Abstract
Distribution of arsenic in the marine environment is associated with its biogeochemical behavior. Indeed, very few studies have been conducted along the seasonal cycle to show its non-conservative behavior in the tropical estuary. The Mahanadi River, one of the major tropical rivers in the peninsular India, drains densely populated and industrialized region of Paradeep port. Over this 1-year study, the variations of inorganic arsenic were examined along the salinity gradient of the Mahanadi estuary, with the aim to provide some insights into the mechanisms that control arsenic concentrations and behavior under estuarine mixing. Arsenic in the estuary was derived from both natural and anthropogenic sources, and it displayed partial removal from the water in the mixing zone. Results of geo-accumulation index indicated that sediments were uncontaminated and they acted as a sink for arsenic. The diffusive fluxes from water to sediment were estimated to be 9.05 μg m−2 day−1 at Chaumohona, 9.83 μg m−2 day−1 at Kaudia, and 11.85 μg m−2 day−1 at Neherubunglow. The findings of the study suggest that both the removal of arsenic by biogeochemical processes and its diffusive transport from water to sediment are of major importance for both the non-conservative behavior of arsenic in the estuary and its export to the coastal water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing concern for arsenic is associated with its potential toxicity and wide contamination in coastal environments (Geiszinger and Goessler, 2002; Smedley and Kinniburgh 2002). Levels of dissolved arsenic (As) in most of the major world rivers range between 1 and 3 ppb (parts per billion). Relatively high As concentrations (72–1400 ppb) could occur (i.e., the Loa and Tocona in Chile, the Waikato in New Zealand and the Madisone in the USA) due to contamination from thermal springs source (Ravenscroft et al. 2009). Elevated arsenic concentration commonly observed in coastal water is strongly influenced by the riverine input, atmospheric deposition, selective uptake by algae, degradability of organisms, adsorption and suspended particles and human activities, and poses increasing concern of potential ecological and health impact (Erry et al. 1999).
Unlike ground water, estuaries do not have the extremely high level of dissolved As for mixing of river water with less concentrated sea waters in addition to its exchange from water to sediment (Smedley and Kinniburgh, 2002). The As could show non-conservative behavior (departures from simple mixing) in estuaries due to processes such as diffusion, co-precipitation, or anthropogenic inputs. These non-conservative behaviors could be related to some possible transfers from dissolved to particulate phases (Andreae and Andreae 1989). Anthropogenic nutrient could enhance Phytoplankton production which could also induce depletion of arsenic in estuarine waters (Featherstone and Butler, 2004). Indeed, very few studies have been conducted on non-conservative behavior of arsenic in the tropical estuary apart those by Mandal et al. 2009 and by Chakraborty et al. 2012.
The Mahanadi River is one of the fourteen major tropical rivers in the Indian peninsula. Paradeep port is located close to the Mahanadi estuary and, along its course, the lower reaches of the estuary receive effluents from several industries, such as Paradeep Phosphates Ltd. (up to 3264 m3/day), Oswal Fertiliser Ltd., Paradeep (4085 m3/day), and the East Coast Breweries and Distilleries Ltd., Paradeep (376 m3/day) (Sundaray et al. 2006). Phosphorus-rich conditions in the water column due to desorption from high sediment loads (154–661 mg L−1) during monsoons are a unique feature of the estuarine geochemistry (Bhattathiri 2001).
The present work provides the measurement of dissolved and sediment As concentration in the Mahanadi, estuary during three contrasting seasons, namely the monsoon, post-monsoon, and pre-monsoon. The data form the basis of both an examination of the geochemical behavior of the metalloid along the salinity gradient and estimates of its flux from water to sediment.
Study site and period
The Mahanadi River ( ~857 km length, ~ 141,600 km2 watershed surface area) is a major Indian estuary in terms of size and material transport from continent to the Bay of Bengal. The tide in the estuary is predominantly semidiurnal. The vertical tide range at the mouth varies from 1.45 to 2.20 m. Climate in the region consists of three seasons; namely the southwest monsoon (June–September), the post-monsoon (October–January), and the pre-monsoon (February–May); 70–80 % of annual rainfall occurs during the southwest monsoon, resulting in high river discharge, which then gradually diminishes during the non-monsoonal months.
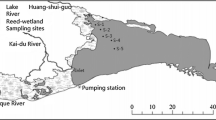
A global positioning system was used to determine the location of monitoring sites [Stn.1, (Neherubunglow) (20° 17′ 38.1″ N; 86° 42′ 30.3″ E), Stn.2, (Kaudia) (20° 18′ 29.5″ N; 86° 41′ 4.4″ E) and Stn.3, (Chaumohona) (20° 20′ 22.9″ N; 86° 36′ 50.8″ E)] along the estuary (Fig. 1). The three stations were selected with the aim of covering the whole freshwater-seawater mixing zone, and their locations were situated between the haline front (Stn.3) and the marine water (Stn.1). Stn.2 was situated about 20 km away from Paradeep Harbor. The water depth ranged 3.5–14.7 m at Neherubunglow, 2.5–9.7 m at Kaudia, and 1.9–10.7 m at Chaumohona.
Sample collection
The sampling expedition was conducted over the year 2010 following seasonal cycle. Each month, at spring, flood tide sampling of surface water was performed with Niskin bottles. The samples were filtered through pre-weighed 0.45 μm Millipore filters. Aliquots for As measurements were transferred into acid-cleaned polypropylene bottles, stabilized by the addition of H2SO4 to yield a 0.1 % v:v and stored in ice box pending analysis. The filters containing suspended particulate matter (SPM) were dried to content weight (75 °C, 48 h).
Sediment core of 30 cm long was collected using a tailor-made Teflon sampler during low tide and was extruded in a glove-box bag in the presence of nitrogen in order to prevent possible aerial oxidation. Thick sediment slices (1 cm each) were freeze dried, sieved through a 63-μm-mesh screen and stored in the dark at 0 °C pending analysis. A Secchi disc was used to measure the transparency of the water column.
Methodology
Air temperature was determined in situ using a thermometer. Chlorinity was estimated by argentometric titration and was converted to salinity (S) using the relation S = 1.80655 Cl (Strickland and Parsons 1968). The determination of As was achieved by means of atomic absorption spectrometry as outlined by Loring and Rantala (1992) and by Yamamoto et al. (1985). Analyses were carried out using a Varian Hydride System-Vapor Generator (Serial No. EL0405–314) connected to a SpectrAA 55B true double beam atomic absorption spectrometer. Arsenic measurements were performed on fine, ground, and homogenized samples of sediment. Sediment aliquots were acid digested (HF, HNO3, HCl) using screw-capped Teflon reactors and digests were evaporated to dryness. The residue was redissolved by adding 20 % HNO3 and heated again for 3 h at 100 °C. The aliquots were then diluted in 20 mL of ultrapure water. Dissolved inorganic arsenic concentration in water was measured after reducing As (V) into As (III). As (III) was directly reacted with KBH4 to form arsine by acidifying sample with 2 mol L−1 HCl and was subsequently stripped out of solution using Ar gas. The accuracy of measurement was checked using reference standard (MERCK K GaA, Germany). Relative accuracy and the coefficient of variation were 96.2 and 9.2 %, respectively, for As. The determination of grain size in sediment was achieved by means of sedimentation method as outlined by Sims and Heckendorn 1991.
In order to quantify the addition or removal of As by biogeochemical processes, we used the following equation (Carpenter et al. 1975):
where Asr, Asest, and Assw are the As concentration in river, estuarine, and sea waters, respectively. Clsw and Clest are the chlorinity of sea and estuarine waters, respectively. If the difference is positive/negative, it indicates that the biogeochemical processes are active for transformation and transport of As in the estuary.
Calculation of water sediment As fluxes (F, μg m−2 day−1) is based on Fick’s first law of diffusion modified for sediments (Berner 1980): F = −φ D s (dC/dZ)z = 0, where φ is the porosity of the sediment (0.5). D s is the whole sediment diffusion coefficient (cm2 s−1) and (dC/dZ)z=0 is the concentration gradient at zero depth. The whole sediment diffusion coefficient (D s ) was calculated using the relation (Iversen and Jorgensen 1993): D s = D 0/1 + n (1 − φ ), where D 0 is the free solution diffusion coefficient and n = 3 (Knab et al. 2009). The positive value of F indicates the downward flux of As.
The index of geo-accumulation (Igeo) was computed using the equation (Loska et al. 2004): Igeo = log2 (AsO/1.5 × AsEC), where AsO is the measured concentration and AsEC is the geochemical background value (average upper continental crust (Henderson and Henderson, 2009a, Henderson and Henderson, 2009b).
Results and discussion
Physico-chemical parameters
During the study period, average air temperature was 30.0 ± 5.86 °C. Secchi disk transparency of the water column increased gradually from 23 cm during the monsoon period to 99 cm in the post-monsoon period and then to 133 cm during the pre-monsoon period. Variations of SPM amounts indicated fluctuations with an average concentration of 88.13 ± 19.1, 509.0 ± 260.0, and 98.13 ± 9.19 mg L−1 in the pre-monsoon, monsoon and post-monsoon, respectively. The salinity gradient ranged from 16.58 psu at Stn.3 to 26.61 psu at Stn.1 in the pre-monsoon. With the onset of the monsoon, the surface salinity gradient declined from its pre-monsoon level to 0.18 psu at Stn.3 and 4.13 psu at Stn.1. Sediment texture pattern was found to be sand dominated with 97.6 % sand followed by 1.74 % silt and 0.64 % clay.
As distribution in water and sediment
In this study, the occurrence level of As in the sediment ( 2.1 mg kg−1, Table 1) was similar to the value determined in the River Ganges (1.2–2.6 mg kg−1), Brahmaputra River (1.4–5.9 mg kg−1), and Meghna River (1.3–5.6 mg kg−1) (Datta and Subramanian 1997) and lower than that determined in the marine sediment (7.7 mg kg−1) (Bowen 1979), Godavari estuary (76.4–347.4 mg kg−1, Chakraborty et al. 2012), and the average upper continental crust (4.79 mg kg−1) (Henderson and Henderson, 2009a, Henderson and Henderson, 2009b). Considerably lower concentration of As (4 mg kg−1) was recently reported for mangrove-dominated estuary, Sundarban, India (Fattorini et al. 2013). Observed Igeo value <0 indicated that the sediment was not contaminated with As (Muller 1981). Vertical distribution of As (Fig. 2) exhibited strong enrichment in the top sediment (2.65–3.06 mg kg−1). Arsenic concentration gradient (dC/dZ) at all stations were negative; namely −30.33 (Neherubunglow), −25.18 (Kaudia), and −23.14 (Chaumohona), suggesting its diffusive transport from water to sediment (Luoma and Raiwbon 2008). From As concentration gradient, water-sediment diffusive flux, F were calculated at 11.85, 9.83, and 9.05 μg m−2 day−1 (Table 1).
Concentration of dissolved As obtained in the present study was 8.0 ± 3.7 μg L−1, which was lower than 10 μg L−1, the World Health Organization (WHO), and US-EPA drinking water limit for dissolved arsenic (U.S. EPA 2001; World Health Organization 1993). The occurrence level of our investigation was close to the maximum range determined in the Tejo Estuary (6.65 μg L−1) (Andreae et al., 1983) and Gironde estuary (5.3 μg L−1) (Deycard et al. 2014) and higher than that determined in the open ocean (0.5–2.0 μg L−1) (Francesconi and Kuchnelt 2002) and the Garonne (2.14 μg L−1), Dordogne (1.05 μg L−1), and Isle (2.82 μg L−1) Rivers, France (Masson et al. 2006). Relatively high concentrations were attributed to various anthropogenic emissions within the extensively urbanized and industrial area.
One-way ANOVA analyses indicated that As concentrations in tidal water differed significantly among seasons (P = 0.001) and stations (P = 0.001). The As concentrations in tidal water was (Table 1) maximum with mean value recorded at 17.2 ± 1.7 μg L−1 during the monsoon at Choumohona and minimum with mean value of 5.88 ± 3.6 μg L−1 at Neherubunglow in the pre-monsoon. Riverine inputs of As could be further contaminated by industrial effluents or sewage. Dilution of coastal water could result in decreases of As concentrations, with increasing water discharge in the monsoon season relative to the pre-monsoon. Fig. 3 depicted decrease of dissolved As concentration along the salinity gradient of the estuary.
Addition/removal of As
Deviation from linear decrease in dissolved As (μg L−1) concentrations with respect to salinity, S (As = 12.92–0.24 S, R 2 = 0.298, n = 42, p < 0.01) suggested its non-conservative behavior and decrease of dissolved As along the salinity gradient was not due to simple mixing of river and sea water in the Mahanadi estuary (Fig. 3). The value of R 2 showed that 29.8 % of the variations in As could be influenced by salinity variation; however, the remaining 70.2 % could be attributed to other factors. At 1 % probability level, the observed value of “t” (4.1) was higher than the critical value (2.576) obtained from the “t” distribution table, suggesting that there was less than 1 % chances of error in drawing this conclusion. As reported for many estuaries (Andreae and Andreae 1989; Vandenhecke et al. 2010), removal of As (0.5–35.3 %) by biotic depletion and abiotic scavenging could be the most probable cause of its non-conservative behavior in the Mahanadi estuary. This abiotic scavenging could occur via adsorption onto sinking suspended particulate matter (Libes 1992; Kits et al. 1994) or by incorporation into biogenic material (Michel et al. 1997). Both suspended particulate matter and As concentration showed maximum level during monsoon in the Mahanadi estuary. This suggests that degradation of organic matter and successive remobilization processes in the low salinity zone within the turbidity maximum could also increase the level of As similar to French estuaries (3 μg L−1) reported in Seyler and Martin 1990.
Conclusion
Concentration of dissolved As in the Mahanadi River was close to or greater than concentration reported for other uncontaminated rivers. From a seasonal perspective, the monsoon period was markedly different to that of other times of the year in terms of As concentration with maximum anthropogenic input. Concentration of As in sediments declined downstream, and it exhibited diffusive transport from water to sediment. Non-conservative behavior of As was found to be associated with its removal via adsorption onto sinking suspended particulate matter or by incorporation into biogenic material in the mixing zone.
References
Andreae, M. O., Byrd, J. T., & Froelich, P. N. (1983). Arsenic, antimony, germanium and tin in the Tejo Estuary, Portugal: modeling a polluted estuary. Environmental Science & Technology, 17(12), 731–737.
Andreae, M. O., & Andreae, T. W. (1989). Dissolved arsenic species in the Schelde estuary and watershed, Belgium. Estuarine, Coastal and Shelf Science, 29, 421–433.
Berner, R. A. (1980). Early diagenesis: a theoretical approach. Princeton: Princeton University Press.
Bhattathiri, P. M. A. (2001). Features at some significant estuaries of India. In R. Sengupta & E. Desa (Eds.), The Indian Ocean—a perspective, Vol 1 (pp. 271–297). New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd.
Bowen, H. J. M. (1979). Environmental chemistry of the elements, Academic Press.
Carpenter, J. H., Bradford, N. L., & Grant, V. (1975). Processes affecting the estuarine waters. In L. E. Cronin (Ed.), Estuarine research (Vol. 1, pp. 188–214). New York: Academic Press.
Chakraborty, P., Jayachandran, S., Rayhunadh Babu, P. V., Karri, S., Tyadi, D., Marcellin, Y. K., et al. (2012). Intra-seasonal variations of arsenic totals and species in tropical estuary surface sediments. Chemical Geology, 322–323,172–180.
Datta, D. K., & Subramanian, V. (1997). Texture and mineralogy of sediments from the Ganges-Brahmaputa Meghna river system in the Bengal basin, Bangladesh and their environmental implications. Environmental Geology, 30, 181–188.
Deycard, V. N., Schafer, J., Blanc, G., Coynel, A., Petit, J. C. J., Lanceleur, L., et al. (2014). Contribution and potential impacts of seven priority substances (As, Cd, Cu, Cr, Ni, Pb and Zn) to a major European Estuary (Gironde Estuary, France) from urban waste water. Marine Chemistry, 167, 123–134.
Erry, B. V., Macuair, M. R., Meharg, A. A., Shore, R. F., & Newton, L. (1999). Arsenic residues in predatory birds from an area of Britain with naturally and anthropogenically elevated arsenic levels. Environmental Pollution, 106, 91–95.
Featherstone, A., Butler, E., & O’Grady, B., (2004). Seasonal cycling of arsenic species in a stratified, microtidal estuary (Huon River, Tasmania). Estuaries 27, 18–35.
Fattorini, D., Sarkar, S. K., Regoli, F., Bhattacharya, B. P., Rakshit, D., Satpathy, K. K., et al. (2013). Levels and chemical speciation of As in representative biota and sediments of a tropical mangrove wetland, India. Environmental Science: Processes and Impacts, 15(4), 773–782.
Francesconi, K.A., & Kuchnelt, D. (2002). Arsenic compounds in the environment. In: Frank, W.T., Jr. (Ed.), Environmental Chemistry of Arsenic. (pp. 51–94) Marcel Dekker, Inc.
Geiszinger A.E., Goessler, W., & Francesconi, K.A., (2002). The marine polychaete Arenicola marina. Its unusual arsenic compound pattern and its uptake of arsenate from seawater. Marine Environmental Research, 53, 37–50.
Henderson, P., & Henderson, G. M. (2009a). Earth Science Data. Cambridge University Press.
Iversen, N., & Jorgensen, B. B. (1993). Diffusion coefficients of sulphate and methane in marine sediments: influence of porosity. Geochimica et Cosmochimica Acta, 57, 571–578.
Kits, H. J., Millward, G. E., Morris, A. W., & Ebdon, I. (1994). Arsenic biogeochemistry in the Humber Estuary, UK. Estuarine, Coastal and Shelf Science, 38(2), 157–172.
Knab, N. J., Cragg, B. A., Hornibrook, E. R. C., Holmkvist, L., Pancost, R. D., Borowski, C., et al. (2009). Regulation of anaerobic methane oxidation in sediments of the Black Sea. Biogeosciences, 5, 1505–1518.
Libes, M. S. (1992). An introduction to marine biogeochemistry. New York: John Wiley & Sons, Inc.
Loring, D. H., & Rantala, R. T. T. (1992). Manual for the geochemical analysis of marine sediment and suspended particulate matter. Earth Science Reviews, 32, 235–283.
Loska, K., Wiechula, D., & Korous, I. (2004). Metal concentration of farming soil affected by industry. Environment International, 30, 159–165.
Luoma, S. N., & Rainbow, P. S. (2008). Metal contamination in aquatic environments: science and lateral management. Cambridge: Cambridge University.
Mandal, S. K., Dey, M., Ganguly, D., Sen, S., & Jana, T. K. (2009). Biogeochemical controls of arsenic occurrence and mobility in the Indian Sundarban mangrove ecosystem. Marine Pollution Bulletin, 58, 652–657.
Masson, M., Schafer, J., Blanc, G., & Pierre, A. (2006). Seasonal variations and annual fluxes of arsenic in the Garonne, Dordogne and Isle Rivers, France. Science of the Total Environment, 21, 325–333.
Michel, P., Boutier, B., Herbland, H., Averty, B., Artigas, L. F., Anger, D., et al. (1997). Behaviour of arsenic on the continental shelf of the Gironde estuary: role of phytoplankton in vertical fluxes during spring bloom conditions. Oceanologica Acta, 21(2), 325–333.
Muller, G. (1981). Die Schwermetallbelastung der sedimenten des Neckars and Sciner. Nebenfluss Chemiker-Zeitung, 6, 157–164.
Ravenscroft, P., Brammer, H., & Richards, K. (2009). Arsenic Pollution: A Global Synthesis, Wiley-Blackwell (ISBN: 978–1–405-18602-5).
Seyler, P., & Martin, J. M. (1990). Distribution of arsenite and total dissolved arsenic in major French estuaries: dependence on biogeochemical processes and anthropogenic inputs. Marine Chemistry, 29, 277–294.
Sims, J. T., & Heckendorn, S. E. (1991). Methods of analysis of the University of Delaware soil testing laboratory. Cooperative bulletin no. 10. Newark: University of Delaware.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Strickland, J. D. H., & Parsons, T. R. (1968). A practical hand book of seawater analysis. Fisheries Research Board of Canada 167.
Sundaray, S. K., Panda, C., Nayak, B. B., & Bhatta, D. (2006). Multivariate statistical techniques for the evolution of spatial and temporal variations in water quality of the Mahanadi River-estuarine system (India)—a case study. Environmental Geochemistry and Health, 28, 317–330.
Henderson, P., & Henderson, G. M. (2009b). Earth Science Data. Cambridge University Press.
U.S. EPA (2001). National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring; final rule. Federal Register, 66(14), 69–76.
Vandenhecke, J., Waeles, M., Cabon, J.-Y., Garnier, C., & Riso, R. D. (2010). Inorganic arsenic speciation in the waters of the Penzé estuary (NW France): seasonal variations and fluxes to the coastal area. Estuarine, Coastal and Shelf Science, 90, 221–230.
World Health Organization. (1993). Guidelines for drinking-water quality. In: Recommendations. Vol. 1. Geneva: WHO
Yamamoto, M., Yasuda, M., & Yamamoto, Y. (1985). Hydride generation atomic absorption spectroscopy coupled with flow injection analysis. Analytical Chemistry, 57, 1375–1382.
Acknowledgment
The authors are thankful to Paradeep Port Trust authorities for the kind permission to conduct this study. Thanks are also due to Dr. Biswapriya Misra, Department of Biology, Genetics Institute, University of Florida, Gainesville, FL 32610, USA, for his kind and generous help to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandal, S.K., Majumder, N., Chowdhury, C. et al. Arsenic distribution in waters and its geochemical behavior in sediment of Mahanadi estuary in India. Environ Monit Assess 188, 461 (2016). https://doi.org/10.1007/s10661-016-5471-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5471-4