level in the blood (less than 180 mg dL-1) could be associated with the fish going through spawning (McDonald and Mulligan 1992).
Our earlier investigation on male C. gariepinus revealed that administration of probiotics B. cereus NP5 also induced reproduction-related gene expression such as follicle-stimulating hormone and luteinizing hormone, therefore increasing male catfish sperm maturation and quality (Enzeline et al. 2022). The ability of probiotics to influence gene expression has also been demonstrated in zebrafish (Gioacchini et al. 2012; Gioacchini et al. 2011; Gioacchini et al. 2010). Zebrafish fed with L. rhamnosus demonstrated increased gene expression of kisspeptin, leptin, and, most crucially, GnRH, impairing the fish's reproductive ability (Gioacchini et al. 2010). Future research should explore gene expression analysis of reproduction-related genes in female C. gariepinus to assist in elucidating the likely molecular mechanism of probiotics in regulating its reproductive performance. The possibility of probiotic secondary metabolites roles should also be studied in order to expand the associated understanding regarding the probiotic's effects on fish reproduction.
Conclusion
These studies confirmed that dietary probiotics B. cereus NP5. have a positive role in reproduction performance and larvae survival of female C. gariepinus; the increasing concentration of probiotics showed a better improvement. The nutritional advantages of probiotics and the induction of plasma estradiol were shown to be the supporting mechanisms of reproductive improvement in this work. Hence, the dietary probiotic B. cereus NP5 treatment can be potentially used to increase the productivity of C. gariepinus aquaculture.
Data availability
No datasets were generated or analysed during the current study.
References
Abduh MY, Koh ICC, Munafi ABA et al (2021) Effects of dietary fish oil and corn oil on gonadosomatic and hepatosomatic index, gonadal histology, 17β-estradiol level and fatty acids profile of mahseer (Tor tambroides) broodstock in captivity. Aquac Nut 27:1448–1459. https://doi.org/10.1111/anu.13282
Amin M, Adams M, Bolch CJ, Burke CM (2017) In vitro screening of lactic acid bacteria isolated from gastrointestinal tract of Atlantic Salmon (Salmo salar) as probiont candidates. Aquac Int 25:485–498. https://doi.org/10.1007/s10499-016-0045-6
AOAC (2012) Official methods of analysis of AOAC International. AOAC International, Maryland, US
Ayuningtyas SQ, Zairin M Jr, Widanarni (2020) Reproductive performance of catfish Clarias sp. with probiotics Bacillus sp. NP5 addition through feed. Jurnal Akuakultur Indonesia 19:74–83. https://doi.org/10.19027/jai.19.1.74-83
Banerjee G, Nandi A, Ray AK (2017) Assessment of hemolytic activity, enzyme production and bacteriocin characterization of Bacillus subtilis LR1 isolated from the gastrointestinal tract of fish. Arch Microbiol 199:115–124. https://doi.org/10.1007/s00203-016-1283-8
Bergmeyer HU (1983) Methods of enzymatic analysis. Academic Press, New York
Borlongan IG (1990) Studies on the digestive lipases of milkfish, Chanos chanos. Aquaculture 89:315–325. https://doi.org/10.1016/0044-8486(90)90135-A
Buruiană CT, Profir AG, Vizireanu C (2014) Effects of probiotic Bacillus species in aquaculture – an overview. The Annals of the University Dunarea de Jos of Galati. Fascicle VI - Food Technol 38:9–17
Chen CY, Wooster GA, Getchell RG et al (2003) Blood chemistry of healthy, nephrocalcinosis-affected and ozone-treated tilapia in a recirculation system, with application of discriminant analysis. Aquaculture 218:89–102. https://doi.org/10.1016/S0044-8486(02)00499-4
Clols-Fuentes J, Nguinkal JA, Unger P et al (2023) Bacterial community in african catfish (Clarias gariepinus) recirculation aquaculture systems under different stocking densities. Front Mar Sci 10. https://doi.org/10.3389/fmars.2023.1073250
Dawood MA, Koshio S, Abdel-Daim MM, Van Doan H (2018) Probiotic application for sustainable aquaculture. Rev Aquacult 11:907–924. https://doi.org/10.1111/raq.12272
Dias JAR, Abe HA, Sousa NC et al (2018) Dietary supplementation with autochthonous Bacillus cereus improves growth performance and survival in tambaqui Colossoma macropomum. Aquac Res 49:3063–3070. https://doi.org/10.1111/are.13767
Djauhari R, Widanarni W, Suprayudi MA, Zairin M Jr (2016) Characterization of Bacillus sp. NP5 and its application as probiotic for common carp (Cyprinus carpio). Res J Microbiol 11:101–111. https://doi.org/10.3923/jm.2016.101.111
Ekasari J, Zairin M Jr, Putri DU et al (2013) Biofloc-based reproductive performance of Nile tilapia Oreochromis niloticus L. broodstock. Aquac Res:1–4. https://doi.org/10.1111/are.12185
Enzeline V, Nasrullah H, Sudrajat AO et al (2022) Spermatogenesis and sperm quality of male African catfish fed with Bacillus sp. NP5 probiotic supplemented diet. AACL Bioflux 15:339–349
Fernandes AST, Petrillo TR, Aguinaga JY et al (2017) Effects of the probiotic Bacillus amyloliquefaciens on growth performance, hematology and intestinal morphometry in cage-reared Nile tilapia. Lat Am J Aquat Res 43:963–971. https://doi.org/10.3856/vol43-issue5-fulltext-16
FAO (2020) Fisheries and aquaculture Statistics. FAO, Rome
García-Fernández C, Domínguez-Petit R, Saborido-Rey F (2022) The Use of Daily Growth to Analyze Individual Spawning Dynamics in an Asynchronous Population: The Case of the European Hake from the Southern Stock. Fishes 7:208. https://doi.org/10.3390/fishes7040208
Ghoname M, El-sayed RS, Ghozlan HA, Sabry S (2020) Application of probiotic bacteria for the improvement of sea bream (Sparus aurata) larval production. Egypt J Aquat Biol Fish 24:371–398. https://doi.org/10.21608/EJABF.2020.70859
Gioacchini G, Giorgini E, Merrifield DL et al (2012) Probiotics can induce follicle maturational competence: the Danio rerio case. Biol Reprod 86:65–75. https://doi.org/10.1095/biolreprod.111.094243
Gioacchini G, Lombardo F, Merrifield DL et al (2011) Effects of probiotic on zebrafish reproduction. J Aquac Res Dev. https://doi.org/10.4172/2155-9546
Gioacchini G, Maradonna F, Lombardo F et al (2010) Increase of fecundity by probiotic administration in zebrafish (Danio rerio). Reproduction 140:953–959. https://doi.org/10.1530/REP-10-0145
Gobi N, Vaseeharan B, Chen JC et al (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellf Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Hara A, Hiramatsu N, Fujita T (2016) Vitellogenesis and choriogenesis in fishes. Fish Sci 82:187–202. https://doi.org/10.1007/s12562-015-0957-5
Hastuti S, Subandiyono S (2018) Haematological parameters of the North African catfish Clarias gariepinus farmed using biofloc technology. AACL Bioflux 11:1415–1424
Hildebrand MC, Rebl A, Nguinkal JA et al (2023) Effects of fe-dtpa on health and welfare of the african catfish Clarias gariepinus (burchell, 1822). Water 15:299. https://doi.org/10.3390/w15020299
Jusmaldi J, Hariani N, Hendra M et al (2020) Some reproductive biology aspects of bonylip barb (Osteochilus vittatus Valenciennes, 1842) in the Waters of Benanga Reservoir, East Kalimantan. Jurnal Iktiologi Indonesia 20:217–233. https://doi.org/10.32491/jii.v20i3.529
Kuebutornye FK, Tang J, Cai J et al (2020) In vivo assessment of the probiotic potentials of three host-associated Bacillus species on growth performance, health status and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture 527:735440. https://doi.org/10.1016/j.aquaculture.2020.735440
Le François NR, Beirão J, Superio J et al (2021) Spotted Wolffish Broodstock Management and Egg Production: Retrospective, Current Status, and Research Priorities. Animals (Basel) 11:2849. https://doi.org/10.3390/ani11102849
Liu H, Wang S, Cai Y et al (2017) Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellf Immunol 60:326–333. https://doi.org/10.1016/j.fsi.2016.12.003
Lombardo F, Gioacchini G, Carnevali O (2011) Probiotic-based nutritional effects on killifish reproduction. Fish Aquac 33:1–11
Lubzens E, Young G, Bobe J, Cerda J (2010) Oogenesis in teleost: how fish eggs are formed. Gen Comp Endocrinol 165:367–389. https://doi.org/10.1016/j.ygcen.2009.05.022
McDonald DG, Mulligan CL (1992) Chemical properties of the blood. In: Fish Physiology Volume XII Part B The cardiovascular system. Academic press, California, pp 58–105
Mehdinejad N, Imanpour MR, Jafari V (2018) Combined or individual effects of dietary probiotic, Pediococcus acidilactici and nucleotide on reproductive performance in goldfish (Carassius auratus). Probiotics Antimicrob Proteins 11:233–238. https://doi.org/10.1007/s12602-017-9377-4
Mehrim AI, Khalil FF, Hassan ME (2015) Hydroyeast Aquaculture® as a reproductive enhancer agent for the adult Nile tilapia (Oreochromis niloticus Linnaeus, 1758). Fish Physiol Biochem 41:371–381. https://doi.org/10.1007/s10695-014-9989-5
Meidong R, Khotchanalekha K, Doolgindachbaporn S et al (2018) Evaluation of probiotic Bacillus aerius B81e isolated from healthy hybrid catfish on growth, disease resistance and innate immunity of Pla-mong Pangasius bocourti. Fish Shellf Immunol 73:1–10. https://doi.org/10.1016/j.fsi.2017.11.032
Muchlisin ZA, Mastura S, Asraf A et al (2014) A preliminary study to evaluate the effects of powder milk solution on the eggs adhesiveness and fertilization rates of African catfish, Clarias gariepinus. AACL Bioflux 7:15–19
Nainggolan A, Sudrajat AO, Utomo BP, Harris E (2014) Ovarian maturation in Asian catfish (Clarias sp.) by combination Oodev and nutrition addition Spirulina plantesis. Int J Sci: Basic and Applied Research 15:564–583
Nandi SK, Suma AY, Rashid A et al (2023) The Potential of Fermented Water Spinach Meal as a Fish Meal Replacement and the Impacts on Growth Performance, Reproduction, Blood Biochemistry and Gut Morphology of Female Stinging Catfish (Heteropneustes fossilis). Life 13:176. https://doi.org/10.3390/life13010176
Nargesi EA, Falahatkar B, Sajjadi MM (2019) Dietary supplementation of probiotics and influence on feed efficiency, growth parameters, and reproductive performance in female rainbow trout (Oncorhynchus mykiss) broodstock. Aquac Nut 26:98–108. https://doi.org/10.1111/anu.12970
Nelson JS, Grande TC, Wilson MV (2016) Fishes of the World. John Wiley & Sons
Nelson ER, Habibi HR (2013) Estrogen receptor function and regulation in fish and other vertebrates. Gen Comp Endocrinol 192:15–24. https://doi.org/10.1016/j.ygcen.2013.03.032
Nuraini N, Tanjung A, Warningsih T, Muchlisin ZA (2017) Induced spawning of siban fish Cyclocheilichthys apogon using Ovaprim. F1000Res 6:1855. https://doi.org/10.12688/f1000research.12885.1
Putra AN, Mustahal M, Syamsunarno MB (2020) Effects of dietary probiotic Bacillus NP5 on the growth performances of catfish (Clarias sp.). Biotropia 27:51–59. https://doi.org/10.11598/btb.2020.27.1.1102
Putra AN, Utomo NBP, Widanarni W (2015) Growth performance of tilapia (Oreochromis niloticus) fed with probiotic, prebiotic and synbiotic in diet. Pak J Nutr 14:263–268. https://doi.org/10.3923/pjn.2015.263.268
Putra AN, Widanarni W (2015) Screening of amylolytic bacteria as candidates of probiotic in tilapia Oreochromis sp. Res J Microbiol 10:1–13. https://doi.org/10.3923/jm.2015.1.13
Radhakrishnan G, Shivkumar MVS et al (2020) Dietary protein requirement for maintenance, growth, and reproduction in fish: A review. J entomol zool stud 8:208–215
Sahraei F, Ahari H, Kakoolaki S (2019) Effect of Bacillus subtilis as a probiotic on protein, lipid content, and trypsin and chymo-trypsin enzymes in rainbow trout biometry (Oncorhynchus mykiss). Aquac Int 27:141–153. https://doi.org/10.1007/s10499-018-0313-8
Sezu NH, Nandi SK, Suma AY et al (2024) Ameliorative Effects of Different Dietary Levels of Fish Protein Hydrolysate (FPH) on Growth and Reproductive Performance, Feed Stability, Tissues Biochemical Composition, Haematobiochemical Profile, Liver Histology, and Economic Analysis of Pabda (Ompok pabda) Broodstock. Aquac Res 1−15. https://doi.org/10.1155/2024/6044920
Sulistyo I, Fontaine P, Rincarh J et al (2000) Reproductive cycle and plasma level of steroid in male eurasian perch (Perca fluviatilis). Aquat Living Resour 13:99–106. https://doi.org/10.1016/S0990-7440(00)00146-7
Torsabo D, Ishak SD, Noordin NM et al (2022) Enhancing Reproductive Performance of Freshwater Finfish Species through Dietary Lipids. Aquac Nutr:7138012. https://doi.org/10.1155/2022/7138012
Tyor AK, Pahwa K (2017) Ovarian development of African sharptooth catfish Clarias gariepinus (Burchell 1822) from Delhi segment of river Yamuna. J Fish Sci 12:117–126. https://doi.org/10.3923/jfas.2017.117.126
Vílchez MC, Santangeli S, Maradonna F et al (2015) Effect of the probiotic Lactobacillus rhamnosus on the expression of genes involved in European eel spermatogenesis. Theriogenology 84:1321–1331. https://doi.org/10.1016/j.theriogenology.2015.07.011
Wuertz S, Schroeder A, Wanka KM (2021) Probiotics in Fish Nutrition—Long-Standing Household Remedy or Native Nutraceuticals? Water 13:1348. https://doi.org/10.3390/w13101348
Yalcin S, Solar K, Akyurt I (2001) Certain reproductive characteristics of the catfish (Clarias gariepinus Burchell, 1822) living in the River Asi. Turkey. Turk J Zool 25:453–460 (https://journals.tubitak.gov.tr/zoology/vol25/iss4/15)
Zaineldin AI, Hegazi S, Koshio S et al (2018) Bacillus subtilis as probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol 79:303–312. https://doi.org/10.1016/j.fsi.2018.05.035.SS
Acknowledgements
We would like to thank the late Prof. Dr. Ir. Muhammad Zairin Junior, M.Sc. for his considerable inspiration, invaluable guidance, and dedication to this research.
Funding
This work was funded by the Ministry of Research, Technology, Higher Education of the Republic of Indonesia under grant No. 261/SP2H/LT/DRPM/2019.
Author information
Authors and Affiliations
Contributions
Valensia Enzeline performed the experiment, analyzed the data, and wrote the first version of the manuscript. Widanarni Widanarni designed the study and wrote the first version of the manuscript. Agus Oman Sudrajat designed the study, reviewed the first version of the manuscript, and approved it for publication. Alimuddin Alimuddin, reviewed the first version of the manuscript and approved it for publication. Hasan Nasrullah wrote the first version of the manuscript, reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval
All experiments in this study associated with fish complied with animal welfare and were conducted according to protocol number 249-2022, approved by the Ethics Committee on Animal Use of the IPB University, October 2022.
Competing of interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Enzeline, V., Widanarni, W., Sudrajat, A.O. et al. Improving reproductive performance and larvae survival by dietary administration of probiotic Bacillus cereus NP5 in female African catfish Clarias gariepinus. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01532-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01532-1