Abstract
The behavior and dynamics of arsenic at Ilha do Mel, Guaraqueçaba, and Paranaguá, located in the Paranaguá Estuarine Complex, Brazil, were investigated in this work. Samples were collected in March, September, and December of 2005. With respect to arsenic behavior, the total concentrations were higher for Paranaguá (22.5 ± 2.5 μg L−1) on average, followed by the samples of Guaraqueçaba (14.4 ± 3.2 μg L−1) and Ilha do Mel (8.7 ± 1.1 μg L−1). The concentrations found in this work were consistently greater when compared to other estuaries. The results can be attributed to geological factors. Nevertheless, it was also possible to realize that higher concentrations were found in Paranaguá, the more impacted environment. These results represent a strong indication that human activities in the area contribute to the enrichment of the estuary with this metalloid. Arsenic was mainly present in the dissolved phase, which corresponded to more than 70% of the total concentration. This behavior can represent a greater residence time of this metalloid in the water column. The marine phytoplankton presence was decisive in dictating the distribution of As in the waters of Paranaguá estuary. Organic species, which are considered the less toxic, were found in higher concentrations in the eutrophic areas such as Guaraqueçaba and Paranaguá. This was a typical behavior during the summer probably due to higher biological activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic is an element with toxic properties, which is recognized by the International Agency for Research on Cancer (IARC) and World Health Organization (WHO) as human carcinogen (Roy and Saha 2002; Melamed 2004; He et al. 2004; ATSDR 2007). Moreover, this element is listed as the highest priority pollutant in the ASTDR/EPA priority list of hazardous substances (Chou and de Rosa 2003). The ranking of substances on this list is based in the frequency, toxicity, and potential for human exposure (CERCLA 2007).
Arsenic is also a ubiquitous element in the environment due to the contribution from natural sources as well as anthropogenic activities (Ren et al. 2010). It has been used in various fields such as medicine, agriculture, electronics, and metallurgy (Kumaresan and Riyazuddin 2001; Melamed 2004; Sharma and Sohn 2009).
In environmental compartments such as air, soil, sediment, and water, arsenic is widely distributed in a variety of chemical forms and species that behave differently in terms of mobility, bioavailability, and toxicity (Gibbon-Walsh et al. 2010). Thus, extensive studies have shown that arsenic speciation is a key aspect in understanding its biogeochemical cycle as well as its toxicity to the biota.
There has been an increasing interest in knowing the behavior of arsenic in estuarine ecosystems because this element has a complex and poorly understood biogeochemistry that involves several physicochemical and biological processes (Michel et al. 2001). Estuaries are dynamic environments where interactions among freshwater, seawater, soil, and atmosphere take place. Estuaries represent transition zones with high variability in their characteristics because of the influence of both salt and freshwaters. Factors such as tide, waves, saline water influx, freshwater flow, sediments, and winds can affect the distribution, transport, and bioavailability of metals and metalloids by changes in pH, temperature, salinity, redox potential, among others. Masson et al. (2009) studied arsenic speciation in the Gironde Estuary (France) and verified that organic matter degradation and redox potential oscillation on different timescales induced profound changes in partition, speciation, and reactivity of arsenic.
The total concentration of arsenic species is generally constant and less than 2.0 μg L−1 in seawater. However, the arsenic concentration in estuarine waters may show more variations due to anthropogenic inputs and hydrodynamics. In estuaries, arsenic occurs as inorganic and organic species, which vary in behavior and toxicity. The organic forms are usually less toxic than inorganic species (Leermakers et al. 2006). Arsenate [As(V)] and arsenite [As(III)] are recognized as the predominant inorganic species in natural waters and can be found in soluble forms in a wide range of pH and redox potential (Smedley and Kinniburg 2002). Arsenate (H2AsO4 − or HAsO4 2−) is generally the most stable species in oxic conditions, while arsenite (H3AsO4 2− or H2AsO3 −) can be present in anoxic environments or as a result of biological processes (Rhine et al. 2005; Karadjova et al. 2008). In addition, arsenic species may be methylated by phytoplankton or microbial action under phosphate-limited conditions to form monomethylarsonic [MMA; CH3AsO(OH)2] and dimethylarsinic [DMA; (CH3)2AsO(OH)] (Maki et al. 2009).
In general, estuaries are the areas of high biological productivity due to nutrient inputs from rivers and so are favorable environments for the biotransformation of arsenic into less toxic forms (methyl arsenicals) by phytoplankton populations. The ability of microorganisms to accumulate, transform, detoxify, and modify the arsenic speciation depends on several parameters such as the phosphate concentration, pH, redox potential, salinity, temperature, and light intensity (Karadjova et al. 2008). Howard et al. (1984) reported that up to 70% of the dissolved arsenic could be found as such transformation products by the action of phytoplankton in the estuaries of the rivers Beaulieu and Tammar, in England.
The biogeochemical behavior of arsenic species has often been studied in oceanic and estuarine environments (Howard et al. 1982, 1988; Howard and Apte 1989; Andreae 1978; Andreae and Andrae 1989; Elbaz-Poulichet et al. 1996; Michel et al. 2001). In Brazil, some studies related to the occurrence of arsenic species in estuarine sediments have been conducted. Mirlean et al. (2003) showed that high concentrations of arsenic were found in the sediments of the Patos Laggon. About 80% of the arsenic that contaminated this region was found in bioavailable forms. Choueri et al. (2009) studied sediments from Paranaguá Estuarine Complex (PEC) and collected evidence of a positive correlation between some contaminants, mainly As and toxicity of samples to the benthic community.
Paranaguá estuary is located in the southern cost of Brazil and it is one of the biggest estuarine systems in America, with 612 km2 of liquid surface and a drainage area of more than 3,000 km2 (de Camargo and Harari 2003). PEC is partially mixed and highly dynamic due to the influence of a tidal regimen and input from shore (Santos et al. 2009). This system is a relevant site due to economic and ecological aspects. The PEC contains numerous environmental protection areas due to a wide variety of environments such as mangroves, islands, inlets, and bays. In this region, urban, port, industrial, tourism, and fishery activities coexist. Such activities can drastically affect the water quality because of discharges of non-treated sewage and industrial wastes, dredging activities, transport and handling of potentially harmful products such as fertilizers, minerals, grain, and oil derivatives (Kolm et al. 2002; Sá et al. 2006; Choueri et al. 2009).
In this context, the aim of the present paper is to evaluate the geochemical behavior of arsenic, through the study of its speciation in surface waters sampled in different areas of the Paranaguá estuary.
2 Experimental
In this study, a set of procedures of clean techniques were adopted to reduce or eliminate contamination of samples from sampling until the final moment of the speciation analysis (U.S.EPA 1996; Sodré et al. 2005). Moreover, recovery tests were always applied in quality assurance and quality control (QA/QC) experiments.
2.1 Reagents and Apparatus
All reagents used in this work were of analytical grade or better. All solutions were prepared with purified water (Milli-Q, Millipore, 18 MΩ cm). The standard solutions of As(V), Cu(II), and Se(IV) were prepared by dilution of standard stock 1,000 mg L−1 (Merck Tritisol). The standard stock solution of 1,000 mg L−1 As(III) was prepared by the dissolution of As2O3 (Merck) in 25% (m/V) NaOH and immediate acidification with concentrated HCl. A 1,000 mg L−1 palladium (as PdCl2-Merck) was used as matrix modifier. The reducing agent solution was prepared with 10 mL HCl (4.5 mol L−1), 20 mL sodium sulfite (1.48 mol L−1), 1.0 g sodium thiosulphate, and 5.0 g potassium iodide (Sperling et al. 1991).
Voltammetric measurements were carried out in an EG&G Princeton Applied Research 394 Electrochemical Trace Analyzer coupled with an EG&G PAR 303 A Static Mercury Drop Electrode. A HMDE working electrode, a Pt auxiliary electrode, and a Ag/AgCl, KClsat reference electrode were used.
An AA-6800 Shimadzu atomic absorption spectrophotometer equipped with GFA 6500 graphite furnace, ASK 6100 autosampler, arsenic hollow cathode lamp, and background correction using deuterium lamp was also used. All analyses were carried out at the 193.7 nm arsenic resonance line with a slit width of 1 nm. Pyrolytic graphite tubes with tungsten filament (75 W, Osram) were also used.
2.2 Study Area and Sampling
The study area located at Paraná State cost (Brazil), north of the coastal plain and foothills of the Serra do Mar is a large subtropical estuarine system connected to the open sea by three tidal channels (Mantavanelli 1999).
Paranaguá Estuarine Complex is characterized as a coastal plain estuary. This system, oriented perpendicularly to the coastline, was formed when a rise in sea level flooded river valleys in the Holocene. The coastal plains are predominantly sandy, usually fine grained, but also they include sediments with organic matter (Hill 1963; Miranda et al. 2002).
PEC is formed by two main axes: north–south by Laranjeiras, Guaraqueçaba, and Pinheiros bays, and east–west by Antonina and Paranaguá bays. PEC has been classified as a partially mixed estuary with lateral heterogeneity, which is usually defined as shallow water environment and with increased salinity from upstream to downstream at all depths. A lateral gradient originates from the freshwater input of river and tidal creeks, creating multiple micro-estuaries (Lana et al. 2001).
Hydrodynamics of the PEC are driven by tidal forcing and river runoff. Tides are semi-diurnal with diurnal inequalities with maximum amplitudes of ca. 2 m, a mean depth of 5.3 m and a residence time of 3.5 days (Lana et al. 2001; Martins et al. 2010). The estuary receives considerable freshwater input from several rivers, mainly in the rainy season. The climate is classified by Köeppen as Cfa, transitional tropical with around 85% mean air humidity and rain in all months of the year. Total annual precipitation is about 2,000 mm and the air temperature ranges from 16°C in the winter to 34°C in the summer (Lana et al. 2001; Martins et al. 2010). A typical rainy season initiates in late spring and lasts during most of the summer, while the dry season lasts from late autumn to late winter, but is usually interrupted by a short and weak rainy period in early winter (Lana et al. 2001).
In this area, several activities such as urban, port, fishing, and industrial (phosphate fertilizers, storage of chemicals, and grains) are present. The PEC has cities and fishermen villages. Paranaguá is the largest city, where is located the port of Paranaguá, the major Brazilian Southern port. The nutrient and trophic states of PEC are the result of the interactions between hydrodynamic processes and different mechanisms such as biological processes, freshwater input, sediment–water interactions, and sewage discharge from the city of Paranaguá (Lana et al. 2001).
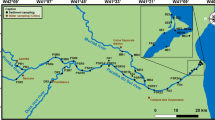
Estuarine water samples were collected in triplicate from Paranaguá Estuarine Complex during three sampling campaigns in 2005, one in March in late summer, other in September at the end of winter, and the third in December in late spring. The sampling campaigns were chosen in order to account for seasonal variations that occur in the PEC throughout the year, especially in rainy and dry seasons. The PEC and the location of sampling points are shown in Fig. 1 (Fávaro et al. 2009).
Three sampling stations were selected because they represent areas with different trophic levels. The first point (P1) was Saco do Limoeiro, in the Ilha do Mel island, situated at the entrance of Paranaguá Bay. This environment is a touristic area with oligotrophic characteristics. The second point (P2) was a natural eutrophic environment called Enseada do Benito, which is located in the Guaraqueçaba Bay. This site presents the influence of urban occupations, mostly small fishermen villages, but there is a low degree of human disturbance when compared to the areas with high urban density (Antonina and Paranaguá). An anthropogenic eutrophic area was chosen as the third point. This is the Anhaia Canal (P3) located near the port of Paranaguá. This site is under the more direct effect of the city and port activities.
At each sampling point, samples were collected in triplicate from surface water using polyethylene bottles. Samples were stored and transported to the laboratory in coolers with ice (4°C) and in the dark. Several physicochemical parameters such as temperature, pH, salinity, and transparency were measured in situ. These parameters were obtained using a thermometer, a pH meter, a refractometer, and a Secchi disk, respectively. On site, the auxiliary parameters were determined to characterize the environment, as dissolved oxygen (OD), total suspended solids (TSS), chlorophyll-a (chlor-a), dissolved inorganic nitrogen, denoted as DIN (NO2 − + NO3 − + NH4 +), and dissolved inorganic phosphorus (DIP) (Strickland and Parsons 1972; Grasshoff et al. 1983; Carmouze 1994).
Samples were transported to the laboratory and were immediately filtered, under vacuum, with cellulose 0.45 μm acetate membranes (Schleicher & Schuell). Samples were analyzed immediately to minimize possible oxidation and microbiological activity.
2.3 Arsenic Speciation
Aliquots of the water samples were filtered to separate the dissolved fraction. Total recoverable arsenic (TRAs) was determined in unfiltered samples, whereas total dissolved arsenic (TDAs) was obtained from the filtered ones, both acidified to pH 1.0 with purified HCl. Determinations of total arsenic were carried out using Tungsten Coil Electrothermal Atomic Absorption Spectrometry (TCA-AAS). The sample injection volume was 20 μL, and the Ar gas flow was 1.0 L min−1. The quantification was performed by standard addition with injections in triplicate. The thermal program was carried out as follows: dry (1) at 150°C for 20 s (ramp mode), dry (2) at 250°C for 10 s (ramp mode), pyrolysis at 1,400°C for 20 s in ramp mode and 25 s in control mode, atomization at 2,200°C for 2 s with temperature control mode, and clean at 2,500°C for 2 s with temperature control mode. The arsenic associated with the solid phase (PAs) was obtained by the difference between TRAs and TDAs concentrations.
Square Wave Cathodic Stripping Voltammetry (SWCSV) at HMDE was used in the determination of inorganic arsenic throughout the formation of an inter-metallic complex involving As-Cu-Se and by standard addition in triplicate (Ferreira and Barros 2002; He et al. 2004; He et al. 2007). Electroactive As(III) was determined in filtered samples by direct SWCSV. The pre-concentration was carried out on the electrode from a solution of HCl in the presence of 4.6 mg L−1 and 3.7 μg L−1 of Cu(II) and Se(IV), respectively. Determination of total inorganic arsenic (TInorgAs) was performed with 10 mL sample, by the reduction of As(V) to As(III), with 350 μL of the reducing agent solution. After the reduction step, the determination of As(III) was performed by SWCSV in the presence of HCl, Cu(II), and Se(IV). As(V) was quantified by the difference between total inorganic As (TInorgAs) and As(III) concentrations. The organic species of arsenic (TOrgAs) were quantified by the difference between TDAs and TInorgAs.
The instrument setup for SWCSV determinations was 600 s purge with purified N2, electrodeposition at −0.38 V versus Ag/AgCl for 30 s (AsIII) or 180 s (TInorgAs) followed by the cathodic scan from −0.38 to −1.0 V with modulation amplitudes 40 mV and 50 Hz of square wave frequency.
The accuracy and precision of the analytical protocol used in the speciation of arsenic by TCA-AAS and SWCSV were confirmed by recovery tests. The results for all recovery tests ranged from 90 ± 2% to 110 ± 5%.
3 Results and Discussion
The data originated from the analyses of some physicochemical parameters to establish the ambient characterization of Paranaguá Estuarine Complex are present in Table 1.
Table 1 shows that the values of most aquatic parameters varied from one location to another. Besides the spatial variation, the temporal variation was also evident, as expected for a dynamic estuarine region. In general, it was observed that the PEC samples collected in the summer (March) were characterized by high pH (>8.0), high salinity (>20), and low nutrient content (DIN and DIP), as a result of the influence of seawater. In addition, maximum chlorophyll-a concentrations in this period indicate a favorable condition for the growth of algal biomass. Despite the variability, the data obtained were consistent for estuarine areas (Howard et al. 1995; Zhang et al. 2007).
Regarding the differences in sampling sites, it was noted that samples of Ilha do Mel had the highest values of pH, salinity, and transparency, indicating the influence of marine waters. Moreover, Table 1 shows that this area is oligotrophic due to low primary productivity, represented by low levels of chlorophyll-a, as a result of low dissolved nutrients such as nitrogen and phosphorus.
Samples from Guaraqueçaba presented lower pH, salinity, and transparency values. These data together with higher levels of TSS suggest that this region is greatly influenced by freshwater inputs. It is recognized that the contribution of freshwater in estuaries is responsible for the input of nutrients, suspended solids, as well as metal species (Howard et al. 1995; Millward et al. 1996). High concentrations of chlorophyll-a and intermediate concentrations of dissolved nutrients were determined in these samples when compared with Ilha do Mel and Paranaguá.
Water samples from the Anhaia Canal (Paranaguá) exhibited intermediate values for most parameters, except for dissolved inorganic nitrogen and phosphorous. DIN and DIP concentrations were up to 2.8 times higher than for the other two sites. These data may indicate that this environment is influenced by freshwater and sewage effluent inputs. It is well known that Paranaguá is the most impacted region in the PEC, by urban, industrial, and port activities (Sá et al. 2006; Choueri et al. 2009). Thus, the aquatic parameters revealed a strong influence of the urbanization process from Paranaguá city. The results for the Paranaguá Bay are consistent with the literature, which indicates that this environment has been influenced by anthropogenic inputs such as domestic discharges and sewage from the port and industries (Choueri et al. 2009; Martins et al. 2010). Kolm et al. (2002) observed that Paranaguá has a poor sewage system and most of it is discharged into rivers or directly into the estuary. The Anhaia, Itiberê, and Correias rivers receive practically all the untreated sewage produced in Paranaguá city (Martins et al. 2010).
3.1 Arsenic Concentrations in the Estuarine Waters of PEC
Total recoverable and dissolved arsenic average concentrations, including the standard deviation, for the samples from the Paranaguá estuary are shown in Fig. 2. Figure 2a presents the total recoverable concentrations of arsenic, and Fig. 2b shows the relative distribution of arsenic between the solid and dissolved phases.
Figure 2a shows TRAs levels for the three sampling sites as well as for the three different seasons. The average TRAs concentrations ranged from 8.7 ± 1.1 to 22.5 ± 2.5 μg L−1 when the spatial aspect is evaluated. TRAs followed the order: Paranaguá > Guaraqueçaba > Ilha do Mel. Taking into account that arsenic levels in seawater are between 1.0 and 2.0 μg L−1; according to the literature, arsenic concentrations determined in this work can be considered slightly higher. However, the literature reports that arsenic concentrations in an estuary can vary as a result of river inputs, salinity and redox gradients, and also via As release from bottom sediments. Typical concentrations are less than 4 μg L−1 in estuarine systems under natural conditions (Sánches-Rodas et al. 2005). Thus, high levels of arsenic in the PEC samples may be related to several factors such as geochemical characteristics of the drainage area, influences of human activities, and also a possible release from sediments that are resuspended during dredging activities that occurs in the PEC (Sá et al. 2006; Choueri et al. 2009). Although the sources of arsenic in the PEC have not been specifically determined until the present moment, it is reasonable to estate that arsenic may be released to aquatic environments from natural weathering of soil and rocks. Anthropogenic sources include mining, smelting, waste water, preservatives, and pesticides (Smedley and Kinniburg 2002). PEC could be naturally rich in arsenic, due to the presence of phosphate rocks in the region, which may contain arsenic in their composition due to geochemical anomaly in the rocks of the area. Fertilizer industries working with mining phosphate are also found in this region. Anthropogenic activities that may contribute with the presence of arsenic in the PEC are intense with traffic of ships and boats with anti-fouling paint, input of untreated domestic and industrial effluents, etc.
Yao et al. (2006) estimated the levels of dissolved arsenic in the range of 1.67–1.93 μg L−1 for samples from the Zhujiang estuary, in China. Seyler and Martin (1990) reported that the maximum dissolved concentrations of arsenic ranged from 1.9 to 3.8 μg L−1 in the Rhône estuary, in France. These authors also reported that human activities contributed to the highest dissolved arsenic levels (16 μg L−1) in the Loire estuary.
Figure 2b shows that higher percentages of arsenic were found in the dissolved fraction for all samples. Dissolved-phase concentrations varied from 72 to 92% of TRAs. The low retention of arsenic by particulate matter may indicate that the residence time for this element in the water column is high for the waters of the Paranaguá estuary. This distribution may contribute to enhance the bioavailability of this element to the aquatic biota.
The distribution of arsenic between the dissolved and particulate phases can also be evaluated by partition coefficients (K D ), which is the quotient between the concentrations found in the particulate matter (in mg kg−1) and in the dissolved phase (mg L−1). Figure 3 presents the log K D for arsenic as a function of TSS concentrations and pH for all collected samples.
The K D values were in the range from 2.3 × 103 to 18.2 × 103 L kg−1. These values were similar to the ones described by Millward et al. (1997), which ranged from 2 to 25 × 103 L kg−1. On average, higher K D values were determined in samples from Anhaia Canal, in Paranaguá, indicating that in this area, arsenic has a slightly higher affinity for the solid phase in comparison with Guaraqueçaba and Ilha do Mel.
The extent of arsenic association with particulate phase depends strongly upon the type and concentration of suspended material and pH. Fig. 3a clearly shows that the partitioning between dissolved and particulate phases exhibited an inverse dependence on total suspend solids. It was observed that K D declines with increasing total suspend solids. This behavior has been commonly described in several papers for marine, estuarine, and freshwater samples (Benoit and Rozan 1999; Sodré and Grassi 2006; Prestes et al. 2006). It may be explained by the particle concentration effect (PCE). PCE is caused by arsenic association with colloidal particles. These particles are counted within the dissolved fraction due to the sample filtration through 0.45 μm pore size membranes.
Arsenic can be sorbed onto solid material, especially clays, iron oxides, aluminum hydroxides, manganese compounds, and organic matter (Smedley and Kinniburg 2002). However, suspended material from PEC has been described as predominantly sandy, usually fine grained (Lana et al. 2001), which can explain the low affinity of the suspended solids for As in the PEC waters.
Regarding the pH influence on the distribution of arsenic between dissolved and particulate phases, Fig. 3b shows that no clear trend was observed between Log K D and pH (R = −0.11). These results may indicate that K D was more influenced by the presence of colloidal particles rather than pH. Based on these aspects, it is believed that the removal of arsenic from the water column by sedimentation does not markedly occur in the samples from the PEC.
3.2 Arsenic Speciation in the Estuarine Waters of PEC
Arsenic speciation in the samples from PEC is presented in Fig. 4. The distribution of arsenic was expressed in terms of the percentage of total recoverable arsenic. Typical standard deviations ranged from 5 to 16%.
Figure 4 depicts that inorganic arsenic (TInorgAs), i.e., the sum of As(III) and As(V) was determined for all samples, representing 16–88% of TRAs. For Ilha do Mel samples, TInorgAs was the major species during the three sampling campaigns, ranging from 54 to 88% of the total arsenic concentration. For Guaraqueçaba and Paranaguá samples, arsenic in inorganic form corresponded to about 64 and 77% of the TRAs concentration, in September and December, respectively.
Among the inorganic arsenic species, on average, the percentage of arsenite followed the order: Ilha do Mel > Paranaguá > Guaraqueçaba. As(III) ranged from 29 to 52% of TRAs for Ilha do Mel, 16–31% for Guaraqueçaba and from 33 to 39% for Paranaguá.
In general, higher percentages of arsenate were observed in September and December for all samples, representing 27–59% of the TRAs. However, in March, As(V) represented less than 3% of the total concentration. The results in Fig. 4 show that the arsenic speciation exhibited a strong seasonal variation, probably due to different biogeochemical characteristics found in the PEC during the studied period.
According to Howard et al. (1995), thermodynamic calculations predict that under low biological activity, arsenate should be the major specie in estuarine systems. However, in temperate regions, climatic variations lead to seasonal changes in biological activity, which result in drastic changes in the speciation of arsenic in natural waters. Sanders (1980) related that the presence of reduced and methylated arsenic species is probably a result of biological mediation processes.
Regarding the organic arsenic fraction, in Fig. 4, it was noted that higher percentages were found in the eutrophic environments, which exhibited higher biological activity, as pointed out by chlorophyll-a levels. In March, arsenic in the organic form corresponded to 43 and 70% of the TRAs for Paranaguá and Guaraqueçaba, respectively. Therefore, these results suggest that biological activity of the microorganisms plays an essential role in the arsenic speciation.
In fact, bioactivity of microorganisms is directly responsible for the conversion process of arsenic species due the ability of the microorganisms to metabolize this species (Maki et al. 2009). Microorganisms such as phytoplankton (microalgae) and bacteria incorporate and accumulate arsenate because As(V) can replace the nutrients that are required in their growth. This substitution occurs because arsenate has chemical similarities with nutrients such as nitrate and phosphate. Thus, microorganisms reduce arsenate into As(III) and methylated (organic) forms and release them throughout detoxification mechanisms (Sharma and Sohn 2009).
Figure 5 shows the behavior of dissolved inorganic phosphorus and As(V) as a function of chlorophyll-a concentrations.
From Fig. 5a, it is possible to observe that dissolved inorganic phosphorous concentrations were inversely related to chlorophyll-a for the samples of the PEC. This behavior indicates that the removal of phosphorus by phytoplankton occurs in the water column when an increase in algal biomass is observed, as reflected in chlorophyll-a levels. During summer, the samples showed higher chlorophyll-a levels and dissolved oxygen saturation were over 105%, indicating phytoplankton in an active growth phase and high primary productivity. Thus, in condition of low DIP concentrations, arsenate assimilation by microorganisms can occur due to chemical similarities between arsenic and phosphate. In fact, Cullen and Reimer (1989) related that arsenate concentration minima often coincide with photosynthetic maxima evidenced by high concentration of chlorophyll-a. This trend is well evidenced in Fig. 5b where an inverse relationship between arsenate concentrations and chlorophyll-a is shown for all samples. Thus, with intense biological activity and uptake of arsenate, the microorganisms can metabolize arsenate producing and releasing As(III) and organic species. Links between organic arsenic and chlorophyll-a concentrations are shown in Fig. 6.
As Fig. 6 shows, total organic arsenic concentrations were directly related to the algal biomass activity (chlorophyll-a) for all the samples. Therefore, these results indicate that phytoplankton activity in the water column of the PEC may be responsible for the production of methylated arsenic forms, which have lower toxicity when compared to the inorganic species. The highest percentages of organic arsenic were determined in samples with high primary productivity during summer, because of the favorable conditions for the growth of algal biomass with low nutrient concentrations and the consequent uptake of As(V). Therefore, arsenic cycle in the Paranaguá estuary is sensitive to biological activity demonstrating the importance of microorganisms such as microalgae in the arsenic speciation in this environment.
In fact, Andreae (1978) found a positive correlation between the concentrations of arsenite and methylated arsenic and the indicators of primary productivity. Maki et al. (2009) also verified that microbial activity in aquatic environments significantly influence arsenic distribution and behavior.
The relationships observed in Figs. 4, 5, and 6 shows that arsenic speciation in estuaries can be highly variable depending on the biogeochemistry of the environment such as nutrient levels, salinity, temperature, dissolved oxygen, microorganism activity. For example, it is possible to observe that the organic arsenic fraction increases with dissolved oxygen saturation and temperature for Guaraqueçaba and Paranaguá, the most biologically productive environments. These results also confirm that high primary productivity can favor the formation of organic arsenic species.
4 Conclusions
In this work, it was evidenced that the arsenic speciation in surface waters of Paranaguá Estuarine Complex was controlled by biogeochemical factors. Regarding the arsenic behavior in this environment, it was observed that the speciation varied seasonally, being influenced by the differences in the three environments. In general, it was observed a low affinity of arsenic for the particulate matter, which can represent a greater residence time of this element in the water column. Less toxic species like organic arsenic were found in highest concentration in the eutrophic regions (Paranaguá and Guaraqueçaba) and during the summer due to higher biological activity. Thus, it is evident that the presence of phytoplankton was decisive in the arsenic behavior.
Besides these aspects, it was concluded that the samples of PEC presented higher arsenic concentrations when compared to results reported for estuarine waters. This occurrence may be related to geological factors, because the PEC could be naturally rich in arsenic as well as due to anthropogenic activities. However, the specific sources of this element are still to be determined. These results represent a first step in understanding the arsenic behavior in a complex environment such as the Paranaguá Estuarine Complex.
References
Andreae MO (1978) Distribution and speciation of arsenic in natural waters and some marine algae. Deep Sea Res 25:391–402
Andreae MO, Andrae TW (1989) Dissolved arsenic species in the Sheldt estuary and watershed, Belgium. Estuar Coast Shelf Sci 29:421–433
ATSDR (2007) Toxicological profile for arsenic. U. S. Department of Health and Human Services, Public Health Service. Agency for toxic substances and disease registry, Atlanta. http://www.atsdr.cdc.gov. Accessed May 2010
Benoit G, Rozan TF (1999) The influence of size distribution on the particle concentration effect and trace metal partitioning in rivers. Geochim Cosmochim Acta 63:113–127
Carmouze JP (1994) O metanolismo de ecossistemas aquátivos: fundamentos teóricos, métodos de estudo e análises químicas. Edgard Blücher, FAPESP, São Paulo
CERCLA (2007) Priority list of hazardous substances ATSDR. http://www.atsdr.cdc.gov/cercla. Accessed May 2010
Chou CHSJ, de Rosa CT (2003) Case studies—arsenic. Int J Hyg Environ Health 206:381–386
Choueri RB, Cesar A, Torres RJ, Abessa DMS, Morais RD, Pereira CDS, Nascimento MRL, Mozeto AA, Riba I, DelValls TA (2009) Integrated sediment quality assessment in Paranaguá Estuarine System, Southern Brazil. Ecotoxicol Environ Saf 72:1824–1831
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
de Camargo R, Harari J (2003) Modeling the Paranaguá Estuarine Complex, Brazil: tidal circulation and cotidal charts. Braz J Oceanogr 51:23–31
Elbaz-Poulichet F, Garnier JM, Guan DM, Martin JM, Thomas AJ (1996) The conservative behavior of trace metals (Cd, Cu, Ni and Pb) and As in the surface plume of stratified estuaries: example of the Rhône river (France). Estuar Coast Shelf Sci 42:289–310
Fávaro LF, de Oliveira EC, Ventura AOB, Verani NF (2009) Environmental influences on the spatial and temporal distribution of the puffer fish Sphoeroides greeleyi and Sphoeroides testudieneus in a Brazilian subtropical estuary. Neotrop Ichthyol 7:275–282
Ferreira MA, Barros AA (2002) Determination of As(III) and arsenic(V) in natural waters by cathodic stripping voltammetry at a hanging mercury drop electrode. Anal Chim Acta 459:151–159
Gibbon-Walsh K, Salaün P, van den Berg CMG (2010) Arsenic speciation in natural waters by cathodic stripping voltammetry. Anal Chim Acta 662:1–8
Grasshoff K, Ehrhardt M, Kremling E (1983) Methods of seawater analysis, 2a edn. Verlag Chemie, Weinheim
He Y, Zheng Y, Ramnaraine M, Locke DC (2004) Differential pulse cathodic stripping voltammetric speciation of trace inorganic arsenic compounds in natural water samples. Anal Chim Acta 511:55–61
He Y, Zheng Y, Locke DC (2007) Cathodic stripping voltammetric analysis of arsenic species in environmental water samples. Microchem J 85:265–269
Hill MN (1963) The sea: ideas and observations on progress in the study of the seas, vol 2. Interscience Publishers, New York
Howard AG, Apte SC (1989) Seasonal control of arsenic speciation in an estuarine ecosystem. Appl Organomet Chem 3:499–508
Howard AG, Arbab-Zavar MH, Apte SC (1982) Seasonal variability of biological arsenic in the estuary of the river Beaulieu. Mar Chem 11:493–498
Howard AG, Arbab-Zavar MH, Apte SC (1984) The behavior of dissolved arsenic in the estuary of the river Beaulieu. Estuar Coast Shelf Sci 19:493–504
Howard AG, Apte SC, Comber SDW, Morris RJ (1988) Biogeochemical control of the summer distribution and speciation of arsenic in the Tamar estuary. Estuar Coast Shelf Sci 27:427–443
Howard AG, Comber SDW, Kifle D, Antai EE, Purdu DA (1995) Arsenic speciation and seasonal in nutrient availability and micro-plankton abundance in Southampton water, U. K. Estuar Coast Shelf Sci 40:435–450
Karadjova IB, Slaveykova VI, Tsalev DL (2008) The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat Toxicol 87:264–271
Kolm HE, Schoenemberger MF, Piemonte MR, Souza PSA, Schnell G, Mucciato MB, Mazzuco R (2002) Spatial variation of bacteria in surface waters of Paranaguá and Antonina Bays, Paraná, Brazil. Braz Arch Biol Technol 45:22–34
Kumaresan M, Riyazuddin P (2001) Overview of speciation chemistry of arsenic. Curr Sci 80:837–845
Lana PC, Marone E, Lopes RM, Machado EC (2001) The subtropical Estuarine Complex of Paranaguá Bay, Brazil. In: Seeliger U, Kjerfve B (eds) Coastal marine ecosystems of Latin America. Ecological Studies. Springer, Berlin, vol. 144, pp 131–145
Leermakers M, Baeyens W, de Gieter M, Smedts B, Meert C, de Bisschop HC, Morabito R, Quevauviller P (2006) Toxic arsenic compounds in environmental samples: speciation and validation. Trends Anal Chem 25:1–10
Maki T, Hirota W, Ueda K, Hasegawa H, Rahman MA (2009) Seasonal dynamics of biodegradation activities for dimethylarsinic acid (DMA) in Lake Kahokugata. Chemosphere 77:36–42
Mantavanelli A (1999) Caracterização da dinâmica hídrica e do material particulado em suspensão na Baía de Paranaguá em em sua bacia de drenagem. Dissertation, Universidade Federal do Paraná
Martins CC, Braun JAF, Seyffert BH, Machado EC, Fillmann G (2010) Anthropogenic organic matter inputs indicated by sedimentary fecal steroids in a large South American tropical estuary (Paranaguá estuarine system, Brazil). Mar Pollut Bull 60:2137–2143
Masson M, Schäfer J, Blanc G, Dabrin A, Castelle S, Lavaux G (2009) Behavior of arsenic and antimony in the surface freshwater reaches of a highly turbid estuary, the Gironde Estuary, France. Appl Geochem 24:1747–1756
Melamed D (2004) Monitoring arsenic in the environment: a review of science and technologies for field measurements and sensors. U. S. Environmental Protection Agency Washington, U.S.EPA. http://www.epa/gov/tio/download/char/arsenic_paper.pdf. Accessed May 2005
Michel P, Averty B, Chiffoleau JF, Romana LA (2001) Biogeochemical behavior of arsenic species in the Seine Estuary in relation to successive high-amplitude primary production, anoxia, turbidity, and salinity events. Estuaries 24:1066–1073
Millward GE, Kitts HJ, Comber SDW, Ebdon L, Howard AG (1996) Methylated arsenic in the southern North Sea. Estuar Coast Shelf Sci 43:1–18
Millward GE, Kitts HJ, Ebdon L, Allen JI, Morris AW (1997) Arsenic species in the Humber Plume U. K. Cont Shelf Res 17:435–454
Miranda LB, Castro BM, Kjerfvr B (2002) Princípios de Oceanografia Física de Estuários. Editora da USP, São Paulo
Mirlean N, Andrus VE, Baisch P, Grieop G, Casartelli MR (2003) Arsenic pollution in Patos Lagoon estuarine sediments, Brazil. Mar Pollut Bull 46:1480–1484
Prestes EC, dos Anjos VE, Sodré FF, Grassi MT (2006) Copper, lead and cadmium loads and behavior in urban stormwater runoff in Curitiba, Brazil. J Braz Chem Soc 17:53–60
Ren JL, Zhang J, Li DD, Cheng Y, Liu SM (2010) Behavior of dissolved inorganic arsenic in the Yellow Sea and East China Sea. Deep Sea Res Part II 57:1035–1046
Rhine ED, Garcia-Dominguez E, Phelps CD, Young LY (2005) Environmental microbes can speciate and cycle arsenic. Environ Sci Technol 39:9569–9573
Roy P, Saha A (2002) Metabolism and toxicity of arsenic: a human carcinogen. Curr Sci 82:38–45
Sá F, Machado EC, Ângulo RJ, Veiga FA, Brandini N (2006) Arsenic and heavy metals in sediments near Paranaguá Port, Southern Brazil. J Coast Res 39:1066–1068
Sánches-Rodas D, Gómez-Ariza JL, Giráldez I, Velasco A, Morales E (2005) Arsenic speciation in river and estuarine waters from southwest Spain. Sci Total Environ 345:207–217
Sanders JG (1980) Arsenic cycling in marine systems. Mar Environ Res 3:257–266
Santos DM, Araújo IP, Machado EC, Carvalho-Filho MAS, Fernandez MA, Marchi MRR, Godoi AFL (2009) Organotin compounds in the Paranaguá Estuarine Complex, Paraná, Brazil: evaluation of biological effects, surface sediment, and suspended particulate matter. Mar Pollut Bull 58:1922–1052
Seyler P, Martin JM (1990) Distribution of arsenite and total dissolved arsenic in major French estuaries: dependence on biogeochemical process and anthropogenic inputs. Mar Chem 29:277–294
Sharma VK, Sohn M (2009) Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environ Int 35:743–759
Smedley PL, Kinniburg D (2002) A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Sodré FF, Grassi MT (2006) Changes in copper speciation and geochemical fate in freshwaters following sewage discharges water. Water Air Soil Pollut 178:103–112
Sodré FF, dos Anjos VE, Prestes EC, Grassi MT (2005) Identification of copper sources to urban surface waters using the principal component analysis based on aquatic parameters. J Environ Monit 7:581–585
Sperling M, Yin X, Wels B (1991) Differential determination of arsenic (III) and total arsenic using flow injection on-line separation and pre-concentration for graphite furnace atomic absorption spectrometry. Spectrochim Acta, Part B 46:1789–1801
Strickland JLH, Parsons TRA (1972) Practical handbook of seawater analysis. Bulletin of the Fisheries Research Board of Canadá, p 167
U.S.EPA (1996) Method 1669—sampling ambient water for determination of trace metals at EPA water quality criteria level. Environmental Protection Agency, Washington, DC
Yao QZ, Zhang J, Qin XG, Xiong H, Dong LX (2006) The behavior of selenium and arsenic in the Zhujiang (Pearl River) Estuary, South China Sea. Estuar Coast Shelf Sci 67:170–189
Zhang J, Li SM, Ren JL, Wu L, Zhang GL (2007) Nutrient gradients from the eutrophic Changjiany (Yangtze River) Estuary to the oligotrophic Kuroshio waters and re-evaluation of budgets for the East China Sea Shelf. Prog Oceanogr 74:449–478
Acknowledgments
The authors would like to thank National Council of Technological and Scientific Development—CNPq for financial support. This research was funded by Edital Universal, Proc. 474713/2003-2 and CT-INFRA/Finep. VEA also thanks Coordenation for the Improvement of the Higher Level Personnel—CAPES and CNPq for the fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
dos Anjos, V.E., da C. Machado, E. & Grassi, M.T. Biogeochemical Behavior of Arsenic Species at Paranaguá Estuarine Complex, Southern Brazil. Aquat Geochem 18, 407–420 (2012). https://doi.org/10.1007/s10498-012-9161-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-012-9161-8