Abstract
The research aimed to evaluate the effects of dietary fructooligosaccharide, probiotics and their combination on the growth performance, antioxidant enzymes and stress resistance of juvenile Eriocheir sinensis. Crabs (average weight: 9.90 ± 0.07 g) were randomly assigned into 4 groups (3 tanks per group) fed experimental diets: basal diet (control group), basal diet supplemented with 5 g/kg FOS, basal diet supplemented with 2 g/kg multi-strain probiotics (each gram contains Lactobacillus acidophilus 106 CFU + Bacillus subtilis 107 CFU + Saccharomyces cerevisiae 1010 CFU) and basal diet supplemented with symbiotic (5 g/kg FOS + 2 g/kg multi-strain probiotics) (designated as control, D1, D2 and D3, respectively). After 8 weeks of feeding trial, crabs were exposed to the transportation process (3 h). Cumulative mortality was recorded at 24 h, 48 h, 72 h and 96 h after transport stress, and pre-transport and post-transport sampling of hepatopancreas occurred for assay. The results indicated that crabs fed D3 had significantly higher weight gain (WG), specific growth rate (SGR) and lower feed conversion ratio (FCR) than crabs fed control diet. Before stress, compared to the control group, crabs fed D1 and D3 diet significantly increased catalase (CAT), superoxide dismutase (SOD) activities and decreased malondialdehyde (MDA) activities, the highest activities of pepsin, trypsin and chymotrypsin were observed in crabs fed D2 diet. After stress, there was a trend that the activities of antioxidants enzymes and proteases in all groups were significantly decreased. And compared to the control group, crabs fed D1 and D3 diet significantly improved SOD and CAT activities, the lowest MDA activity was observed in crabs fed D3 diet, no significant difference was obtained in activities of total antioxidant capacity (T-AOC) and three proteases among all groups (P > 0.05). At 96 h after transportation process, the lowest cumulative mortality was observed in crabs fed D3 diet, the difference among all groups before and at 24 h, 48 h and 72 h after stress was not significant (P > 0.05). In summary, a basal diet supplemented with synbiotic could improve the growth performance and protect the hepatopancreas of Eriocheir sinensis more effectively than singular supplementation with prebiotics or probiotics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chinese mitten crab, Eriocheir sinensis is one of the most important and productive crab species cultured in China, and its annual production reached 756,877 tons in 2018 (Ministry of Agriculture and Rural Affairs, China Society of Fisheries, 2019). This crab species is a popular and high-value aquaculture crustacean because of its nutritional value, delicious flavor and the food culture in China (Gao et al., 2014; Wang et al., 2016; Lin et al., 2020). However, the rapid expansion of the production has resulted in the emergence of some problems. Since 2015, a diseased called hepatopancreatic necrosis disease (HPND) spread rapidly in major aquaculture areas of crab, especially in Jiangsu province in 2016, and in the past years, causing a mortality rate of 40%-50% which leading to serious economic losses (Chen et al., 2017a, b). Although revealing the risk factors for HPND of crab has been a research hotspot, its pathogenesis remains unclear (Gao et al., 2018). However, there are two things that can be determined: firstly, there are many factors related to hepatopancreas disease, such as nutrition supply, genetic degeneration stress and environments (Gao et al., 2018); secondly, the hepatopancreas of diseased crabs appear white, atrophied and necrotic, and as a detoxification organ in crustaceans, the hepatopancreas participates in many important life activities, which include energy storage and nutritional metabolism (Huang et al., 2015; Gao et al., 2018). Therefore, monitoring the hepatopancreas related indicators plays an important and essential role in evaluating the host health of Eriocheir sinensis.
Nowadays, instead of antibiotics, one of the most common ways to enhance aquatic immunity is the application of vaccine, prebiotics, probiotics, and immunostimulants (Yu et al., 2014). According to published literatures, prebiotics and probiotics are classified as non-digestible food ingredient that beneficially improve the intestinal balance of other organisms, when consumed in adequate amounts (Jia et al., 2017). Fructooligosaccharide (FOS) is one of the most common prebiotics studied in aquaculture (Ringø et al., 2010), and dietary administration with FOS has been proved to promote growth performance (Lima Paz et al., 2019), antioxidant capability (Chen et al., 2017a, b), innate immunity (Jia et al., 2019), disease and stress resistance (Soleimani et al., 2012). Among the probiotics used in aquaculture Lactobacillus acidophilus (L. acidophilus), Bacillus subtilis (B. subtilis) and Saccharomyces cerevisiae (S. cerevisiae) are the most well- established ones (Hosseini et al., 2016; Interaminensea et al., 2018; Zhou et al., 2018). These three probiotics have been shown to positively affect the growth performance, immunity and stress resistance of various aquatic species (Talpur et al., 2013; Jiang et al., 2017; Führ et al., 2016).
Generally, probiotics and prebiotics are mostly studied separately. However, since probiotic is lack of ability to form stable masses and maintain the advantage in intestinal flora (Ringø et al., 2010), the synbiotics, as a combination of probiotics species with appropriate prebiotics that have positively influence on the host by enhancing the survival and implantation of live microbial dietary supplements in the gastrointestinal tract has been suggested (Roberfroid, 2000; Bielecka et al., 2002). Meanwhile, there is limited information about the effect of dietary synbiotic administration in Chinese mitten crab. Therefore, the aim of present research was to evaluate the effects of dietary synbiotic (FOS and probiotics) on the growth performance, antioxidant and anti-stress ability of juvenile Eriocheir sinensis.
Materials and methods
Experimental diets
There are four experimental diets in this study: basal diet (control group), basal diet supplemented with 5 g/kg FOS, basal diet supplemented with 2 g/kg multi-strain probiotics (each gram contains Lactobacillus acidophilus 106 CFU + Bacillus subtilis 107 CFU + Saccharomyces cerevisiae 1010 CFU) and basal diet supplemented with symbiotic (5 g/kg FOS + 2 g/kg multi-strain probiotics) (designated as control, D1, D2 and D3, respectively). The doses were selected according to the results from previous studies (Hoseinifar et al., 2016; Dawood and Koshio, 2016). The FOS product was provided by Jiangsu Ease Biotech Co., Ltd (China), and consisted of 95% 1–3fructose. The heat resistance Lactobacillus acidophilus (L. acidophilus) used in the study was supplied by SKF (Beijing) Bio-Technology Co., Ltd., Bacillus subtilis (B. subtilis) and yeast Saccharomyces cerevisiae (S. cerevisiae) was obtained from Jiangsu Ease Biotech Co., Ltd (China). Probiotics were prepared based upon the method suggested by (Merrifield et al. (2011), their viability were confirmed by culturing on de Man, Rogosa & Sharpe (MRS; Merck, Germany) agar during preservation (Hoseinifar et al., 2015a, b).
Test diets were produced by the commercial feed processing method at the Nanjing Shuaifeng feed Co., LTD (China). The basal diet (38.12% crude protein and 5.21% crude lipid) formulation and composition were showed in Table 1. Experimental diets were sealed in plastic bags and stored at − 20 °C until use. The crabs were fed two times a day (7:00 and 19:00) at a ration of 4% of body weight for 8 weeks.
Experimental design
Chinese mitten crabs from one brood (one mother) were provided by Pukou fish farm of Freshwater Fisheries Research Institute of Jiangsu province, China. Before this study, crabs were acclimated two weeks fed with the basal diet. After the conditioning period, 900 healthy male crabs (initial body weight 9.90 ± 0.07 g) were randomly assigned into 12 outdoor rectangular tanks (4.0 m × 4.0 m × 2.0 m, water height: 0.2 m) with floating underground water. During the rearing period, the mean water quality indices were: water temperature ranged from 27 ℃ to 31 ℃, DO > 6.50 mg L-1 and pH 7.5 ~ 8.0. The exuvial and dead crabs were checked daily. After 8 weeks, all crabs were weighed and counted before sampling.
Transport stress test
Upon completion of the rearing experiment, after the first sampling, 18 crabs of similar size were sampled from each tank and exposed to the transportation stress. The crabs were kept in nylon net bags with ice and then transported on paved road for 3 h inside a van. At the end of the transportation, each group of cabs were distributed into per indoor plastic tank (1.0 m × 0.4 m × 0.4 m, height of water: 0.2 m), respectively. Meanwhile, no feeding, water exchanging and human interference was allowed during the stress test. Water temperature ranged from 28 ℃ to 31 ℃, DO > 6.40 mg/L and pH 7.8 ~ 8.1. Cumulative mortality was recorded at 24 h, 48 h, 72 h and 96 h after stress.
Sampling and processing
At termination of the feeding trail and transport stress test (96 h after stress), three crabs from each tank were randomly sampled and dissected to collect hepatopancreas samples, then they were frozen in liquid nitrogen immediately and stored at -80 °C for analysis of antioxidant capacity and protease activity.
Analysis and measurement
The growth parameter in this study was calculated as follows:
Weight gain rate (WGR, %) = 100 × (Wt-W0) / W0.
Specific growth rate (SGR) = 100 × (ln Wt − ln W0) / T.
Feed conversion ratio (FCR) = F/ W.
Survival rate (SR, %) = 100 × Nt / N0.
Where W0 is the initial body weight, Wt is the final body weight, T is the number of days in feeding trail, F is the total feed intake, W is the total weight gain, N0 is the number of initial crab, Nt is the number of final crab.
Hepatopancreas antioxidant enzyme and protease activities were measured by assay kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's instruction. The level of total antioxidant capacity (T-AOC), pepsin, trypsin and chymotrypsin were determined with colorimetric method, superoxide dismutase (SOD) were measured with hydroxylamine method, catalase (CAT) were determined with spectrophotometric method, and malondialdehyde (MDA) was measured with TBA method.
Data statistics and analysis
Duncan’s multiple range test was applied to rank the means after statistical analysis were subjected to one-way analysis of variance (ANOVA) in SPSS 22.0. All results were presented as mean ± standard error of the mean (X ± SEM).
Results
Growth performance
The growth performance of crab was shown in Table 2. After 8 weeks of feeding trial, compared to the control group, crabs fed D3 significantly increased WG and SGR, and significantly decreased FCR, respectively (P < 0.05). Moreover, SR exhibited no significant alteration among four groups (P > 0.05).
Antioxidant capacity before and after stress
The effects of dietary FOS and probiotics on T-AOC, SOD, CAT and MDA levels in hepatopancreas before and after transport stress were shown in Fig. 1. It can be seen in Fig. 1A that there was no statistical difference among the T-AOC levels before and after stress (P > 0.05). Figure 1B presents that before and after stress, compared to control diet, crabs fed D1 and D3 significantly improved SOD activities and CAT activities showed a similar tend (P < 0.05) (Fig. 1C). As shown in Fig. 1D, before and after stress, MDA activities in the crabs of control group were significantly higher than those in D1, D3 and D3 diet, respectively (P < 0.05). Meanwhile the activities of SOD, T-AOC, CAT and MDA in all groups appeared to significantly decreased after stress (P < 0.05).
Effects of dietary fructooligosaccharides, probiotics and their combination on T-AOC (A), SOD (B), CAT (C) and MDA (D) levels of juvenile Chinese Mitten Crab hepatopancreas before and after transport stress. Different capital letters above the bars indicate significant differences (P < 0.05) at different time points in the same group in Duncan’s multiple-range test; different small letters above the bars indicate significant differences (P < 0.05) in different groups at the same time point in Duncan’s multiple-range test; data are expressed as mean ± SEM (n = 9)
Protease activity before and after stress
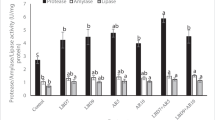
The activities of pepsin, trypsin and chymotrypsin in hepatopancreas before and after stress were shown in Fig. 2. As can be seen in Fig. 2A and Fig. 2B, before stress, the highest activities of pepsin and trypsin were observed in D2 (P < 0.05), and compared to the control and D1 group, the chymotrypsin activity in D2 group was also significant higher (Fig. 2C) (P < 0.05). It showed that there was no significant alteration between the treatments and the control group after stress (P > 0.05). Compared to the activities before stress, there was a trend that three proteases activities in all experimental groups were significantly decreased after stress (P < 0.05).
Effects of dietary fructooligosaccharides, probiotics and their combination on pepsin (A), trypsin (B) and chymotrypsin (C) activities of juvenile Chinese Mitten Crab hepatopancreas before and after transport stress. Different capital letters above the bars indicate significant differences (P < 0.05) at different time points in the same group in Duncan’s multiple-range test; different small letters above the bars indicate significant differences (P < 0.05) in different groups at the same time point in Duncan’s multiple-range test; data are expressed as mean ± SEM (n = 9)
Cumulative mortality after stress
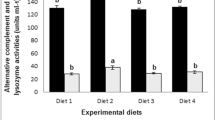
The observed cumulative mortality was recorded at 24 h, 48 h, 72 h and 96 h after transport stress, respectively (Fig. 3). Cumulative mortality in crabs fed D3 diet was significant lower than those in control and D2 group at 96 h after stress (P < 0.05). While the difference among all groups at 24 h, 48 h and 72 h after stress was not significant (P > 0.05).
Effects of dietary fructooligosaccharides, probiotics and their combination on cumulative mortality of juvenile Chinese Mitten Crab after transport stress. Values as mean ± SEM (n = 3), diverse little letters show significant differences (Duncan’s multiple-range test, P < 0.05) in different groups of each sampling point
Discussion
Synbiotic has been shown to have positively influence on growth, immunity, intestinal health, disease and anti-stress in some aquatic species (Azimirad et al., 2016; Hoseinifar et al., 2015a, b; Ai et al., 2011; Hamsah et al., 2019). In the present work, WGR, SGR and FCR of crabs were significantly affected by the combination of FOS and probiotics, which indicates that dietary administration of synbiotic (FOS and probiotics) at an appropriate level could be beneficial to the growth performance of juvenile Eriocheir sinensis. The possible reason might be that compared to separate prebiotic or probiotic diets, the combination of those two can increase digestibility of prebiotic, improve the survival and colonization of probiotic, furthermore, elevate health status. Previous studies also have shown dietary FOS could improve the growth by improving the digestion and uptake of the feed (Soleimani et al., 2012; Guerreiro et al., 2016). However, in this experiment, the crabs fed the diet with FOS (5 g/kg) didn’t show a significant improvement in growth performance, this might be due to the high level of dietary FOS causing excessive prebiotics, which are defined as non-digestible food ingredients that can’t be fermented by intestinal microbiota, and the indigestible substances will accumulate in the intestine which may irritate the gut (Olsen et al., 2001; Jantarathin et al., 2017). In accordance with the present study, Grisdale-Helland et al. (2008) revealed that Atlantic salmon (Salmo salar) fed with 10-g FOS/kg feed without probiotic for 4-month showed no significant difference in feed efficiency or energy retention, which demonstrated that the improvement of growth performance in symbiotic group might due to the successful colonization of probiotics in the gut and modulated gut microbiota from synbiotics can produce nutrients and enzymes rather than the dietary supplementation of FOS (Suzer et al., 2008). Moreover, Hosseini et al. (2016), Aly et al. (2008) reported that supplementation with L. acidophilus and combination of B. subtilis and L. acidophilus didn’t significantly affect the growth and feed uptake. But contrary to these findings, administration of L. acidophilus, B. subtilis, S. cerevisiae had a significant impact in black swordtail (Hoseinifar et al., 2015a, b), tilapia (Liu et al., 2017) and juvenile beluga (Hoseinifar et al., 2011), respectively. These contradictory results across studies may be attributed in particular to differences in species, supplementation regime and sampling strategy.
Under stable living conditions or certain environment fluctuations without severe stress, the removal and production of reactive oxygen species (ROS) are maintained in a balanced state in animal cells. Meanwhile, several antioxidant defense mechanisms are designed in the body to deal with it. The antioxidant system includes enzymes such as total antioxidant capacity (T-AOC), catalase (CAT) and superoxide dismutase (SOD), which play an important role in the immunity and anti-stress ability of aquatic animals by eliminating the ROS to protect the host from oxidative damage. In addition, previous studies reported that the administration of the probiotic, prebiotic and synbiotics can affect the antioxidant defense through different mechanisms: ability to chelate metal ions, modulation of gut microbiota, production antioxidant metabolites and prevention of ROS production (Hoseinifar et al., 2020; Hasyimi et al., 2020). In the current study, compared to the control group, crabs fed dietary supplementation with FOS and synbiotic, the CAT and SOD activities in hepatopancreas increased and the MDA activity decreased significantly before and after stress. Demonstrate that the immune system of juvenile Chinese mitten crab was stimulated by oral administration of FOS and synbiotic, and anti-oxidative responses was affected by the FOS- and synbiotic- induced immune system, which indicates that synbiotic and FOS could restrain the enhancement of lipid peroxide under transport stress. The antioxidant effect of FOS administration is closely related to its bifidogenic effect (Zhang et al., 2014a, b) and the primary role of prebiotics is to improve the survivability and implantation of the probiotic (Huynh et al., 2017). It is assumed that the prebiotic could increase stimulation of CAT activity may contribute to enhancing the phagocytic activity and increasing the production of reactive oxygen metabolites by macrophages (Reyes-Becerril et al., 2008). Besides, probiotic are reported to be plays an important role in increasing the assimilation of dietary antioxidants from the feed (Castex et al., 2008).
The antioxidant enzyme activity mentioned above is significantly affected by stress for aquatic animals during breeding (Zhang et al., 2012; Wongsasak et al., 2015). Data from the current study showed that the effects of FOS, probiotics and their synbiotic on anti-oxidative system followed the same trends between before and after transport stress treatment. Similar results reported in the case of supplementation with FOS and synbiotic in blunt snout bream (Zhang et al., 2014a, b) and Pacific white shrimp (Wongsasak et al., 2015). Few prior studies have reported that probiotics could positively affect oxygen radical production in aquatic animal (Biswas et al., 2012; Andrews et al., 2011). However, the results of this study represented that the dietary administration of probiotics didn’t significantly influence the antioxidant capacity. This discrepancy might be due to the probiotics dose and the feeding duration. Immunostimulatory and antioxidant activity of probiotics vary with dose, and differences in specific immune parameters stimulated by the same probiotics also depends on the feeding time (Kumar et al., 2008; Panigrahi et al., 2004, 2005; Andrews et al., 2011).
Generally, the stress response also is accompanied by a variety of protease activity changes, such as the levels of pepsin, trypsin and chymotrypsin. In this study, the results demonstrated that the transport stress led to decreased protease activity in hepatopancreas of crab. Wang et al (2009) reported that the activity of protease in hepatopancreas of Cyprinus carpio tended to increase at first and then decrease with the prolongation of starvation stress. Owing to the fact that relevant research is quite limited, the mechanism involved in this process is still unclear. The decrease of protease activity in this study may be due to the damage of hepatopancreas after stress (Liang et al., 2016). Further investigations will be required to elucidate the effects of protease activity and stress on crabs.
In addition, significant improvements in protease activities were both observed in crabs fed synbiotic diet and probiotic diet in our present study, however, compared to the control diet, dietary supplementation with FOS in crabs didn’t produce any significant effects on protease activity. The similar phenomenon was also observed by Wang et al. (2017) who investigated that the effect of probiotic (Bacillus lincheniformis) and synbiotic (Bacillus lincheniformis and alginate oligosaccharides) on sea cucumber Apostichopus japonicas and showed that probiotic and synbiotic can significantly improve protease activity, no significant difference was observed between the control and those fed with the prebiotic diet. Soltani et al. (2019) also found that the activity of chymotrypsin was greater in Caspian Roach (Rutilus Frisii kutum) fry fed with synbiotics (FOS + Pediococcus acidilactici and Lactococcus lactis) than in control fish. Talpur et al. (2013) showed that supplementation of Lactobacillus plantarum significantly increased the protease activity of blue swimming crab. Probiotics can enhance the level of protease in aquatic animals might due to the ability to produce proteolytic, amylolytic, lipolytic enzymes and improve the microbial metabolism by accumulation of supply probiotics in the gut of juvenile.
Stress test has been commonly used to evaluate the health state of fitness of aquatic animal in nutritional studies (Caipang et al., 2014; Zhao et al., 2014; Liu et al., 2019). The results of the present study revealed significant increase in resistance against transport stress in juvenile Chinese mitten crab after feeding with synbiotic diet and prebiotic diet. In conformity to our research, Azimirad et al. (2016) reported that the maximum stress resistance was shown in the synbiotic treatment (Pediococcus acidilactici and fructooligosaccharide) during acute decrease of temperature and increase salinity stress. According to prior research, FOS supplements could cause immune mechanism after stress through adjusting stress response, which improve tolerance to stress, for instance, FOS can reduce cortisol production, HSP70 and HSP90 mRNA expression levels (Zhang et al., 2014a, b). Furthermore, FOS is able to selectively stimulating the growth and/or activity of a part of bacteria like Bacillus spp and lactic acid bacteria which could beneficially affect the host (Meyer and Stasse-Wolthuis, 2009). The increase in resistance against stress in crabs fed with synbiotic and FOS can be possibly attributed to the basis of increased antioxidant capacity. The improvement of stress resistance caused by synbiotic may be due to the enhancement of health status.
In conclusion, our study indicated that dietary synbiotic (FOS and L. acidophilus, B. subtilis, S. cerevisiae) supplementation could improve the growth performance, antioxidant capability, protease activity in hepatopancreas, as well as the resistance to transport stress in juvenile Chinese mitten crab.
References
Ai QH, Xu HG, Mai KS, Xu W, Wang J, Zhang WB (2011) Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 317:155–161
Aly SM, Ahmed YAG, Ghareeb AAA, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunology 25:128–136
Andrews SR, Sahu NP, Pal AK, Mukherjee SC, Kumar S (2011) Yeast extract, brewer’s yeast and spirulina in diets for Labeo rohita fingerlings affect haematoimmunological responses and survival following Aeromonas hydrophila challenge. Res Vet Sci 91:103–109
de Lima A, Paz M, da Silva J, Marques da Silva KM, Val AL (2019) Protective effects of the fructooligosaccharide on the growth performance, hematology, immunology indicators and survival of tambaqui (Colossoma macropomum, Characiformes: Serrasalmidae) infected by Aeromonas hydrophila. Aquaculture Reports 15:2352–5134
Azimirad M, Meshkini S, Ahmadifard N, Hoseinifar SH (2016) The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellfish Immunology 54:516–522
Bielecka M, Biedrzycka E, Majkowska A (2002) Selection of probiotics and prebiotics for synbiotics and confirmation of their in vivo effectiveness. Food Res Int 35:125–131
Biswas G, Korenaga H, Takayama H, Kono T, Shimokawa H, Sakai M (2012) Cytokine responses in the common carp, Cyprinus carpio L. treated with baker’s yeast extract. Aquaculture 356–357:169–175
Caipang CMA, Fatira E, Lazado CC, Pavlidis M (2014) Short-term handling stress affects the humoral immune responses of juvenile Atlantic cod, Gadus morhua. Aquacult Int 22:1283–1293
Castex M, Chim L, Pham D, Lemaire P, Wabete N, Nicolas JL, Schmidely PH, Mariojouls C (2008) Probiotic P. acidilactici application in shrimp Litopenaeus stylirostris culture subject to vibriosis in New Caledonia. Aquaculture 275:182–193
Chen WW, Romano N, Ebrahimi M, Natrah I (2017a) The effects of dietary fructooligosaccharide on growth, intestinal short chain fatty acids level and hepatopancreatic condition of the giant freshwater prawn (Macrobrachium rosenbergii) postlarvae. Aquaculture 469:95–101
Chen XW, Wang J, Yue WC, Liu JS, Wang CH (2017b) Hepatopancreas transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with white hepatopancreas syndrome. Fish Shellfish Immunol 70:302–307
Dawood MAO, Koshio S (2016) Recent advances in the role of probiotics and prebiotics in carp aquaculture: A review. Aquaculture 454:243–251
Führ F, Tesser MB, Rodrigues RV, Pedron J, Romano LA (2016) Artemia enriched with hydrolyzed yeast improves growth and stress resistance of marine pejerrey Odontesthes argentinensis larvae. Aquaculture 450:173–181
Gao TH, Xu Y, Wang K, Deng YF, Yang YJ, Lu QP, Pan JL, Xu ZQ (2018) Comparative LC-MS based non-targeted metabolite profiling of the Chinese mitten crab Eriocheir sinensis suffering from hepatopancreatic necrosis disease (HPND). Aquaculture 491:338–345
Gao XC, Gu SQ, Tao NP, Wang XC, Zhuang KJ, Wu N (2014) Comparison of volatile compounds in Chinese mitten crab (Eriocheir sinensis) hepatopancreas and crab gonad by monotrap adsorption. Science and Technology of Food Industry 31:324–329 ((in Chinese with English abstract))
Grisdale-Helland B, Helland SJ, Gatlin DM III (2008) The effects of dietary supplementation with mannanoligosaccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar L.). Aquaculture 283:163–167
Guerreiro I, Serra CR, Enes P, Couto A, Salvador A, Costas B, Oliva-Teles A (2016) Effect of short chain fructooligosaccharides (scFOS) on immunological status and gut microbiota of gilthead sea bream (Sparus aurata) reared at two temperatures. Fish Shellfish Immunology 49:122–131
Hamsah H, Widanarni W, Alimuddin A, Yuhana M, Junior MZ, Hidayatullah D (2019) Immune response and resistance of Pacific white shrimp larvae administered probiotic, prebiotic, and symbiotic through the bio-encapsulation of Artemia sp. Aquacult Int 27:567–580
Hasyimi W, Widanarni W, Yuhana M (2020) Growth Performance and Intestinal Microbiota Diversity in Pacific White Shrimp Litopenaeus vannamei Fed with a Probiotic Bacterium, Honey Prebiotic, and Synbiotic. Curr Microbiol 77:2982–2990
Hosseini M, Miandare HK, Hoseinifar SH, Yarahmadi P (2016) Dietary Lactobacillus acidophilus modulated skin mucus protein profile, immune and appetite genes expression in gold fish (Carassius auratus gibelio). Fish Shellfish Immunology 59:149–154
Hoseinifar SH, Eshaghzadeh H, Vahabzadeh H, Mana NP (2016) Modulation of growth performances, survival, digestive enzyme activities and intestinal microbiota in common carp (Cyprinus carpio) larvae using short chain fructooligosaccharide. Aquac Res 47:3246–3253
Hoseinifar SH, Mirvaghefi A, Amoozegar MA, Sharifian M, Esteban MÁ (2015a) Modulation of innate immune response, mucosal parameters and disease resistance in rainbow trout (Oncorhynchus mykiss) upon synbiotic feeding. Fish Shellfish Immunol 45:27–32
Hoseinifar SH, Mirvaghefi A, Merrifield DL (2011) The effects of dietary inactive brewer’s yeast Saccharomyces cerevisiae var. ellipsoideus on the growth, physiological responses and gut microbiota of juvenile beluga (Huso huso). Aquaculture 318:90–94
Hoseinifar SH, Roosta Z, Hajimoradloo A, Vakili F (2015b) The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri). Fish Shellfish Immunology 42:533–538
Hoseinifar SH, Yousefi S, Van Doan H, Ashouri G, Gioacchini G, Maradonna F, Carnevali O (2020) Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Reviews in Fisheries Science & Aquaculture. https://doi.org/10.1080/23308249.2020.1795616
Huang S, Wang J, Yue W, Chen J, Gaughan S, Lu W, Lu G, Wang C (2015) Transcriptomic variation of hepatopancreas reveals the energy metabolism and biological processes associated with molting in Chinese mitten crab. Eriocheir Sinensis Scientific Reports 5:14015
Huynh TG, Shiu YL, Nguyen TP, Truong QP, Chen JC, Liu CH (2017) Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: A review. Fish Shellfish Immunology 64:367–382
Interaminense JA, Vogeley JL, Gouveia CK, Portela RWS, Oliveira JP, Andrade HA, Peixoto SM, Soares RB, Buarque DS, Bezerra RS (2018) In vitro and in vivo potential probiotic activity of Bacillus subtilis and Shewanella algae for use in Litopenaeus vannamei rearing. Aquaculture 48:114–122
Jantarathin S, Borompichaichartkulb C, Sanguandeekulb R (2017) Microencapsulation of probiotic and prebiotic in alginate-chitosan capsules and its effect on viability under heat process in shrimp feeding. Materials Today: Proceedings 4:6166–6172
Jia ET, Li ZQ, Xue YF, Jiang GZ, Li XF, Liu WB, Zhang DD (2017) Effects of dietary fructooligosaccharide on the growth, antioxidants, immunity and disease resistance of Chinese mitten crab. Aquaculture 481:154–161
Jia ET, Zheng XC, Cheng HH, Liu J, Li XF, Jiang GZ, Liu WB, Zhang DD (2019) Dietary fructooligosaccharide can mitigate the negative effects of immunity on Chinese mitten crab fed a high level of plant protein diet. Fish Shellfish Immunology 84:100–107
Jiang HY, Chen TJ, Sun HC, Tang ZL, Yu JY, Lin ZP, Ren PL, Zhou XY, Huang Y, Li XR, Yu XP (2017) Immune response induced by oral delivery of Bacillus subtilis spores expressing enolase of Clonorchis sinensis in grass carps (Ctenopharyngodon idellus). Fish Shellfish Immunology 60:318–325
Kumar R, Mukherjee SC, Ranjan R, Nayak SK (2008) Enhanced innate immune parameters in Labeo rohita (Ham.) following oral administration of Bacillus subtilis. Fish Shellfish Immunology 24:168–172
Liang ZX, Liu R, Zhao DP, Wang LL, Sun MZ, Wang MQ, Song LS (2016) Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunology 54:523–528
Lin ZD, Han FL, Lu JT, Guo JL, Qi CL, Wang CL, Xiao SS, Bu XY, Wang XD, Qin JG, Chen LQ (2020) Influence of dietary phospholipid on growth performance, body composition, antioxidant capacity and lipid metabolism of Chinese mitten crab. Eriocheir sinensis. Aquaculture 516:734653
Liu BL, Fei F, Li XT, Wang XY, Huang B (2019) Effects of stocking density on stress response, innate immune parameters, and welfare of turbot (Scophthalmus maximus). Aquacult Int 27:1599–1612
Liu HT, Wang SF, Cai Y, Guo XH, Cao ZJ, Zhang YZ, Liu SB, Yuan W, Zhu WW, Zheng Y, Xie ZY, Guo WL, Zhou YC (2017) Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunology 60:326–333
Merrifield D, Bradley G, Harper G, Baker R, Munn C, Davies S (2011) Assessment of the effects of vegetative and lyophilized Pediococcus acidilactici on growth, feed utilization, intestinal colonization and health parameters of rainbow trout (Oncorhynchus mykiss Walbaum). Aquac Nutr 17:73–79
Meyer D, Stasse-Wolthuis M (2009) The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr 63:1277–1289
Ministry of Agriculture and Rural Affairs, China Society of Fisheries (2019) China Fishery Statistical Yearbook 2019. Beijing
Olsen RE, Myklebust R, Kryvi H, Mayhew TM, Ringø E (2001) Damaging effect of dietary inulin on intestinal enterocytes in Arctic charr (Salvelinus alpinus L.). Aquac Res 32:931–934
Panigrahi A, Kiron V, Kobayashi T, Puangkaew J, Satoh S, Sugita H (2004) Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunopathol 102:379–388
Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, Sugita H (2005) The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture 243:241–254
Reyes-Becerril M, Salinas I, Cuesta A, Meseguer J, Tovar-Ramírez D, Ascencio-Valle F, Esteban MA (2008) Oral delivery of live yeast Debaryomyces hansenii modulates the main innate immune parameters and the expression of immunerelevant genes in the gilthead seabream (Sparus aurata L.). Fish Shellfish Immunology 25:731–739
Ringø E, Olsen RE, Gifstad TØ, Dalmo RA, Amlund H, Hemre GI, Bakke AM (2010) Prebiotics in aquaculture: a review. Aquac Nutr 16:117–136
Roberfroid MB (2000) Prebiotics and probiotics: are they functional food? Am J Clin Nutr 71:1682–1687
Soleimani N, Hoseinifar SH, Merrifield DL, Barati M, Abadi ZH (2012) Dietary supplementation of fructooligosaccharide (FOS) improves the innate immune response, stress resistance, digestive enzyme activities and growth performance of Caspian roach (Rutilus rutilus) fry. Fish Shellfish Immunology 32:316–321
Soltani M, Badzohreh G, Mirzargar S, Farhangi M, Shekarabi PH, Lymbery A (2019) Growth Behavior and Fatty Acid Production of Probiotics, Pediococcus acidilactici and Lactococcus lactis, at Different Concentrations of Fructooligosaccharide: Studies Validating Clinical Efficacy of Selected Synbiotics on Growth Performance of Caspian Roach (Rutilus Frisii kutum) Fry. Probiotics and Antimicrobial Proteins 11:765–773
Suzer C, Coban D, Kamaci HO, Saka S, Firat K, Otgucuoglu O, Kucuksari H (2008) Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: effects on growth performance and digestive enzyme activities. Aquaculture 280:140–145
Talpur AD, Ikhwanuddin M, Abdullah MDD, Bolong AMA (2013) Indigenous Lactobacillus plantarum as probiotic for larviculture of blue swimming crab, Portunus pelagicus (Linnaeus, 1758): Effects on survival, digestive enzyme activities and water quality. Aquaculture 416–417:173–178
Wang AM, Fang YQ, Wei XJ, Li J, Xu HF, Han GM (2009) Effect of Starvation Stress on the Activity of Protease and Amylase in Intestine and Hepatopancreas of Cyprinus carpio. Chin J Ecol 30:94–97 ((in chinese))
Wang S, He Y, Wang Y, Tao N, Wu X, Wang X, Qiu W, Ma M (2016) Comparison of flavour qualities of three sourced Eriocheir sinensis. Food Chem 200:24–31
Wang XT, Sun YX, Wang LL, Li XY, Qu KL, Xu YP (2017) Synbiotic dietary supplement affects growth, immune responses and intestinal microbiota of Apostichopus japonicus. Fish Shellfish Immunology 68:232–242
Wongsasak U, Chaijamrus S, Kumkhong S, Boonanuntanasarn S (2015) Effects of dietary supplementation with β-glucan and synbiotics on immune gene expression and immune parameters under ammonia stress in Pacific white shrimp. Aquaculture 436:179–187
Yu HH, Han F, Xue M, Wang J, Tacon P, Zheng YH, Wu XF, Zhang YJ (2014) Efficacy and tolerance of yeast cell wall as an immunostimulant in the diet of Japanese seabass (Lateolabrax japonicus). Aquaculture 432:217–224
Zhang CN, Li XF, Jiang GZ, Zhang DD, Tian HY, Li JY, Liu WB (2014a) Effects of dietary fructooligosaccharide levels and feeding modes on growth, immune responses, antioxidant capability and disease resistance of blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunology 41:560–569
Zhang CN, Tian HY, Li XF, Zhu J, Cai DS, Xu C, Wang F, Zhang DD, Liu WB (2014b) The effects of fructooligosaccharide on the immune response, antioxidant capability and HSP70 and HSP90 expressions in blunt snout bream (Megalobrama amblycephala Yih) under high heat stress. Aquaculture 433:458–466
Zhang J, Liu Y, Tian L, Yang H, Liang G, Xu D (2012) Effects of dietary mannan oligosaccharide on growth performance, gut morphology and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunology 33:1027–1032
Zhao W, Liang M, Liu Q, Yin XW (2014) The effect of yeast polysaccharide (YSP) on the immune function of Apostichopus japonicus Selenka under salinity stress. Aquacult Int 22:1753–1766
Zhou M, Liang RS, Mo JF, Yang S, Gu N, Wu ZH, Babu VS, Li J, Huang YM, Lin L (2018) Effects of brewer’s yeast hydrolysate on the growth performance and the intestinal bacterial diversity of largemouth bass (Micropterus salmoides). Aquaculture 484:139–144
Funding
This research was supported by funding from the Jiangsu Agricultural Industry Technology System (Chinese mitten crab) (JATS [2019] 384).
All procedures performed in studies involving animals were in accordance with the guidelines and ethical standards of Chinese Academy of Fishery Sciences and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wan, JJ., Pan, Jl., Shen, MF. et al. Changes in the growth performance, antioxidant enzymes and stress resistance caused by dietary administration of synbiotic (fructooligosaccharide and probiotics) in juvenile Chinese Mitten Crab, Eriocheir sinensis. Aquacult Int 30, 467–481 (2022). https://doi.org/10.1007/s10499-021-00811-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00811-5