Abstract
We investigated the dissolved major elements, \( {}^{87}{\text{Sr/}}{}^{86}{\text{Sr}},\;\delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } ,\;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) composition of the Min Jiang, a headwater tributary of the Chang Jiang (Yangtze River). A forward calculation method was applied to quantify the relative contribution to the dissolved load from rain, evaporite, carbonate, and silicate reservoirs. Input from carbonate weathering dominated the major element composition (58–93%) and that from silicate weathering ranged from 2 to 18% in unperturbed Min Jiang watersheds. Most samples were supersaturated with respect to calcite, and the CO2 partial pressures were similar to or up to ∼5 times higher than atmospheric levels. The Sr concentrations in our samples were low (1.3–2.5 μM) with isotopic composition ranging from 0.7108 to 0.7127, suggesting some contribution from felsic silicates. The Si/(Na* + K) ratios ranged from 0.5 to 2.5, which indicate low to moderate silicate weathering intensity. The \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) for five select samples showed that the source of dissolved sulfate was combustion of locally consumed coal. The silicate weathering rates were 23–181 × 103 mol/km2/year, and the CO2 consumption rates were 31–246 × 103 mol/km2/year, which are moderate on a global basis. Upon testing various climatic and geomorphic factors for correlation with the CO2 consumption rate, the best correlation coefficients found were with water temperature (r 2 = 0.284, p = 0.009), water discharge (r 2 = 0.253, p = 0.014), and relief (r 2 = 0.230, p = 0.019).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Weathering is a key process that affects the Earth’s surface. It influences the geomorphic evolution of the Earth, formation of soils, and the ionic composition of rivers. Among chemical weathering reactions involving various mineral groups, that of silicates is of particular interest. The incongruent dissolution of silicate minerals is in most cases facilitated by hydrogen ions ultimately originating from hydration of atmospheric CO2. In this manner, silicate weathering acts as a major sink of atmospheric CO2 on geologic time scales.
Raymo and Ruddiman (1992) suggested that the Cenozoic cooling of the global climate was initiated by unusual continental uplift, via enhanced silicate weathering and CO2 consumption, and by change in the atmospheric and oceanic circulation patterns. Their hypothesis has initiated plentiful debates and research in various fields ranging from general circulation models and uplift histories to geochemical search for supporting evidence. The most significant uplift during the past ∼50 Ma was of the Himalayas and the Tibetan Plateau, the latter with average height of 4,500 m and areal extent of 1,000,000 km2. Therefore, this unique terrain has been a focus of extensive study regarding the weathering process in uplifted regions (Galy and France-Lanord 1999; Karim and Veizer 2000; Dalai et al. 2002; Wu et al. 2005).
The Min Jiang originates from the Min Shan, a mountain located in the eastern margin of the Tibetan Plateau (Fig. 1a, b). It flows southward to merge with the Dadu He and Qingyi Jiang at Leshan and finally joins the Chang Jiang (Yangtze River) at Yibin. Chen et al. (2002) and Qin et al. (2006) have investigated the fluvial geochemistry of the Min Jiang. However, in Chen et al. (2002)’s study, detailed estimate of the weathering sources was unattainable due to the incomplete major elements data set, whereas Qin et al. (2006) focused on the temporal variations rather than partitioning the different weathering sources.
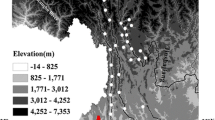
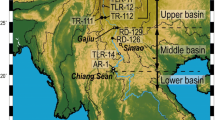
(a) Elevation map of the study area. (b) Sample location map of the Min Jiang. Circles: samples from this study (CJ300 series), Triangles: samples from Qin et al. (2006) (CJ0200 series), Stars: cities
Based upon published (Qin et al. 2006) and newly acquired data, we quantified the relative contribution of the solute sources (silicate, carbonate, evaporite, rain) using a forward calculation method. New isotopic data (\( {}^{87}{\text{Sr/}}{}^{86}{\text{Sr}},\;\delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } ,\;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \)) for select samples were useful in the source discrimination process. We then calculated the silicate weathering rates, CO2 consumption rates, and the total dissolved solid (TDS) fluxes and tested for potential relationships between CO2 consumption rates and basin-specific climatic and geomorphic parameters (temperature, precipitation, relief, etc.), the latter obtained using the geographic information system (GIS).
2 Study Area
2.1 Topography and Climate
The Min Jiang is a 793-km-long major tributary of the upper Chang Jiang and has a total drainage area of 133,500 km2. Its headwaters rise from mountains with elevation ∼5,500 m, and its tributary, the Dadu He, originates from the Tibetan Plateau at ∼4,900 m (Fig. 1a). The river flows south through the 1∼3 km deep valleys of the Min Shan, Qionglai Shan, and Daxue Shan (Fig. 1b). A significant elevation change is observed when it meets the Sichuan Basin, an alluvial plain with an average altitude of ∼500 m (Burchfiel et al. 1995) (Fig. 1a).
The climate is subtropical except for the upper Dadu He region. The average winter temperatures are 5.5°C for Chengdu and 0.5°C for Maerkang (Fig. 1a) (Map Publishing House of China 1998; Chinese Academy of Sciences Comprehensive Research Team 1985). The average summer temperatures are 26°C for Chengdu and 15–17°C for Maerkang. The mean annual precipitation is 500–800 mm on the eastern Tibetan Plateau and 1,200–1,500 mm in the Sichuan Basin. Half of the annual rainfall is focused between June and September (Bureau of Geology and Mineral Resources of Sichuan Province 1991).
2.2 Geology
The mountainous middle to upper drainage basin is divided into two geologic terrains, the Songpan-Garze fold belt and the Longmenshan fold belt (Fig. 1a). The Songpan-Garze fold belt is almost exclusively Triassic marine flysch deposits 5–15 km thick (Xiao et al. 2007). The lithology is sandstone, shale, and slate with sparse carbonate and volcanic rocks. Minor outcrops of sandstone and mudstone interbedded with coal seams are also present. Constituting the Longmenshan fold belt is a series of nappe of Paleozoic and Precambrian rocks. It is composed of meta-igneous and meta-sedimentary rocks such as slate and phyllite with intercalated marble, or carbonate with clastic rocks, or coal-bearing sandstone and mudstone (Commission on the Annals of Sichuan Province 1998). The Sichuan Basin is an undeformed terrain which belongs to the Yangtze Platform (Burchfiel et al. 1995). The area southwest of Leshan consists of carbonate intercalated with halite or gypsum, sandstone, slate, granite, and the Emeishan basalt. The rest of the Sichuan Basin belonging to the study area is covered with fluvial deposits, reddish sandstone and mudstone (Bureau of Geology and Mineral Resources of Sichuan Province 1991; Pan et al. 1997).
3 Sampling and Analytical Methods
Samples were collected during an expedition in August 2005. Water temperature and pH were measured at the time of sampling. Aliquots for major element analyses were filtered through 0.45-μm cellulose nitrate filters and those for Sr through 0.4-μm polycarbonate filters in the field within 24 h of collection. Samples for Si and Sr were acidified with ultrapure HNO3 and stored in pre-cleaned HDPE bottles. For \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) analyses, a stable S carrier (0.02 g Na2SO4) was added to ∼20 l of filtered water and passed through an anion exchange column (Amberlite IRA 400, 15–50 mesh) using a peristaltic pump (Hong and Kim 2005). Major cations were analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES), alkalinity by Gran titration, and Cl, NO3, and SO4 by ion chromatography (IC) at Seoul National University. Si was analyzed by colorimetry using the silicon-molybdenum blue complex method. Sr concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS) and Sr isotope ratios by thermal ionization mass spectrometry (TIMS) at the Korean Basic Science Institute (KBSI). Sulfur isotopes of dissolved sulfate (\( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \)) were measured by stable isotope ratio mass spectrometry (SIRMS) at KBSI, and oxygen isotopes (\( \delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \)) were analyzed by continuous flow isotope ratio mass spectrometry (CF-IRMS) at University of Calgary. The S and O isotope ratios are normalized with respect to CDT and V-SMOW standards, respectively.
4 Results and Discussion
Where we make comparisons, data from this work are referred to as the “CJ300 series” and that of Qin et al. (2006) as the Station samples or the “CJ0200 series”. The Station samples were sampled monthly at four locations in 2001, the CJ0200 series in May of 2002 during the initial rising stage of the river, and the CJ300 series in August of 2005 during the second rising stage (see Fig. 2 of Qin et al. 2006). The dates in Tables 1 and 2 of Qin et al. (2006) should be 2002 and 2001, respectively. Spatially, the Station samples were at four locations on the Min Jiang, Qingyi Jiang, and Dadu He, the CJ0200 series was mostly in the headwaters, while the CJ300 series was in the lower reaches (Fig. 1b). There were a few spatially matched samples: four CJ300–CJ0200 pairs that had a slight seasonal difference (CJ302 and CJ209; CJ306 and 0203; CJ307 and 0202; CJ308 and 0201), and four CJ300-Station pairs that were sampled in the same months though in different years (CJ301 and YA0108; CJ302 and LD0108; CJ304 and GC0108; CJ308 and XK0108) (Table 1) (Fig. 1b).
4.1 Major Elements, \( {}^{87}{\text{Sr/}}{}^{86}{\text{Sr}},\;\delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } ,\;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \)
In the CJ300 series, the water temperatures ranged from 14.5°C in the upper Min Jiang to 22.6°C in the Sichuan Basin, and the pH values were between 7.8 and 8.3 (Table 1). Total dissolved cations (TZ+ = Na+ + K+ + 2Mg2+ + 2Ca2+) varied from 1,578 to 2,813 μEq (10−6 charge equivalent units per liter), moderately higher than the world average of ∼1,250 μEq (Meybeck 1979). Normalized inorganic charge balance (NICB = (TZ+ − TZ−)/TZ+ × 100%, where TZ− = Cl− + 2SO4 2− + HCO3 − in μEq) was less than ±5%, which ensured the quality of the analyses (Table 1). Total dissolved solid (TDS = Na + K + Mg + Ca + Cl + SO4 + CO3 + SiO2) was from 122 to 215 mg/l, comparable to the Ganges and Indus draining the Himalayas (Galy and France-Lanord 1999; Dalai et al. 2002; Karim and Veizer 2000), but lower than the upper Huang He where most samples were in the range of 200 to 400 mg/l (Wu et al. 2005). On a global scale, the TDS of the Min Jiang is higher than the other rivers draining orogenic zones or shield terrains. The rivers draining orogenic zones are the Amazon (average 41 mg/l) and Orinoco (average 82 mg/l) draining the Andes, Mackenzie (average 160 mg/l) draining the Rockies, and East Siberian rivers (average 70 mg/l) draining the Verkhoyansk and Cherskiy ranges (Moon et al. 2007 and references therein). Rivers draining shield terrains are the Amazon (average 11 mg/l) draining the Brazilian Shield, Orinoco (average 9 mg/l) draining the Guayana Shield, St. Lawrence (average 32 mg/l) draining the Canadian Shield, and the Lena (average 90 mg/l) draining the Aldan Shield (Moon et al. 2007 and references therein).
On ternary diagrams, the samples plotted in a narrow range indicative of carbonate weathering (Fig. 2a). This Ca-HCO3 dominance is a general feature of the rivers draining the eastern Tibetan Plateau (Wu et al. 2005; Qin et al. 2006; Moon et al. 2007). Even so, the Min Jiang had especially low Na + K and Si proportions (Fig. 2a).
Sample CJ304 is the furthest downstream sample which represents the Min Jiang system. Sample CJ305 is the Jinsha Jiang, another name for the upper Chang Jiang above confluence with the Min Jiang (Fig. 1b). It is not part of the Min Jiang system, but we used it as a reference sample for the Chang Jiang before it joins the Min Jiang. The main difference between the two samples was the elevated Na and Cl concentrations in CJ305 (Fig. 2a). CJ305 also had the highest TZ+ and TDS of the CJ300 series samples. Jinsha Jiang carries signatures of evaporite weathering from its arid headwaters in the Tibetan Plateau interior.
The ternary diagrams normalize for difference in water discharge and are useful for comparing the six sets of matched samples (Fig. 2b). The CJ300 series samples are slightly more (Na + K)-rich, but in terms of anions a consistent trend is difficult to discern. The differences can be taken as natural inter-annual variation in chemical composition.
The samples from the lower elevations (CJ 301, 303, 304) showed higher nitrate and phosphate concentrations (27–41 μM NO3 and >0.10 μM PO4) compared to those from the upper reaches (6–22 μM NO3 and <0.10 μM PO4). We suspect an anthropogenic input in the vicinity of the heavily populated Sichuan Basin. The high nitrate/phosphate molar ratios (CJ301 = 261, CJ303 = 114, CJ304 = 29) are characteristic of the agricultural pollution (Berner and Berner 1996).
4.1.1 Carbonate Weathering
The calcite saturation indices (CSI) and the CO2 partial pressures were calculated using the Geochemist’s Workbench® v.4.0 (Bethke 2002). The riverine pCO2 is expressed with respect to the atmospheric pCO2 corrected for elevation (Andrews et al. 1996).
where P z is the atmospheric pCO2 at altitude Z (km), P o is the atmospheric pCO2 at sea level (370 μatm), and H is the scale height (∼8.4 km).
Sample CJ307, a headwater tributary of the Min Jiang, is the only sample undersaturated with respect to calcite, and the rest are supersaturated as is usual for natural waters in carbonate terrains (Huh et al. 1998a; Dalai et al. 2002) (Fig. 3).
For pCO2, the CJ300 series samples are up to ∼5 times higher than atmospheric levels, whereas many of the CJ0200 series samples are close to atmospheric levels (Fig. 3). Partial pressures of CO2 in the range of 1–10 times atmospheric levels are quite common for swift flowing rivers (Stallard and Edmond 1987; Huh et al. 1998a, 1998b; Huh and Edmond 1999). In their study of the Amazon, Stallard and Edmond (1987) observed low CO2 saturation levels (<10 times atmospheric) in high productivity lakes and swift Andean rivers and higher levels (10–100 times atmospheric) in high respiration lakes and main channel Amazon or flat land tributaries. In the case of the Min Jiang too, the subtle differences in the saturation of pCO2 could be explained by two factors: (1) supply of external DOC and POC of terrestrial origin, which are oxidized within rivers to produce CO2 (Richey 2004); and (2) water turbulence and flow velocity facilitating outgassing from supersaturated river waters (Wanninkhof et al. 1990). Internal processes like production or respiration of plankton and aquatic plants can both consume and produce CO2 and do not lead to sustained supersaturation on their own (Richey 2004). The lower precipitation, lower temperatures, and the resultant lower biomass plus higher elevation, relief, and more dynamic river flow of the CJ0200 series may be responsible for close to atmospheric levels of pCO2.
We observed some differences in the saturation states of both calcite and pCO2 for the matched samples (Fig. 3). The CJ300 samples have lower CSI and higher \( P_{{\text{CO}}_{{\text{2,river}}} } /P_{{\text{CO}}_{{\text{2,atm}}} } \) values than samples collected at similar locations 3 or 4 years earlier. The pH was lower by 0.1–0.5 units in the samples collected in year 2005.
4.1.2 Evaporite Weathering and Sources of Sulfur
Except for two samples (CJ 303, 304), Cl concentrations were low (<19 μM) in the CJ300 Min Jiang samples, which indicated that halite dissolution was generally minor in this area (Table 1). The two samples (40, 80 μM Cl) could have been influenced either by halite that is intercalated in the carbonate matrix or by anthropogenic input from the Sichuan Basin, but further speculation is not warranted with available information. This is still much lower than in the Jinsha Jiang above confluence with the Min Jiang (CJ305, 431 μM Cl) (Table 1).
The dissolved sulfate concentrations ranged between 110 and 306 μM (Table 1), with the low elevation samples (CJ301 = 203 μM, CJ303 = 306 μM, CJ304 = 234 μM) higher than the other samples (CJ302 = 110 μM, CJ306 = 123 μM, CJ307 = 130 μM, and CJ308 = 152 μM). Other CJ0200 series samples also had lower sulfate concentrations except for one sample CJ0221. To discern between the three potential sources of dissolved sulfate—(1) dissolution of gypsum and anhydrite, (2) sulfide oxidation, and (3) anthropogenic input from coal combustion—we measured the sulfur and oxygen isotope ratios of dissolved sulfate. The \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \) values of the samples were 1.9–7.5‰ (Table 1) (Fig. 4). The range of the δ34S values for the three sources mentioned above are—(1) Lower to Middle Triassic marine sulfate of the Sichuan Basin: +14.5 to +32.5‰ (Chen and Chu 1988), (2) sulfide minerals of Sichuan-Yunnan-Guizhou district: +10.9 to +17.7‰ (Li et al. 2006a), and (3) sulfur released from combustion of locally consumed coal and subsequently dissolved in water (excluding outliers): −6.1 to +7.4‰ (Li et al. 2006b). The \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \) values of the four Min Jiang samples are within the upper range for coal combustion. This indicates that the anthropogenic input is the dominant source of dissolved sulfate for these five samples. This result is consistent with the geographical proximity to the heavily populated Sichuan Basin and the relatively high population densities for these sample drainage areas (>40 indiv/km2) (Table 4). The rain in the Sichuan basin had extremely high sulfate concentration (125 μM) (Aas et al. 2007) compared to the upper reaches (2.6–3.0 μM) (Zhang et al. 2003) (Table 2), and the \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \) value of the rain in the Leshan and Yibin area (Fig. 1a) was +4.8 to +5.5‰ (Li et al. 2006b) which overlaps the range of the samples.
The \( \delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) values for the dissolved sulfate were −0.5 to +5.2‰ (Table 1) (Fig. 4). The \( \delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) values of the gypsum and anhydrite from the Yangtze Platform are mostly between 13 and 15‰ (Goldberg et al. 2005), and we can rule out this source. For both sulfide oxidation and coal combustion, the oxygen atoms in sulfate are incorporated from meteoric water and atmospheric oxygen. Therefore, it is not possible to distinguish between the two processes with \( \delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) alone. However, as a back-of-the-envelope-type exercise, we used the \( \delta {}^{18}{\text{O}}_{{\text{O}}_{\text{4}} } \) to calculate the relative contributions from the two ultimate sources of oxygen: O2 and H2O. Atmospheric oxygen has a rather constant \(\delta ^{18}\rm {O_{\rm {o}_{2}}} \) value of 23.5‰ (Hoefs 1997), and the meteoric water at Chendgu (1997–1998) has an average \(\delta ^{18}\rm {O_{\rm {H}_{2}\rm O}} \) value of −4.8‰ (IAEA/WMO 2004). There is 8.7–11.4‰ fractionation during incorporation of O2 into SO4, whereas the fractionation is negligible when O from H2O is incorporated into SO4 (Lloyd 1968; Toran and Harris 1989). The average \( \delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) value of our samples was 3.1‰. Assuming that the oxygen in SO4 (3.1‰) is a binary mixture between oxygen from O2 (23.5–10‰) and oxygen from H2O (−4.8‰), we can roughly estimate the relative contribution of the two sources: 43% from O2 and the rest (57%) from H2O. Thus, one or two of the four oxygen atoms in SO4 was formed in an oxygenated environment, but may have exchanged up to three O atoms with the surrounding water molecules in the intermediate sulfite step of oxidation (Bottrell and Tranter 2002).
In summary, the \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \) values indicated that the anthropogenic input from coal combustion was the main source of dissolved sulfate in the five samples analyzed, and the \( \delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) data indicated that the oxidation step happened in an oxic environment.
4.1.3 Silicate Weathering and Strontium Isotopes
The Si/(Na* + K) ratio is often used as an index of silicate weathering intensity. The assumption is that the Na* (Na corrected for halite contribution) and K originated from silicate minerals. More intensive weathering would yield higher Si/(Na* + K) ratios in the dissolved load. For example, weathering of Na-feldspar to beidellite gives a ratio of 1.7, weathering of Na-feldspar to kaolinite a ratio of 2.0, and weathering of Na-feldspar to gibbsite a ratio of 3.0 (Huh et al. 1998a). The Si/(Na* + K) ratios of the samples ranged from 0.5 to 2.5, which means that the silicate weathering intensity tends to be very low for the CJ300 series and very low to moderate for the CJ0200 and Station series (Fig. 5). Sometimes, the very low Si/(Na* + K) ratios are attributed to the biologic uptake of Si (e.g. diatom or phytolith growth) if there are swampy or stagnant waters. However, considering that the Min Jiang flows through the mountain valleys until it meets the Sichuan Basin, biogenic uptake of Si does not seem to be important. We think the low values of Si/(Na* + K) are due to the underlying lithology of the Min Jiang drainage being sediments that have already experienced previous cycle(s) of weathering and not fresh bedrock. As a reference, weathering of average shale to kaolinite gives the Si/(Na* + K) ratio of 1.0 (Huh et al. 1998b).
Si versus (Na* + K), where Na* = Na − Cl. Guidelines for Si/(Na* + K) ratios of 0.5 and 2.5 are shown. For reference, Si/(Na* + K) ratios for Na-feldspar to beidellite is 1.7, Na-feldspar to kaolinite is 2.0, and Na-feldspar to gibbsite is 3.0. Arrows connect the spatially matched samples, pointing from Station samples and CJ0200 series to CJ300 series
The matched samples showed that the inter-annual difference was due mainly to higher Na* + K in the CJ300 series while the Si levels remained similar (Fig. 5). It is difficult to conclude whether the increase in Na* + K of ∼100 μM truly constitutes a decrease in weathering intensity or an input of Na and K that is unaccounted for. Only two samples CJ302 and CJ307 from the headwaters had increases in both Na* + K and Si, and in this case the weathering intensity was maintained.
The Sr isotope ratios of Phanerozoic marine limestones vary between 0.7065 and 0.7090 (Burke et al. 1982), and those of felsic silicates are more radiogenic (>0.710) because of substitution of parent 87Rb in K sites. Therefore, the dissolved 87Sr/86Sr can provide a guide to carbonate vs. silicate contribution. Other minor sources to be considered are the evaporites (∼0.7072 to ∼0.7102) (Palmer et al. 2004) and mafic silicate minerals (∼0.702 to ∼0.705) (Smith and Compston 1982; Kurz and Kammer 1991). In our case, based on lithologic information and major element data we could overlook significant mafic silicate or evaporite contributions and make a qualitative estimate of carbonate and felsic silicate contribution based on the Sr isotope data.
The CJ300 series samples had Sr concentrations between 1.3 and 2.5 μM and 87Sr/86Sr ranging from 0.711 to 0.713 (Fig. 6) (Table 1). The plot of Sr isotope ratio and 1/Sr showed a mixing trend between carbonate (high Sr concentration, low 87Sr/86Sr) and felsic silicate (low Sr concentration, high 87Sr/86Sr) (Fig. 6). The Jinsha Jiang above confluence with the Min Jiang had the highest concentration and the least radiogenic value probably due to the evaporite and carbonate dominant lithology upstream. The lowest concentration and the most radiogenic values were from the headwaters of the Min Jiang. Even though the major element composition of the river waters was Ca-HCO3 type, contribution from felsic silicates was identifiable with the Sr isotopes.
4.2 Forward Calculation
Weathering of different rock types is the major source of the dissolved load in pristine rivers. The three major categories—silicate, carbonate, and evaporite—are distinctive in chemical composition, and contributions from each can be back-calculated based on the riverine chemical composition. One more category that is included as an initial correction is the rain contribution. To evaluate the contribution from the four reservoirs, we used a forward calculation method which sequentially allocates the major elements to the four sources. By determining the relative contribution from the reservoirs, we can calculate the CO2 consumption rate by silicate weathering. We included the CJ0200 series samples of Qin et al. (2006) to cover the relatively uncontaminated area with low population density (Table 4).
A flow chart of the calculation method is shown in Fig. 7 and the data set used is listed in Table 3. Normally, the lowest Cl concentration of the sample data set (in our case 7 μM) is taken to be derived entirely from rain, and this lowest Cl value is multiplied by the X/Cl ratios (X = Na, K, Mg, Ca, Cl, SO4) of rain to yield the rain contribution of the different chemical species. Local rain composition was acquired by averaging the rain from Lhasa, Amdo, Dangxiong, and Dingri (Table 2). When the above procedure was applied to our data set and the rain contribution subtracted from the original concentrations, the K content of two samples (CJ0218, 0220) yielded negative values. So, an alternative Cl concentration was chosen (4.3 μM) such that the lowest K concentration (CJ0218) became 0 μM after the rain correction. Thus, we assume no K sources other than rainwater for this sample (CJ0218).
We attributed the remaining Cl to halite dissolution (Clevap = Clriver − 4.3 = Naevap) and the remaining SO4 to gypsum dissolution (SO4,evap = SO4,river − SO4/Clrain × 4.3 = Caevap). The residual Na after rain and evaporite correction was considered to be from silicate weathering (Nasil). We calculated Casil and Mgsil by multiplying Nasil by Ca/Na and Mg/Na molar ratios of local silicate rock, respectively. Here, we adopted average Ca/Na (0.54) and Mg/Na (0.26) molar ratios of granitoids from the eastern Songpan-Garze fold belt (Xiao et al. 2007) (Table 3). One potential disadvantage with this approach is that the differential weathering among elements Na, Ca, and Mg is not specifically taken into account. An alternative method is to use Ca/Na and Mg/Na ratios of silicate monolithologic streams. However, in drainage basins of the spatial scale and geologic complexity as the Min Jiang, such monolithologic streams are difficult to find. When one resorts to data from monolithologic streams elsewhere, it also introduces uncertainty. Thus, we opted to use the local rock composition. The Mg remaining after rain and silicate correction was taken to be Mgcarb, and the Ca remaining after rain, evaporite, and silicate correction Cacarb.
The forward model results for the Gaochang Station sample (GC0108) are not reliable. The Cl levels were anomalously high, even higher than Na. Thus, no Na could be attributed to silicate origin, and since the silicate portion is calculated using Ca/Na and Mg/Na ratios, no cations could be apportioned to silicate origin for this sample. We have excluded it in our discussions of fluxes and yields.
The cations from each reservoir can be summed up: e.g. Cationsil = Nasil + Ksil + Mgsil + Casil (in molar units). The relative contributions from each reservoir normalized to 100% are shown in Fig. 8 and Table 5. The silicate contributions ranged from 0 to 25%, carbonate 39 to 93%, evaporite 2 to 37%, and rain 3 to 10%. The Jinsha Jiang above confluence with the Min Jiang (CJ305) had the highest relative contribution from evaporites (37%) and lowest from carbonate (39%). Discharge-weighted averages of silicate, carbonate, evaporite, and rain for the Min Jiang basin samples were 17, 61, 17, and 5%, respectively.
Thus far, we have neglected the role of sulfide weathering and acid rain from coal combustion, both of which introduce non-CO2-derived acids that can facilitate weathering. The sulfur isotope data, supported by the geographic locations, population density, and NO3 and PO4 concentrations, indicated that all samples from the Sichuan Basin analyzed for \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \) and \( \delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) (CJ 301, 303, 304, 305, and 308) were influenced by atmospheric deposition of locally consumed coal (Fig. 4). Those samples had some of the highest silicate contributions (Fig. 8), and if they are excluded, the discharge-weighted averages of silicate, carbonate, evaporite, and rain for the Min Jiang basin samples are 14, 69, 11, and 6%, respectively.
4.3 Flux Calculations
With the ArcGIS® v.9.0 software, we calculated the basin area and runoff, which were used for calculating the weathering rates and fluxes, as well as other climatic (temperature, precipitation, potential evapotranspiration) and geomorphic (elevation, relief) parameters used to evaluate the controlling factors for the weathering rates and fluxes (Table 4). The drainage basin area was modified from the Hydro1k database for Asia obtained from the Land Process Distributed Active Archive Center (LP DAAC) of USGS. The air temperature, precipitation, and potential evapotranspiration data were from Leemans and Cramer (1991), and runoff data were from UNEP/GRDC composite Runoff Fields v1.0 with 30-min resolution (Fekete et al. 2002). The mean local relief was calculated from the 10-min gridded modal height data for land masses provided by the US Navy Fleet Numerical Oceanography Center (FNOC). The digital elevation model (DEM) was obtained by the 3-arc-s elevation dataset from the Shuttle Radar Topography Mission (SRTM). The population density with 30-min resolution was from the Land Scan Global Population 2000 Database developed by Oak Ridge National Laboratory (Hearn et al. 2001).
The calculated fluxes and yields for all samples are reported in Table 5. The Jinsha Jiang sample (CJ305) had the highest TDS, silicate, and CO2 fluxes, which is as expected given the highest water discharge (Table 5). Area-normalized yields are still high, but not as extreme. The Min Jiang before it joins the Chang Jiang (CJ304) for the same reason is the second highest in terms of fluxes. In this case, the yields are also high, but we suspect that at this location one cannot rule out anthropogenic effect and cannot assume that the silicate weathering rate and CO2 consumption rate thus calculated represent natural rates. The samples which were suspected of anthropogenic impact based on the sulfur isotope data had high silicate weathering yield (132–342 × 103 mol/km2/year) and CO2 consumption yield (183–482 × 103 mol/km2/year). This is because the forward model does not distinguish between the sulfuric acid and CO2 as the agent of weathering. Disregarding those samples, the TDS flux (0.03−6.95 × 106 tons/year) varied widely among samples depending on water discharge, while the TDS yield was more uniform at 78–187 tons/km2/year (Table 5). The silicate weathering flux was 0.01−8.14 × 109 mol/year, and the silicate weathering yield was 22.6−181 × 103 mol/km2/year. The CO2 consumption flux ranged 0.02−11.3 × 109 mol/year, and the CO2 consumption yield was 31−246 × 103 mol/km2/year (Table 5). A headwater tributary of the Min Jiang draining the Longmen Shan (CJ307) had the highest silicate weathering and CO2 consumption rates per unit area. The lowest yields were observed in the extreme headwater tributary sample of the Min Jiang draining the tufa deposits (CJ0209).
If we compare the matched samples for the area-averaged yields, the TDS yields are similar, but silicate weathering and CO2 consumption rates are two to three times higher in the CJ300 series. This is puzzling, because if there had been a significant increase in anthropogenic activity in the Min Jiang basin during the 3–4 years, the CJ300 series should also have higher TDS yield from the abundant carbonates which weather fast.
In comparison to other orogenic rivers, the CO2 consumption rate of the Min Jiang (31−246 × 103 mol/km2/year) was lower than the Orinoco (145−234 × 103 mol/km2/year), Amazon (102−347 × 103 mol/km2/year), Ganges (111−572 × 103 mol/km2/year), and Brahmaputra (164−196 × 103 mol/km2/year), but higher than the Siberian rivers (4−26 × 103 mol/km2/year), Yukon (28−29 × 103 mol/km2/year), Mackenzie (5−57 × 103 mol/km2/year), and Upper Huang He (6−116 × 103 mol/km2/year) (Wu et al. 2005 and references therein). Lerman et al (2007) proposed a dissolution model of a mixed sediment-crust source and estimated a global CO2 consumption rate of 22 × 1012 mol C/year. Using 100 × 106 km2 as the continental area drained to oceans, this translates to 220 × 103 mol/km2/year, which is consistent with the above results within the uncertainty of such estimates.
4.4 Factors Affecting the CO2 Consumption Rate
Various climatic and geologic factors (air temperature, precipitation, runoff, potential evapotranspiration, area, relief, elevation) (Table 4) were tested for correlation with the CO2 consumption yield to find out the parameters responsible for higher silicate weathering rate (i.e. CO2 consumption rate). No single parameter had a strong correlation. In the unperturbed watersheds of the Min Jiang, the best correlation, albeit with still quite low r 2 values of 0.284 (p = 0.009) was found with water temperature, the second best with discharge (r 2 = 0.253, p = 0.014), and the third with relief (r 2 = 0.230, p = 0.019). Warm and humid climatic conditions and rugged physiology together account for the rates of silicate weathering found in the Min Jiang drainage basin.
5 Summary
Using new data on major elements, \( {}^{87}{\text{Sr/}}{}^{86}{\text{Sr}},\;\delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } ,\;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) and a forward model, we constrained the relative contributions of rain, evaporite, carbonate, and silicate reservoirs to the Min Jiang. In unperturbed watersheds of the Min Jiang, carbonate weathering (58–93%) was dominant but silicate weathering, the major sink of atmospheric CO2 over geologic time scales, was also significant, from 2 to 18%. Most of the samples were supersaturated with respect to calcite, and up to ∼5 times supersaturated in CO2 with respect to the atmosphere. The \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \) values of the five samples from the lower reaches (1.9–7.5‰) were within the range for locally consumed coal, suggesting that anthropogenic contribution is the dominant source of the dissolved sulfate for these samples. The \( \delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) values were between −0.5 and 5.2‰ and indicated that sulfur oxidation occurred in an oxic environment. We attributed the major source of the dissolved sulfate to anthropogenic input by coal combustion. The Sr isotopic ratio (0.7108–0.7127) indicated some contribution from felsic silicate rocks, and the Si/(Na* + K) ratios of 0.5–2.5 suggested low to moderate silicate weathering intensity. The silicate weathering rates were 23−181 × 103 mol/km2/year and the CO2 consumption rates 31−246 × 103 mol/km2/year, moderate values on a global scale. The combined effect of water temperature, discharge, and relief was responsible for the variation in silicate weathering rates in this region.
References
Aas W, Shao M, Jin L, Larssen T, Zhao D, Xiang R, Zhang J, Xiao J, Duan L (2007) Air concentrations and wet deposition of major inorganic ions at five non-urban sites in China, 2001–2003. Atmos Environ 41:1706–1716
Andrews JE, Brimblecombe P, Jickells TD, Liss PS (1996) An introduction to environmental chemistry. Blackwell Science, Oxford
Berner EK, Berner RA (1996) Global environment: water, air, and geochemical cycles. Prentice-Hall, Englewood Cliffs
Bethke CM (2002) The Geochemist’s Workbench 4.0. University of Illinois, USA
Bottrell SH, Tranter M (2002) Sulphide oxidation under partially anoxic conditions at the bed of the Haut Glacier d’Arolla, Switzerland. Hydrol Process 16:2363–2368
Burchfiel BC, Chen Z, Liu Y, Royden LH (1995) Tectonics of the Longmen Shan and adjacent regions, Central China. Int Geol Rev 37:661–735
Bureau of Geology, Mineral Resources of Sichuan Province (1991) Regional geology of Sichuan Province. Geological Publishing House, Beijing
Burke WH, Denison RE, Hetherington EA, Koepnick RB, Nelson HF, Otto JB (1982) Variation of seawater 87Sr/86Sr throughout phanerozoic time. Geology 10(10):516–519
Chen JS, Chu XL (1988) Sulfur isotope composition of Triassic marine sulfates of south China. Chem Geol 72:155–161
Chen J, Wang F, Xia X, Zhang L. (2002) Major element chemistry of the Changjiang (Yangtze River). Chem Geol 187:231–255
Chinese Academy of Sciences Comprehensive Research Team (1985) Hydrologic geography for the western Sichuan and northern Yunnan area. Sciences Publishing House, Beijing
Commision on Annals of Sichuan Province (1998) Annals of Sichuan Province (geology part). Science and Technological Publishing House of Sichuan Province, Chengdu
Dalai TK, Krishnaswami S, Sarin MM (2002) Major ion chemistry in the headwaters of the Yamuna river system: chemical weathering, its temperature dependence and CO2 consumption in the Himalaya. Geochim Cosmochim Acta 66(19):3397–3416
Fekete BM, Vörösmarty CJ, Grabs W (2002) High resolution fields of global runoff combining observed river discharge and simulated water balances. Global Biogeochem Cycles 16(3):1042. doi:10.1029/1999GB001254
Galy A, France-Lanord C (1999) Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem Geol 159(1–4):31–60
Goldberg T, Poulton SW, Strauss H (2005) Sulphur and oxygen isotope signatures of late Neoproterozoic to early Cambrian sulphate, Yangtze Platform, China: Diagenetic constraints and seawater evolution. Precambrian Res 137:223–241
Hearn P, Hare T, Schruben P, Sherrill D, Lamar C, Tsushima P (2001) Global GIS database: digital atlas of South Asia. U.S. Geological Survey
Hoefs J (1997) Stable isotope geochemistry, 4th edn. Springer-Verlag, Berlin
Hong YL, Kim G (2005) Measurement of cosmogenic 35S activity in rainwater and lake water. Anal Chem 77:3390–3393
Huh Y, Edmond JM (1999) The fluvial geochemistry of the rivers of Eastern Siberia: III. Tributaries of the Lena and Anabar draining the basement terrain of the Siberian Craton and the Trans-Baikal Highlands. Geochim Cosmochim Acta 63:967–987
Huh Y, Tsoi MY, Zaitsev A, Edmond JM (1998a) The fluvial geochemistry of the rivers of Eastern Siberia: I. Tributaries of the Lena River draining the sedimentary platform of the Siberian Craton. Geochim Cosmochim Acta 62(10):1657–1676
Huh Y, Panteleyev G., Babich D, Zaitsev A, Edmond JM (1998b) The fluvial geochemistry of the rivers of Eastern Siberia: II. Tributaries of the Lena, Omoloy, Yana, Indigirka, Kolyma, and Anadyr draining the collisional/accretionary zone of the Verkhoyansk and Cherskiy ranges. Geochim Cosmochim Acta 62(12):2053–2075
IAEA/WMO (2004) Global network of isotopes in precipitation. The GNIP database. http://isohis.iaea.org. Cited 22 July 2007
Karim A, Veizer J (2000) Weathering processes in the Indus River Basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem Geol 170(1–4):153–177
Kurz MD, Kammer DP (1991) Isotopic evolution of Mauna Loa volcano. Earth Planet Sci Lett 103:257–269
Leemans R, Cramer W (1991) The II ASA database for mean monthly values of temperature, precipitation and cloudiness on a global terrestrial grid. II ASA Research Report RR-91-18. International Institute of Applied Systems Analyses
Lerman A, Wu L, Mackenzie FT (2007) CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar Chem 106:326–350
Li XB, Huang ZL, Li WB, Zhang ZL, Yan ZF (2006a) Sulfur isotopic compositions of the Huize super-large Pb-Zn deposit, Yunnan Province, China: implications for the source of sulfur in the ore-forming fluids. J Geochem Explor 89:227–230
Li XD, Masuda H, Kusakabe M, Yanagisawa F, Zeng HA (2006b) Degradation of groundwater quality due to anthropogenic sulfur and nitrogen contamination in the Sichuan Basin, China. Geochem J 40:309–332
Lloyd RM (1968) Oxygen isotope behaviour in the sulfate-water system. J Geophys Res 73:6099–6110
Map Publishing House of China (1998) Atlas of the Chinese natural geography plates, Beijing
Meybeck M (1979) Concentrations des eaux fluviales en éléments majeurs et apports en solution aux océans. Rev Géol Dyn Géogr Phys 21:215–246
Moon S, Huh Y, Qin J, van Pho N (2007) Chemical weathering in the Hong (Red) River basin: rates of silicate weathering and their controlling factors. Geochim Cosmochim Acta 71(6):1411–1430
Palmer MR, Hevalci C, Fallick AE (2004) Sulfur, sulfate oxygen and strontium isotope composition of Cenozoic Turkish evaporites. Chem Geol 209:341–356
Pan G.T, Cheng ZL, Li XZ, Yan YJ, Xu XS, Xu Q, Jiang XS, Wu YL, Luo JL, Zu TX, Pen YM (1997) Geological-tectonic evolution in the eastern Tethys. Geological Publishing House, Beijing
Qin J, Huh Y, Edmond JM, Du G., Ran J (2006) Chemical and physical weathering in the Min Jiang, a headwater tributary of the Yangtze River. Chem Geol 227(1–2):53–69
Raymo ME, Ruddiman WF (1992) Tectonic forcing of late Cenozoic climate. Nature 359:117–122
Richey JE (2004) Emission of CO2 from riverine systems. In: Steffan E (ed) Global change and the Earth system: a planet under pressure. Springer-Verlag, Berlin, New York, pp 172–173
Smith IE, Compston W (1982) Strontium isotopes in Cenozoic volcanic rocks from southeastern Papua New Guinea. Lithos 15:199–206
Stallard RF, Edmond JM (1987) Geochemistry of the Amazon 3. Weathering chemistry and limits to dissolved inputs. J Geophys Res 92(C8):8293–8302
Toran L, Harris RF (1989) Interpretation of sulfur and oxygen isotopes in biological and abiological sulphide oxidation. Geochim Cosmochim Acta 53:2341–2348
Wanninkhof R, Mulholland PJ, Elwood JW (1990) Gas exchange rates for a first-order stream determined with deliberate and natural tracers. Water Resour Res 26(7):1621–1630
Wu L, Huh Y, Qin J, Du G., van der Lee S (2005) Chemical weathering in the Upper Huang He (Yellow River) draining the eastern Qinghai-Tibet Plateau. Geochim Cosmochim Acta 69(22):5279–5294
Xiao L, Zhang HF, Clemens JD, Wang QW, Kan ZZ, Wang KM, Ni PZ, Liu XM (2007) Late Triassic granitoids of the eastern margin of the Tibetan Plateau: geochronology, petrogenesis and implications for tectonic evolution. Lithos 96:436–452
Zhang DD, Peart M, Jim CY, He YQ, Li BS, Chen JA (2003) Precipitation chemistry of Lhasa and other remote towns, Tibet. Atmos Environ 37:231–240
Acknowledgment
Financial support was provided by the US NSF EAR0134966 and KOSEF grant No. R01-2006-000-10019-0 to Y. Huh and Brain Korea 21 Graduate Student Fellowships to S. Moon, H. Noh, and J. Yoon.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, J., Huh, Y., Lee, I. et al. Weathering Processes in the Min Jiang: Major Elements, \( {}^{87}{\text{Sr/}}{}^{86}{\text{Sr}},\;\delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } ,\;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) . Aquat Geochem 14, 147–170 (2008). https://doi.org/10.1007/s10498-008-9030-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-008-9030-7