Abstract
This study investigated the effect of Bacillus subtilis IS02 as a probiotic bacterium in four different treatment conditions, namely T1 (no probiotic), T2 (106), T3 (107), and T4 (108) cfu/g of B. subtilis IS02 in the diet of rainbow trout for 30 days, on the protein content, lipid content, trypsin and chymotrypsin enzyme activity, and the bioassay. The protein content and lipid content were measured using the Kjeldahl and Soxhlet methods, respectively.T4 had the highest amount of measured protein (25.30%) while T1 had the lowest amount (15.90). The T4 treatment had a significant effect (P value = 0.001) on improving the protein content as well as the trypsin and chymotrypsin enzyme activity. In contrast, there was no significant difference in terms of the weight gain and specific growth rate between the treatments. Additionally, the results showed that the T3 treatment had a significant effect on increasing the protein content of the carcass, although the effect was not significant in relation to the lipid content. B. subtilis IS02 in a concentration range of 107–108 cfu/g could be employed to improve the protein productive value, trypsin and chymotrypsin enzyme activity, and protein content of the carcass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase in demand for fish seen in recent years is due to rapid population growth worldwide as well as an increased awareness of the nutritional value of fish, a preference for fish over animal proteins, and recognition that fish has both a low-calorie count and low lipid level. In order to meet the increased global demand, increasing aquaculture production, including fish, and improving the rate of production per unit area are vital considerations (Daniels et al. 2010). The use of probiotics represents a novel aquaculture technology. The probiotics typically applied in aquaculture include strains of Lactobacillus, Streptococcus, Enterococcus, Bifidobacterium, Pseudomonas, Rhodomonas, Bacillus, Carnobacterium, Lactococcus, Micrococcus, Nitrobacter, and rhodopseudomonas. Probiotics are usually added to the environment and diet of breeding animals in order to enhance growth and survival rates and also help to increase digestion and the uptake of nutrition (Irianto and Austin 2002).
B. subtilis is one of the probiotics capable of properly metabolizing food due to its ability to break down proteins and carbohydrates and produces vitamins belonging to the B group, including biotin and B12 (Mombelli and Gismondo 2000).
Many studies have previously been conducted in this area. For instance, Rengpipat et al. (1998) examined the effect of the probiotic Bacillus S11 in three different treatments on Nile tilapia over 100 days. The bacterium was used in fresh cells, soluble in normal saline, and lyophilized forms. The results showed no significant difference between the three forms used in the diet, although there was a significant difference between the three treatment groups and the control group (Rengpipat et al. 1998).
In addition, Wang et al. (2008) investigated the effect of Bacillus spp. on shrimp and found significant positive effects (P < 0.05) on both growth rate and digestive enzymes protease and amylase between the three treatment groups examined over 28 days (Wang et al. 2008).
Farhangi and Carter (2007) evaluated the effect of probiotic bacilli on the feed efficiency and carcass quality of fingerling rainbow trout. In their study, 560 fingerling rainbow trout obtained from a breeding center. The fingerling rainbow trout were fed a diet supplemented with probiotics. The results of this study showed that the probiotics in the experimental treatment groups had a positive and significant effect on the protein efficiency ratio, lipid efficiency ratio, and protein productive value when compared to the control group (P < 0.05) (Farhangi and Carter 2007).
Seenivasan et al. (2012) investigated the effect of the probiotic B. subtilis on shrimp larvae (M. rosenbergii). According to their results, after 90 days, growth parameters such as the survival rate, weight gain, specific growth rate, food conversion ratio, feed efficiency, and efficiency ratio were all increased significantly in the samples fed with the probiotic (P < 0.05). There was also a significant increase in the amount of amino acids, carbohydrates, lipids, and ash (P < 0.05). The results therefore indicated that B. subtilis, as a probiotic, could be used to increase the survival, growth, and energy production of the shrimp (Seenivasan et al. 2012).
Merrifield et al. (2010) examined the effect of various probiotics (B. subtilis, B. licheniformis, and Enterococcus faecium) on the feeding of rainbow trout (Oncorhynchus mykiss Walbaum) previously treated with oxolinic acid. Their results showed that the use of B. subtilis and B. licheniformis in the rainbow trout feed significantly improved the food conversion ratio (FCR), specific growth rate (SGR), and protein efficiency ratio (PER). The results of this study indicated the potential role of probiotics in stabilizing and enhancing gastrointestinal microbes following treatment with antibiotics, strengthening the mechanism of the intestinal barrier function, and protecting against potential secondary pathogens (Merrifield et al. 2010).
Therefore, the present study was conducted in order to determine the effect of B. subtilis IS02 at four different concentrations during 30 days of rearing on the protein content, lipid content, trypsin and chymotrypsin enzyme activity, and bioassay of rainbow trout.
Materials and methods
Preparation of the diet
The utilized water was analyzed and compared with the standard values in terms of the chlorine, pH, dissolved oxygen, total dissolved solids (TDS), total suspended solids (TSS), ammonia, nitrate, nitrite, salinity, total hardness, turbidity, conductivity, and microbial indices (Olsen 2004). Based on the recommendations of Newaj-Fyzul et al. (2007), the probiotic dosage was considered to be 107 cfu/g, which has previously shown the best response to the pathogenic bacterium (Aeromonas hydrophila) found in rainbow trout. The concentrations of 106, 107, and 108 cfu/g of the probiotic B. subtilis IS02 (prepared by Takgene Zist Co., Iran) were evaluated to determine the most appropriate dosage. The four chosen treatments were T1 (106 cfu/g), T2 (107 cfu/g), T3 (108 cfu/g), and T4 (control, no probiotic) (Newaj-Fyzul et al. 2007).
The primary probiotic powder contained 1011 cfu/g of B. subtilis IS02. As a result, concentrations of 106, 107, and 108 cfu/g were sprayed onto EXF1 feed (prepared by Kimiyagaran-e-Taghziyeh Co., Iran). After drying, soy oil was sprayed onto the food in order to prevent rapid dissolution. The food was then kept in a refrigerator until testing (Newaj-Fyzul et al. 2007).
Fish experiments
In total, 490 female rainbow trout (6.5 ± 2), whose feeding was discontinued 24 h prior to the transfer, were transferred to the hall of a fish farm located in Firuzkuh, Iran. The fish were equally divided into three bathtubs and, 48 h after the transfer, they were fed with probiotic-free foods to help them adapt to their new environmental conditions (temperature, oxygen, and pH) for 1 week. After this time, the samples were equally divided into 12 bathtubs. Subsequently, bathtubs 1 to 3 were used for treatment T1, 4 to 6 for treatment T2, 7 to 9 for treatment T3, and 10 to 12 for treatment T4. By the end of the research period, each treatment group was fed to satiation with its own special diet, and the values obtained were recorded in the respective tables.
Sampling and bioassay
Sampling was carried out using Torstensen method. During the first sampling, four fish were harvested randomly from each of the three utilized bathtubs. In order to evaluate the digestive enzymes, three random samples were selected from each replication in the second and third samplings some 30 min prior to feeding and 2 h after feeding. The samples were anesthetized using clove extract at a concentration of 100 mg/l. All biological indices were determined using the models provided by (Torstensen et al. 2008).
Preparation of the samples
The specimens were disinfected using alcohol and then placed inside the sponge that had previously been cut. A longitudinal incision was made in their abdomen. The gastrointestinal (GI) tract was completely dissected, and the specimens were washed with distilled water and then encoded. After separating the head and fins, the fish meat was also separated from the skin, packed, and transferred to a freezer at − 20 °C (Kanani et al. 2014; Hosseini et al. 2017).
Preparation of the enzyme extraction
In order to prepare the enzyme extraction, a buffer containing 100 mM tris acid, 0.1 M ethylenediaminetetraacetic acid (EDTA), and 0.1% Triton X-100 with a pH of 7.8 was first prepared and then adjusted to a pH of 7.8. Each of the digestive tract samples was removed separately from the freezer and mixed with the others using a stirrer. One portion of the visceral tissue (1 g) was homogenized in nine portions of buffer (9 ml) using a homogenizer on an ice surface for 30 s. The resulting mixture was centrifuged at 20,000×g for 5 min at 4 °C. The supernatant was then stored in a freezer (− 80 °C) until the pancreatic enzymes were measured (Babaei et al. 2011).
Trypsin measurement
The Bradford protein assay (1976) was used to measure both the trypsin and chymotrypsin enzymes. The trypsin was measured according to the recommendations of Erlanger et al. (1961) using 43.5 mg of a benzoyl-DL-arginine-p-nitroanilide (BAPA) substrate (Sigma Aldrich Co.) in 1 ml of dimethyl sulfoxide, which reached the final volume with a calcium chloride buffer (0.05 M tris acid, 0.02 M CaCl2.2H2O, and a pH of 8.2). Then, 25 μl of the extracted enzyme solution was mixed with 1.25 ml of freshly prepared substrate solution and incubated for 10 min at 37 °C. The absorption rate was read at a wavelength of 410 nm using a spectrophotometer. The results were replaced in the following equation, and the relative activity of the trypsin enzyme was obtained (Erlanger et al. 1961).
\( \mathrm{Relative}\ \mathrm{activity}\ \mathrm{of}\ \mathrm{the}\ \mathrm{enzyme}\ \mathrm{trypsin}=\frac{\mathrm{measured}\ \mathrm{solution}\ \mathrm{volume}\times 1000\times \mathrm{Sample}\ \mathrm{absorbance}\ \mathrm{at}\ 410\;\mathrm{nm}\ }{\mathrm{Mg}\ \mathrm{of}\ \mathrm{protein}\ \mathrm{in}\ \mathrm{measured}\ \mathrm{solution}\ } \)
Chymotrypsin measurement
To measure the chymotrypsin enzyme, a buffer containing 10.55 g of CaCl2.2H2O in 250 ml of 0.2 M tris(hydroxymethyl)aminomethane buffer was first prepared and adjusted to a pH of 7.8 using HCl. It was then diluted to 1 l and 432 ml of methanol was added. The 15.7 mg of substrate N-benzoyl-l-tyrosine ethyl ester (BTEE) (Sigma Aldrich Co.) was reached in the final volume with the buffer prepared during the previous step and then incubated for 5 min at 30 °C. The absorption rate was read at a wavelength of 256 nm (Hummel 1959).
Measurement of the carcass protein and lipid content
The protein content of each rainbow trout carcass was measured using the Kjeldahl method and according to Standard 924 (AOAC 1990). Further, the lipid content was measured according to Standard 742–2003.
Data analysis methods
The results were analyzed by means of SPSS version 24 software using a univariate analysis of variance, a one-way ANOVA with the Bonferroni test, and a repeated measures ANOVA in a completely randomized design. A P value < 0.05 was considered to be significant, and the data were reported as ± SD (standard deviation).
Results and discussion
Effect of the treatments on the growth factors
The different concentrations of the probiotic B. subtilis IS02 included in the diet of the rainbow trout were found to significantly increase the protein productive value (PPV). This result is similar to that of a study conducted by Farhangi and Carter (2007), who evaluated the effect of probiotic bacilli on rainbow trout fry. However, the results obtained in relation to the other factors were different from the results of both those researchers and Wang et al. (2008), who reported a significant difference (P < 0.05) between the control group and the treatment groups of tilapia after 40 days of feeding with the probiotic.
There was no significant difference in terms of the other factors, including WG, SGR, FCR, PER, LER, VFI, FE, LPV, and DGI, between the control group and the treatment groups fed with different concentrations of the probiotic B. subtilis IS02, which is most likely due to the relatively short feeding period (30 days). In practical terms, the highest percentage of weight gain was related to treatment T3 (225.02 ± 73.36), while the lowest percentage was seen in the control group (170.62 ± 51.17). The highest and lowest SGR values were related to treatment T4 (3.85 ± 0.28) and the control group (3.18 ± 0.71), respectively. The highest and lowest FCR values were related to the control group (23.71 ± 9.85) and treatment T2 (17.98 ± 8.91), respectively, while the highest and lowest protein efficiency ratios were seen for treatment T3 (2.03 ± 0.69) and the control group (1.36 ± 0.66), respectively. In terms of the lipid efficiency ratio, the highest and lowest values were related to treatment T3 (5.67 ± 1.92) and the control group (1.31 ±), respectively. Further, the highest and lowest lipid productive values were seen for treatment T2 (0.23 ± 0.07) and the control group (0.08 ± 0.03), respectively, while the highest and lowest voluntary feed intake values were related to the control group (98.72 ± 15.53) and treatment T4 (63.13 ± 21.71), respectively. The highest and lowest feed efficiency values were seen for treatment T3 (0.85 ± 0.29) and the control group (0.65 ± 0.20), respectively, while the highest and lowest daily growth index values were related to treatment T3 (0.66 ± 0.13) and treatment T4 (0.52 ± 0.18), respectively (Table 1). These results indicate that there was a difference between the groups. Bagheri et al. (2008) examined the effect of a probiotic on rainbow trout and observed a significant difference (P < 0.05) in relation to the SGR, PER, and CF factors.
Ziaei-Nejad et al. (2006) investigated the effect of a prebiotic, namely bionic yeast cell wall, on rainbow trout and reported a significant increase in the specific growth rate, body weight gain, condition factor, and protein efficiency ratio in the treatment groups fed with the bionic yeast cell wall (P < 0.05).
Merrifield et al. (2010) used B. subtilis and B. licheniformis to feed rainbow trout, and they observed a significant improvement in the FCR, SGR, and PER (P < 0.05) (Merrifield et al. 2010).
Signs of the same letters indicate no significant difference between the groups, a = 0.05.
The increase in the growth factors can be attributed to the synthesis of vitamins and cofactors as well as the increased enzyme activity caused by the probiotics, which serve to improve the gastrointestinal tract and thus enhance growth (Gatesoupe 1999).
Rengpipat et al. (1998) examined the effect of Bacillus S11 on shrimp. After 100 days, their results indicated no significant difference between the groups fed with a probiotic in terms of the growth rate (P > 0.05), although there was a significant difference between the treatment groups and the control group (Rengpipat et al. 1998).
Naseri et al. (2013) observed positive results when using the probiotic Bioplus 2B to feed rainbow trout. In their study, the final weight, FCR, SGR, and PER all showed a significant difference between the treatment groups (P ≤ 0.05). Meanwhile, the statistical analysis indicated no significant difference in terms of the total length and survival rate between the treatment groups (P ≥ 0.05), while a significant difference was observed in relation to the PPV (Naseri et al. 2013).
Effect of the treatments on the digestive enzymes
A repeated measures ANOVA was used during 3 weeks of sampling (week zero, beginning of the third week, and beginning of the fifth week) to investigate the interaction of time and the probiotic concentration on the changes in the trypsin digestive enzyme. Based on the multivariate test, the lambda value was 0.045, which was significant (P < 0.05). According to Mauchly’s sphericity test (Table 3), the frequency of sampling is generally effective in relation to the level of trypsin, with the test values (P = 0.269, X2 = 2.62, W = 0.687) showing that the main effect of time can reject the assumption of sphericity. Therefore, its corrective values (p = 0.0, F = 38.71 and p = 0.02, F = 3.50) were significant for the time variable (week) as well as the interaction between the time variable and the probiotic concentration (Table 2).
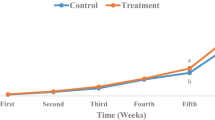
According to both Fig. 1 and Table 3, the lowest and highest trypsin activity at the beginning of the third week was related to treatment T3 (0.025 ± 0.001) and treatment T4 (0.051 ± 0.006), respectively.
The estimated value in the treatment T4 group showed no significant difference to that of the treatment T2 group (0.047 ± 0.006) (P > 0.05). These values at the beginning of the fifth week were related to treatment T1 (0.017 ± 0.001), with no significant difference being seen in relation to the values for treatment T3 (0.019 ± 0.007) and only a slight increase being seen in relation to treatment T4 (0.052 ± 0.007), which indicates that the values were linear. In terms of the enzyme chymotrypsin, based on the multivariate test, the lambda value was 0.242, which was significant (P < 0.05). According to Mauchly’s sphericity test (Table 3), the frequency of sampling is generally effective in relation to the level of chymotrypsin, with the test values (P = 0.167, X2 = 3.583, W = 0.599) showing that the main effect of time can reject the assumption of sphericity. Therefore, its corrective values (P = 0.0, F = 14.146 and P = 0.0, F = 10.453) were significant for the time variable (week) as well as the interaction between the time variable and the probiotic concentration (Table 4).
According to Fig. 2 and Table 5, the lowest and highest chymotrypsin activity at the beginning of the third week was related to treatment T3 (0.552 ± 0.180) and treatment T4 (2.436 ± 0.180), respectively. These values at the beginning of the fifth week were related to treatment T4 (0.395 ± 0.238), with no significant difference being seen in relation to the values for treatment T2 (0.415 ± 0.238) and only a slight increase being seen in relation to treatment T4 (0.859 ± 0.003), which indicates that the values were linear. The digestive enzymes are among the most important factors affecting and improving the feed efficiency of fish as well as the hydrolysis of carbohydrates, lipids, and protein in the diet (Lemieux et al. 1999).
Nevertheless, only limited information is currently available regarding the extracellular enzymes secreted by the intestinal bacteria and their biochemical significance (Bairagi et al. 2002).
In the present study, the use of different probiotic concentrations of B. subtilis IS02 in the diet of rainbow trout was found to result in a significant difference in the level of the digestive enzymes (trypsin and chymotrypsin) between the control group and the treatment groups, which was also the case in the study by Essa et al. (2010), who investigated the effect of probiotics on the digestive enzymes (amylase, protease, and lipase) in tilapia. In our study, the highest level of trypsin was related to treatment T4 (0.052 ± 0.006), while the lowest level was observed in treatment T1 (0.007 ± 0.001). Additionally, the highest level of the chymotrypsin enzyme was associated with treatment T4 (436.2 ± 0.180), while the lowest level was seen in treatment T3 (0.555 ± 0.180) (Essa et al. 2010).
Adding probiotics as a living supplement to the diet can help them to become established in the GI tract (Fuller and Perdigon 2003). These microorganisms as well as the enzymes secreted by them play an important role in the digestive process (Munilla-Moran et al. 1990).
Further, they stimulate the production of intracellular enzymes (Ziaei-Nejad et al. 2006) and thus increase digestibility. The increase in the digestive enzymes seen in this study may be due to the probiotic activity of B. subtilis IS02 and the production of the associated extracellular enzymes. However, it is difficult to distinguish between the intracellular enzymes produced by the fish and the extracellular enzymes produced by the probiotics in the GI tract (Essa et al. 2010).
The effect of the treatments on the carcass protein and lipid content
The sampling was performed at the end of the study period in order to compare the protein and lipid content in the various concentrations of the probiotic B. subtilis IS02. The changes were studied using a one-way analysis of variance with the Bonferroni test. In terms of the protein content, according to Table 6, the various concentrations of the probiotic B. subtilis IS02 appear to have had different effects on the protein content of the rainbow trout during the 30-day research period (p = 0.001). The significance limit of the ANOVA for the lipid content was greater than 0.05 (p = 0.414), which indicates that the different concentrations of the probiotic B. subtilis IS02 had no effect on the variation in the lipid content seen in the rainbow trout (Table 6).
Naseri et al. (2013) observed positive results in relation to the use of the probiotic Bioplus 2B in rainbow trout, so that there was a significant difference in the protein and lipid content of the carcass between the different treatment groups at the end of the experimental period (P < 0.05). The results of using different concentrations of the probiotic B. subtilis IS02 in the diet of rainbow trout indicated an increase in the crude protein content when analyzing the composition of the carcass. This result is similar to the findings obtained by (Farhangi and Carter 2007) and Azevedo et al. (2012) in relation to the effect of B. subtilis and mannan-oligosaccharide on tilapia, which is likely due to the production of protease enzymes in the GI tract (Irianto and Austin 2002). This serves to improve both digestion and the uptake of protein compounds (Fuller and Perdigon 2003). The findings of the study conducted by El-Haroun et al. (2006) are also consistent with the results of the abovementioned research, showing a significant difference in the protein content of tilapia under the influence of probiotics (P ≤ 0.01) (El-Haroun et al. 2006).
The change in the carcass lipid content was not significant in the different treatment groups, which is consistent with the findings of Daniels et al. (2010) in relation to the effect of B. subtilis, B. licheniformis, and E. faecium on rainbow trout as well as those of Ghaljaei et al. (2016), although it differs from the results of Farhangi and Carter (2007), who found a significant difference between the treatment groups (P < 0.05). The changes in the lipid and protein content could be related to the changes in their synthesis and storage in the fish muscles (Abdel-Tawwab et al. 2005).
Conclusion
The aim of this study was to investigate the effects of different concentrations of the probiotic Bacillus subtilis IS02 on the protein content and digestive enzymes of rainbow trout using a spectrophotometer and a bioassay. In order to determine the proper dosage of the probiotic B. subtilis IS02 as well as its effect on the protein content, growth, and trypsin and chymotrypsin enzymes in rainbow trout, four treatment groups were created, namely T1 (106 cfu/g), T2 (107 cfu/g), T3 (108 cfu/g), and T4 (control, no probiotic). Finally, based on the results of the bioassay and the measurement of the digestive enzymes, proteins, and lipids in the composition of the carcass, the dose that had the best effect on the rainbow trout was selected as the optimal dosage of the probiotic B. subtilis IS02. The results concerning the PPV factor indicated that the different concentrations of the probiotic B. subtilis IS02 had various effects on the rainbow trout during the 30-day study period when compared to the control treatment (p = 0.001). There was also a significant difference between the groups in terms of the trypsin and chymotrypsin enzyme activity (p = 0.05). Additionally, the effects of the probiotic concentrations on the protein content of the rainbow trout differed during the 30-day study period (P = 0.001). The significance limit of the ANOVA in relation to the lipid content was greater than 0.05 (P = 0.414), which indicates that the different concentrations of the probiotic B. subtilis IS02 had no effect on the variation in the lipid content seen in the rainbow trout (Table 4). The present findings demonstrated that a concentration of 108 cfu/g is most suitable for use in the diet of rainbow trout due to the positive effects on the trypsin and chymotrypsin enzyme activity as well as the protein content seen in treatment T4. In this regard, 107 cfu/g of the probiotic B. subtilis IS02 also had the best effect on the carcass protein content of the rainbow trout in treatment T3. Hence, it can be concluded that the most appropriate concentration of B. subtilis IS02 for use in the diet of rainbow trout is within the range of 107 to 108 cfu/g, which leads to the stimulation of the digestive enzymes, the elevation of the carcass protein content, and, consequently, the high-feed efficiency of the rainbow trout.
References
Abdel-Tawwab M, Mousa MA, Sharaf SM, Ahmad MH (2005) Effect of crowding stress on some physiological functions of Nile tilapia, Oreochromis niloticus (L.) fed different dietary protein levels. International Journal of Zoological Research 1:41–47
AOAC (1990). Official Methods of Analysis. Washington, DC: Asso-ciation of Ocial Analytical Chemists
Azevedo AS, Sousa S, Jacinto A (2012) An amputation resets positional information to a proximal identity in the regenerating zebrafish caudal fin. BMC Dev Biol 12:24
Babaei SS, Kenari AA, Nazari R, Gisbert E (2011) Developmental changes of digestive enzymes in Persian sturgeon (Acipenser persicus) during larval ontogeny. Aquaculture 318:138–144
Bagheri T, Hedayati SA, Yavari V, Alizade M, Farzanfar A (2008) Growth, survival and gut microbial load of rainbow trout (Onchorhynchus mykiss) fry given diet supplemented with probiotic during the two months of first feeding. Turk J Fish Aquat Sci 8:43–48
Bairagi A, Ghosh KS, Sen SK, Ray AK (2002) Enzyme producing bacterial flora isolated from fish digestive tracts. Aquac Int 10:109–121
Daniels CL, Merrifield DL, Boothroyd DP, Davies SJ, Factor JR, Arnold KE (2010) Effect of dietary Bacillus spp. and mannan oligosaccharides (MOS) on European lobster (Homarus gammarus L.) larvae growth performance, gut morphology and gut microbiota. Aquaculture 304:49–57
El-Haroun E, Goda AS, Chowdhury K (2006) Effect of dietary probiotic Biogen® supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.). Aquac Res 37:1473–1480
Erlanger BF, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278
Essa MA, El-Serafy S, El-Ezabi MM, Daboor SM, Esmael NA, Lall SP (2010) Effect of different dietary probiotics on growth, feed utilization and digestive enzymes activities of Nile tilapia, Oreochromis niloticus. Int J Zoo Res 5:143–162
Farhangi M, Carter CG (2007) Effect of enzyme supplementation to dehulled lupin-based diets on growth, feed efficiency, nutrient digestibility and carcass composition of rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 38:1274–1282
Fuller R, Perdigon G (2003) Gut flora, immunity and health. Blackwell Publishing, Hoboken
Gatesoupe F (1999) The use of probiotics in aquaculture. Aquaculture 180:147–165
Ghaljaei FA, Khara H, Shenavar MA (2016) Effect of lactobacillus plantarum (kc426951) bacteria isolated from the intestine of rainbow trout guilan on hematological indices and immune of rainbow trout (Oncorhynchus mykiss Walbaum, 1792) The quarterly journal of animal physiology and development (quarterly journal of biological sciences) winter 2016, 9(32):61-68
Hosseini R, Ahari H, Mahasti P, Paidari S (2017) Measuring the migration of silver from silver nanocomposite polyethylene packaging based on (TiO2) into Penaeus semisulcatus using titration comparison with migration methods. Fish Sci 83(4):649–659
Hummel BC (1959) A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol 37:1393–1399
Irianto A, Austin B (2002) Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 25:333–342
Kanani HG, Nobahar Z, Kakoolaki S, Jafarian, H (2014) Effect of ginger-and garlic-supplemented diet on growth performance, some hematological parameters and immune responses in juvenile Huso huso. Fish Physiol Biochem 40(2):481–490
Lemieux H, Blier P, Dutil J-D (1999) Do digestive enzymes set a physiological limit on growth rate and food conversion efficiency in the Atlantic cod (Gadus morhua)? Fish Physiol Biochem 20:293–303
Merrifield D, Bradley G, Baker R, Davies S (2010) Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria postantibiotic treatment. Aquac Nutr 16:496–503
Mombelli B, Gismondo MR (2000) The use of probiotics in medical practice. Int J Antimicrob Agents 16:531–536
Munilla-Moran R, Stark J, Barbour A (1990) The role of exogenous enzymes in digestion in cultured turbot larvae (Scophthalmus maximus L.). Aquaculture 88:337–350
Naseri S, Khara H, Shakoori M (2013) Effects of probiotics and Fe ion on the growth and survival and body composition of rainbow trout, Oncorhynchus mykiss (Walbaum, 1792) frys. J Appl Anim Res 41:318–325
Newaj-Fyzul A, Adesiyun AA, Mutani A, Ramsubhag A, Brunt J, Austin B (2007) Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 103:1699–1706
Olsen Y (2004) Live food technology of cold-water marine fish larvae. Culture of cold-water Marine Fish 73–128
Rengpipat S, Phianphak W, Piyatiratitivorakul S, Menasveta P (1998) Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture 167:301–313
Seenivasan C, Radhakrishnan S, Muralisankar T, Bhavan PS (2012) Bacillus subtilis on survival, growth, biochemical constituents and energy utilization of the freshwater prawn Macrobrachium rosenbergii post larvae. Egypt J Aquat Res 38:195–203
Torstensen B, Espe M, Sanden M, Stubhaug I, Waagbø R, Hemre G-I, Fontanillas R, Nordgarden U, Hevrøy E, Olsvik P (2008) Novel production of Atlantic salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 285:193–200
Wang Y-B, Tian Z-Q, Yao J-T, Li W-F (2008) Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture 277:203–207
Ziaei-Nejad S, Rezaei MH, Takami GA, Lovett DL, Mirvaghefi A-R, Shakouri M (2006) The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252:516–524
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sahraei, F., Ahari, H. & Kakoolaki, S. Effect of Bacillus subtilis as a probiotic on protein, lipid content, and trypsin and chymotrypsin enzymes in rainbow trout biometry (Oncorhynchus mykiss). Aquacult Int 27, 141–153 (2019). https://doi.org/10.1007/s10499-018-0313-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-018-0313-8