Abstract
A detailed analysis of the major ion chemistry, chemical weathering and CO2 consumption was conducted in the Songhua River basin, which is the largest basin with a draining area of 557 thousand km2 in Northeast China. The dataset used in this study included major ion concentrations came from 56 hydrological stations from the year of 1962 to 1984. The median of the total dissolved solid concentration of the Songhua River was 104.8 mg/L, about two times higher than the global average (65.0 mg/L), but lower than that of other Chinese Rivers. Prevalent ions were Na+, Ca2+ and HCO3 −, and the average Ca2+/Na+ and Mg2+/Na+ molar ratios were 0.70 and 0.42, which were close to silicate weathering end-member. Long-term trend analysis by seasonal MK test showed that from 1962 to 1984, Ca2+, Na+ + K+ and HCO3 − were significantly increasing, and the increasing rates calculated by linear fit slopes for Ca2+, Na+ + K+ and HCO3 − ions were 0.22, 0.63 and 2.35 mg/year, respectively. An inverse model showed that the dissolved loads primarily came from rock weathering (91.5 %), including silicate weathering (66.4 %) and a small portion from carbonates (16.1 %) and evaporites (9.0 %). Other origins consisted of anthropogenic inputs (5.3 %) and atmospheric inputs (3.2 %). Average silicate and carbonate weathering rates were estimated as 4.03 and 1.76 t km−2 year−1, and CO2 consumption rates of silicates and carbonates were 17.1 × 104 and 1.85 × 104 mol km–2 year−1, respectively. These results are consistent with the lithologies of the study area, which mainly consisted of silicate rocks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
From the view of the global carbon cycle, it has been recognized that the flux of CO2 consumed by carbonate dissolution on the continents is balanced by the flux of CO2 released into the atmosphere from the oceans by carbonate precipitation on the geological time scale (Berner et al. 1983). Chemical weathering of silicate rock acts as a net sink for atmospheric CO2, and its rate and feedback mechanisms, which are controlled by climatic and geologic factors, function as regulators of atmospheric CO2 levels over geologic time scales (Berner 1991; Edmond 1992; Raymo and Ruddiman 1992; Walker et al. 1981). Numerous studies on major ions and dissolved loads of larger rivers have been carried out in the Changjiang River (Chen et al. 2002; Ran et al. 2010), the Huanghe River (Zhang et al. 1995), the Pearl River (Xu and Liu 2010; Zhang et al. 2007), the Huai River (Zhang et al. 2011) and the rivers of the Qinghai-Tibet Plateau (Wu et al. 2008) to examine hydrochemical characteristics, chemical erosion and CO2 consumption rates, and long-term climatic evolution of the Earth. A general conclusion of CO2 consumption rate for Chinese large rivers showed that it decreased from South China to North China due to climate and hydrological factors (Gaillardet et al. 1999).
The Songhua River as the largest tributary of the Heilong River is the largest basin with a drainage area of 557 thousand km2 in Northeast China. Although the work of Gaillardet et al. (1999) gave an overall understanding on rock weathering and CO2 consumption rates in the Heilong River basin, it was considered to be rough for the Songhua River basin due to rock weathering and associated CO2 consumption rates varying with temperatures, runoff, geology and topographic relief. The average TDS, 55 mg/L for the Heilong River and 132 mg/L for the Songhua River, implies the hydrochemical difference of these two rivers. Consequently, a different weathering pattern in the Songhua River is expected compared to the overall weathering characteristics in the Heilong River.
In this study, the objectives are to (1) examine the characteristics of temporal and spatial distribution of major ion chemistry in the Songhua River basin, (2) identify the mechanisms controlling the major ion chemistry and determine the contributions of different end-members to the hydrochemical composition based on an inverse method, and (3) assess the chemical weathering rates of silicate, and carbonate and corresponding CO2 consumption rates.
Study area
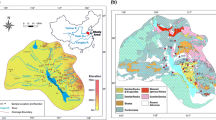
The Songhua River, located in Northeast China, is the largest tributary of Heilong River, flowing through the Jilin and Heilongjiang provinces (Fig. 1). The river originates in the Changbai Mountains, travels from southeast to northwest until the Nenjiang River joins near Zhaoyuan city. Then it turns its direction toward the northeast, and finally flows into the Heilong River at Tongjiang city. The main channel of Songhua River, with a length of 1897 km, is connected by a large number of branches, including Mudan River, Huifa River, Hulan River, Lalin River, Mayi River, Yinma River, Tangwang River and Woken River (Table 1).
The Songhua River basin is located in the temperate continental monsoon zone with annual mean temperature ranging from 3 to 5 °C. Mean temperatures in July and January are 22.5 and −20 °C, respectively. Annual precipitation of the Songhua River basin is around 500 mm, and about 79 % occurs from June to September. The average annual runoff at the hydrological station Jiamusi near the outlet is about 6.32 × 1,010 m3 and the runoff from July to October takes up almost 60 % of the annual runoff.
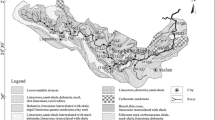
The Songhua River basin is a Meso-Cenozoic sedimentary basin developed on a pre and upper Paleozoic basement (Fig. 1). The basement rocks, which mainly outcrop on the south and north-east edges of the basin, are composed of gneiss with inclusions of marble and metamorphic peridotite. Igneous rocks such as granite, diorite and basalt show banded distribution extending from the north to the south at the center of the basin. Sandstones scatter in the entire basin. Quaternary sediments are found in the northeast part of the basin with a thickness from 50 to 120 m. No significant outcrops of carbonates are observed in the Songhua River basin and they are mainly disseminated along with other rock types.
Materials and methods
Data sources
Since 1956, the Ministry of Water Resources of the People’s Republic of China has established more than 900 hydrochemical stations along more than 500 rivers in China including the Songhua River to monitor major element concentrations, as well as other hydrological and water quality parameters (Chen et al. 2002). Available data of the Songhua River basin used in this study were from a total of 56 stations in the basin from 1962 to 1984. Among these stations, Gaolicheng, Hongshizhe, Jilin, Songhuajiang, Fuyu, Haerbin, Tonghe, Yilan and Jiamusi are located along the main channel from the upstream to downstream (Fig. 1; Table 1). Other stations are along the tributaries of the Songhua River. The data are given in the unit of both mg/L and meq/L, which are convenient for chemical stoichiometric calculations. In this study, only data with the charge balance (∑cation − ∑anion)/∑total ions) <5 % was accepted. Those that did not satisfy the criterion were taken as outliers due to misprint and excluded from following analysis. Analytical methods for major element were summarized as Ca2+ and Mg2+ by EDTA titration, Na+ (K+) by flame spectrometry, HCO3 − by acid titration, SO4 2− by BaCl2 titration, Cl− by AgNO3 titration and SiO2 by molybdenum blue method. TDS was calculated by the sum of Ca2+, Mg2+, Na+, K+, SO4 2−, Cl− and HCO3 −, and SiO2 was not included due to too much missing values. Quality assurance and quality control information about the data sources was introduced and examined by Chen et al. (2002).
Statistical methods
One-way analysis of variance (ANOVA) was performed to compare seasonal differences in major ion concentrations by IBM SPSS 18.0 for windows, and p values smaller than 0.05 were considered significant. The long-term trend analysis of major ion concentrations was performed using the seasonal Mann–Kendall trend analysis, following the approaches of Hirsch and Slack (1984). Trends were considered statistically significant when p < 0.01. When there was a statistically significant trend, the changing rate was quantified by linear fit. The seasonal Mann–Kendall trend test was performed by statistical software R.
Result
Spatial distribution
Mean values of physical and hydrochemical parameters for the main channel of Songhua River and its seven major tributaries are shown in Table 1. In the Songhua River basin, pH ranged from 6.5 to 7.8, with an average of 7.1. TDS varied from less than 50.0 mg/L to higher than 300.0 mg/L. The median of TDS was 104.8 mg/L which was higher than the world median (65.0 mg/L) (Meybeck and Helmer 1989) and lower than other major rivers in China such as the Changjiang River (205.9 mg/L), the Yellow River (486.4 mg/L), the Pearl River (192.0 mg/L) and the Huai River (214.2 mg/L). The highest TDS was found in the Yinma River on the southwest corner of the Songhua River basin (Fig. 1).
Prevalent cations were K+ + Na+ > Ca2+ > Mg2+, and these chemical features showed a typical cationic composition of rivers draining silicate bedrocks. Na+ (plus K+) and Ca2+ were the most abundant cations with concentrations of 8.4–31.6 and 8.8–30.7 mg/L, respectively. HCO3 − was the most abundant anion; and its concentration ranged from 60.3 to 174.3 mg/L. Cl−, as the second most abundant anion, varied from 2.8 to 15.8 mg/L. SO4 2− ranging from 2.7 to 14.9 mg/L was the least abundant anion among the anions. Similar with TDS, the highest cations and anions were observed in the southwest of the study area.
Variations of TDS values and hydrochemical pattern along the main channel of Songhua River are shown in Fig. 2. For convenience, the Gaolicheng station and the Jiamusi station were set as the starting and ending point, respectively, along the main channel in this paper. TDS was less than 100 mg/L in the upstream with mountains and increased up to 300 mg/L after the Yinma tributary joins about 400 km downstream from the Gaolicheng station. A sudden decrease of TDS was observed after the Nenjiang River flows in before the Haerbin station. Then TDS increased again at the lower reach until the river flows through the Jiamusi station. The increasing trend along the river shows the tributaries had substantial contributions to the increased TDS of the main channel; and this trend does not seem to be consistent with trends observed in the Changjiang River and Huai River, where the TDS concentrations of the main channel showed a general decreasing trend due to dilution effect by large tributaries (Chen et al. 2002). Hydrochemical pattern changed from Ca–Na–HCO3 type in the headwater to Ca–HCO3 type in the middle and upper reaches.
The variations of TDS and hydrochemical pattern along the main channel. The boxplot showed the TDS ranges of each station. White square gave the mean value of TDS, upper and lower edge of the box showed the 25th and 75th percentiles of TDS. The line in the box gave the median of TDS. The whiskers are determined by the 5th and 95th percentiles. The asterisk showed the “outliers”
Temporal variations
Seasonal variations of major ions were illustrated by the monthly averaged hydrochemistry data at the Jiamusi station (Fig. 3). All the ions showed significant seasonal variations, with variation factors (VF: the ratio of the highest to the lowest value during a year) for Ca2+, Mg2+, Na+ + K+, Cl−, SO4 2− and HCO3 − being 2.16, 2.28, 2.26, 1.78, 2.23, and 2.61, respectively. All the ions showed significant decreases in wet season due to dilution, which was similar to Amazon River (Gibbs 1972) and the Lena River (Gordeev and Sidorov 1993), but different from the Changjiang River where the major element did not vary greatly in different seasons (Chen et al. 2002).
Furthermore, the time series data from 1962 to 1984 at the Jiamusi station were used to evaluate the long-term trend of major ions (Fig. 4). Ca2+, Na+ + K+ and HCO3 − showed significant increasing trends during this period. No significant trends were detected for other ions. The increasing rates calculated by linear fit for Ca2+, Na+ + K+ and HCO3 − ions were 0.22, 0.63 and 2.35 mg/year, respectively. The long-term variations observed in the Songhua River basin were different from the Changjiang River with significant increasing trend of Cl− and SO4 2− due to intensified acid deposition caused by increases in coal consumptions (Chen et al. 2002).
Discussion
Statistical difference for seasonal change of major ions
The one-way ANOVA procedure of pairwise multiple comparisons was used to examine the seasonal differences of major ions (Table 2). In spring, major ions were significantly higher than that in summer but lower than that in winter except for SO4 2−, and not statistically differed from fall except for Cl− and SO4 2−. In summer, all the ions had the lowest concentrations due to dilution effect. The ion concentrations between summer and winter had a large gap, and the values of the deviation were −10.11, −2.62, −10.20, −55.44, −4.28 and −3.45 mg/L for Ca2+, Mg2+, Na+ + K+, HCO3 −, Cl− and SO4 2−, respectively. The concentrations of the major ions in fall were similar with that in spring, significantly higher than that in summer and lower than that in winter. Moreover, all the ions showed strong condensed features in winter, contrary to the dilution effect in summer.
Mechanisms controlling the major ion chemistry
Atmospheric inputs
Rainwater has been recognized as an important source of dissolved species (major ions) in surface waters (Appelo and Postma 2005; Gibbs 1970; Stallard and Edmond 1981). The contribution of Cl− to dissolved salt loads in rivers is expected to decrease with increasing distance from the sea when there are no terrestrial sources effects (Stallard and Edmond 1981). However, in the Songhua River basin, elevated Cl− concentrations were found in some inland stations located in the southwest rather than in the seaward stations (Fig. 5), implying that in addition to precipitation inputs other origins such as weathering of evaporite rocks or anthropogenic inputs existed in the study area.
Contributions from precipitation inputs can be estimated as Cl−, which behaves conservatively through the hydrological cycle (Appelo and Postma 2005; Ollivier et al. 2010). Li et al. (2010) reported chemical data of precipitation at the Longfengshan station located around the center of the study area. It was found that the SO4 2−/Cl− molar ratio in precipitation was about 3.65, showing a sulfate enrichment of rainwater with respect to sea origin salt (SO4 2−/Cl− = 0.05). The Ca2+ and Mg2+ also suggested terrestrial sources with a high Cl-normalized ratio (1.75 and 0.49, respectively) compared to sea origin salt (0.02 and 0.01, respectively).
Runoff coefficient of the Songhua River was about 0.21 (Song et al. 2010); therefore, the evaporation factor (the reciprocal of the runoff coefficient) of the major ions was 4.72. Accordingly, major ions came from precipitation and their contributions to river chemical compositions were estimated. It was found that as a whole the atmospheric inputs contributed 16.5 % of Ca2+, 8.9 % of Mg2+, 11.6 % of K+ + Na+ and 17.3 % of Cl− in the Songhua River basin.
Anthropogenic inputs
The major ion chemistry can also be affected by a variety of human activities (Meybeck and Helmer 1989), which have become particularly important in China due to drastically intensified industrial and agricultural activities (Chen et al. 2002). Based on the Cl-normalized ratio of precipitation, it was found that the atmospheric loading in the Songhua River had a significant terrestrial component, especially for SO4 2− as a result of extensive and intensive use of S-enriched coal and power production via coal combustion in the study area. In addition, Cl− is also considered to be an indicator of the impact of land use and human activity on water chemistry. In this study, three reservoirs were considered for Cl− as atmospheric inputs, anthropogenic inputs and evaporite weathering, and two reservoirs were considered for SO4 2− as cyclic salt origin from sea and anthropogenic inputs from coal combustion. The contribution of anthropogenic inputs was calculated by deducting the TDS came from other sources from the total TDS.
Rock weathering inputs
Ternary diagrams were employed to develop an overall understanding of the water–rock reactions which control the major chemical compositions of river water in the study area (Fig. 6). Samples defined a narrow range on the cation ternary diagram, and most clustered in the center of the plot which implied that chemical weathering of silicates dominated in the study area. The dominance of silicate rock weathering is not surprising, given the fact that silicate rocks are well-spread in the Songhua River basin (Fig. 1). Outcrops of carbonate rocks sporadically existed; therefore, it was reasonable to recognize the contribution of the carbonate rock weathering to the river chemical compositions. In addition, evaporites were also considered to contribute the chemical compositions of the river due to only 17.3 % of Cl− explained by atmospheric inputs.
Beside the ternary diagram, the plot of the relation between the molar ratio of (Na+ + K+)/HCO3 − and (2Ca2+ + 2Mg2+)/HCO3 − also showed the dominance of silicate dissolution in the Songhua River basin (Fig. 7). The line of Na+ + K+ + 2Ca2+ + 2Mg2+ = HCO3 − reflects the contributions of silicate weathering to water chemistry (Li et al. 2009). Obviously, river water samples fall into the section between the silicate weathering line and the carbonate weathering line, and had a tendency to be close to the silicate weathering line.
Rock weathering inputs were evaluated in terms of mixing between three main end-members corresponding to the weathering products of carbonates, silicates and evaporites (Gaillardet et al. 1997; Negrel et al. 1993; Ollivier et al. 2010). The approach used by Ollivier et al. (2010) was followed to determine rock weathering contributions to major cations (Na+, Ca2+ and Mg2+). The end-members and samples expressed by Na-normalized molar ratios (Ca2+/Na+ and Mg2+/Na+) are showed in Fig. 8. Carbonate end-member was characterized with high ratios of Ca2+/Na+ and Mg2+/Na+ in the top-right corner of Fig. 8. In the Songhua River basin, the highest values of Ca2+/Na+ and Mg2+/Na+ were 2.03 and 1.56, much less than the Na-normalized molar ratios of carbonate end-member due to no tributaries flow through purely carbonate-dominated area. The influence of silicate weathering end-member was reflected by lower Ca2+/Na+ and Mg2+/Na+ ratios compared to the carbonate and it was found that most of the samples located around the silicate end-member. Evaporite end-member was usually difficult to be constrained due to the diversity of salt rock types, but it is recognized that this end-member was reflected by the lowest Ca2+/Na+ and Mg2+/Na+ ratios. In this study, the molar ratios of Ca2+/Na+ and Mg2+/Na+ for the three end-members were selected as 55 and 15 for carbonates, 0.35 and 0.24 for silicates and 0.17 and 0.02 for evaporites, respectively.
Chemical budget and CO2 consumption
Contributions of different end-members
Contributions of weathering to major cations (Na+, Ca2+ and Mg2+) were estimated followed by the method used by Ollivier et al. (2010), and the chemical budget estimation for other elements (anions and silica) was calculated by using a forward method based on stoichiometry.
The proportions of Ca2+, Mg2+ and Na+ originating from different rock weathering reservoirs are given in Table 3. Overall, in the Songhua River Basin, the dissolved cation load of rivers was dominated by silicate weathering, and specifically 63.2 % of Ca2+, 79.5 % of Mg2+ and 84.4 % of Na+ came from silicates weathering. Carbonates contributed to Ca2+ and Mg2+ with a value of 30.7 and 18.8 % in the dissolved cation load. Evaporites weathering contribute 6.2 % of Ca2+, 1.7 % of Mg2+ and 15.3 % of Na+.
The contributions of atmospheric inputs, anthropogenic inputs, and rock weathering (sum of carbonates, silicates and evaporites) to the total TDS for all the stations were shown in Fig. 9. Here, chemical budget calculated according to the hydrochemistry data at the Jiamusi station (the basin outlet station) was used to give an overall understanding. Generally, rock weathering contributed 91.5 % of the total TDS including 66.4 % from silicates, 16.1 % from carbonates and 9.0 % from evaporites. About 5.3 % of the TDS came from anthropogenic inputs, and atmospheric inputs only contributed 3.2 % of the TDS. The significant dominance of silicate weathering with an average of 66.4 % was quite higher than the global silicate weathering proportions (<40.0 %) (Gaillardet et al. 1999).
Chemical weathering and CO2 consumption rates
Chemical weathering and CO2 consumption rates of silicates and carbonates were estimated by mass budget, surface area and runoff of the basin. The calculation method of silicates weathering (R sil) and carbonates weathering (R carb) rates were followed from the work of Xu and Liu (2010). Because no data for K was available, the equation was slightly modified by eliminating the K item as follows:
where X sil (X refers to Na, Ca, Mg and SiO2) is the composite came from silicate weathering and Y carb (Y refers to Ca, Mg and HCO3 −) is the composite came from carbonate weathering. Q annual is the annual runoff and A is the draining area of the station.
The equations describing the calculation of CO2 consumption rates of silicates (ΦCO2sil) and carbonates (ΦCO2carb) were shown as follows.
where the symbols are the same with the above equations.
The results of chemical weathering and CO2 consumption rates for main channel and tributaries, grouped based on lithology, are listed in Table 4. In the Songhua River basin, the average silicate (R sil) and carbonate weathering (R carb) rates at the river outlet station Jiamusi were 4.03 and 1.76 t km−2 year−1, respectively; and the corresponding CO2 consumption rates for silicates and carbonates were 17.1 × 104 and 1.85 × 104 mol km−2 year−1. It is obvious that lithology plays an important role in chemical weathering by comparing chemical weathering rates among the tributaries with different lithology (Table 4). Igneous rocks had the highest R sil with an average of 7.61 t km–2 year−1 than other rocks. Quaternary sediment had the highest R carb with an average of 4.19 t km−2 year−1. The area covered by sandstones showed the lowest R carb with an average of 0.50 t km−2 year−1 due to carbonates deficiency. In addition, the climatic influence (here mainly refer to runoff) to the silicate weathering in the Songhua River basin was illustrated by Fig. 10. The log–log correlation plot gave a positive correlation trend between Rsil and runoff, which agreed with the silicate weathering rate law of other world rivers.
In comparison with other Chinese rivers (Table 5), for R sil, the Songhua River was the same as the Lancang Jiang (4.1 t km–2 year−1), but lower than most of other Chinese rivers such as the Changjiang, the Xijiang and the five rivers originating in the Qingha-Tibet. Only the Huanghe had a lower R sil (3.0 t km−2 year−1) than the Songhua River due to its small runoff. For R carb, the Songhua River was one order of magnitude lower than other Chinese rivers because of little carbonate outcrops in this study area. In addition, cold temperature (3–5 °C) and low annual precipitation (500 mm) also have a strong influence on the rock weathering rate. For the CO2 consumption rates, the Songhua River had a higher ΦCO2sil than the Changjiang, Huanghe, Xijiang and Jinsha Jiang Rivers, but a lower ΦCO2carb than all the Chinese rivers. In addition, compared to the result of the Heilong River (Gaillardet et al. 1999), the Songhua River basin showed a higher silicate and a lower carbonate weathering rate, and this further indicates that the spatial heterogeneity of the lithology and climatic factors such as runoff and temperature have a strong influence on the chemical weathering of rivers.
Conclusions
In this study, the major ions chemistry, chemical weathering rates and CO2 consumption rates in the Songhua River basin were examined. Main conclusions are provided as follows:
-
1.
The Songhua River had a low TDS with a median of 104.8 mg/L, and the prevalent ions were Na+ + K+ and Ca2+ for cations, and HCO3 − for anions. All the ions showed significant seasonal variations with a decrease in wet seasons and an increase in dry seasons. From 1962 to 1984, Ca2+, Na+ + K+ and HCO3 − showed significant increasing trends in the study area. Increasing rates for Ca2+, Na+ + K+ and HCO3 − ions were 0.22, 0.63 and 2.35 mg/year, respectively. No significant long-term trends were detected for other ions.
-
2.
The major ion chemistry was dominated by silicate dissolution in the Songhua River basin. The average Ca2+/Na+ and Mg2+/Na+ molar ratios were 0.70 and 0.42, respectively, which were close to silicate weathering end-member. Generally, rock weathering contributed 91.5 % of the total TDS including 66.4 % from silicates, 16.1 % from carbonates and 9.0 % from evaporites. About 5.3 % of the TDS can be attributed to anthropogenic inputs, and atmospheric inputs only contributed 3.2 % of the TDS in the Songhua River basin.
-
3.
The average silicate (Rsil) and carbonate weathering (Rcarb) rates of the Songhua River basin were 4.03 and 1.76 t km−2 year−1, respectively. The CO2 consumption rates of silicate and carbonate were 17.1 × 104 and 1.85 × 104 mol km−2 year−1, respectively. Both of the weathering and CO2 consumption rates for carbonates were lower than other Chinese Rivers.
References
Appelo C, Postma D (2005) Geochemistry, groundwater and pollution. Taylor & Francis, London
Berner RA (1991) A model for atmospheric CO2 over phanerozoic time. Am J Sci 291(4):339–376
Berner RA, Lasaga AC, Garrels RM (1983) The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am J Sci 283(7):641–683
Chen J, Wang F, Xia X, Zhang L (2002) Major element chemistry of the Changjiang (Yangtze River). Chem Geol 187(3–4):231–255
Edmond JM (1992) Himalayan tectonics, weathering processes, and the strontium isotope record in marine limestones. Science (New York, NY) 258(5088):1594–1597
Gaillardet J, Dupre B, Allegre CJ, Négrel P (1997) Chemical and physical denudation in the Amazon River Basin. Chem Geol 142(3):141–173
Gaillardet J, Dupré B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159(1):3–30
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Gibbs RJ (1972) Water chemistry of the Amazon River. Geochim Cosmochim Acta 36(9):1061–1066
Gordeev VV, Sidorov IS (1993) Concentrations of major elements and their outflow into the Laptev Sea by the Lena River. Mar Chem 43(1–4):33–45
Hirsch RM, Slack JR (1984) A nonparametric trend test for seasonal data with serial dependence. Water Resour Res 20(6):727–732
Li S, Xu Z, Wang H, Wang J, Zhang Q (2009) Geochemistry of the upper Han River basin, China: 3 Anthropogenic inputs and chemical weathering to the dissolved load. Chem Geol 264(1–4):89–95
Li Y, Yu X, Cheng H, Lin W, Tang J, Wang S (2010) Chemical characteristics of precipitation at three Chinese regional background stations from 2006 to 2007. Atmos Res 96(1):173–183
Meybeck M, Helmer R (1989) The quality of rivers: from pristine stage to global pollution. Global Planet Change 1(4):283–309
Negrel P, Allegre CJ, Dupre B, Lewin E (1993) Erosion sources determined by inversion of major and trace element ratios and strontium isotopic ratios in river water: the Congo Basin case. Earth Planet Sci Lett 120(1–2):59–76
Ollivier P, Hamelin B, Radakovitch O (2010) Seasonal variations of physical and chemical erosion: a three-year survey of the Rhone River (France). Geochim Cosmochim Acta 74(3):907–927
Ran X, Yu Z, Yao Q, Chen H, Mi T (2010) Major ion geochemistry and nutrient behaviour in the mixing zone of the Changjiang (Yangtze) River and its tributaries in the Three Gorges Reservoir. Hydrol Process 24(17):2481–2495
Raymo ME, Ruddiman WF (1992) Tectonic forcing of late Cenozoic climate. Nature 359(6391):117–122
Song X, Mu X, Gao P, Wang F, Wang S (2010) Analysis on historical evolution and driving force of rainfall and runoff of Harbin Station in Songhua River. Sci Soil Water Conserv 8(2):46–51
Stallard RF, Edmond JM (1981) Geochemistry of the Amazon 1 Precipitation chemistry and the marine contribution to the dissolved load at the time of peak discharge. J Geophys Res 86(C10):9844–9858
Walker JCG, Hays PB, Kasting JF (1981) A negative feedback mechanism for the long-term stabilization of the Earth’s surface temperature. J Geophys Res 86(C10):9776–9782
Wu W, Xu S, Yang J, Yin H (2008) Silicate weathering and CO2 consumption deduced from the seven Chinese rivers originating in the Qinghai-Tibet Plateau. Chem Geol 249(3–4):307–320
Xu Z, Liu CQ (2010) Water geochemistry of the Xijiang basin rivers, South China: chemical weathering and CO2 consumption. Appl Geochem 25(10):1603–1614
Zhang J, Huang W, Letolle R, Jusserand C (1995) Major element chemistry of the Huanghe (Yellow River), China-weathering processes and chemical fluxes. J Hydrol 168(1–4):173–203
Zhang S, Lu X, Higgitt DL, Chen C, Sun H, Han J (2007) Water chemistry of the Zhujiang (Pearl River): natural processes and anthropogenic influences. J Geophys Res 112(F1):F01011
Zhang L, Song X, Xia J, Yuan R, Zhang Y, Liu X, Han D (2011) Major element chemistry of the Huai River basin China. Appl Geochem 26(3):293–300
Acknowledgments
This work was funded by the Main Direction Program of Knowledge Innovation of Chinese Academy of Sciences: Study on water cycle, water resource carrying capacity and optimization configuration (No. KZCX2-YW-Q06-1). The authors wish to thank Dr. Long Di for the suggestions to this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, Y., Tang, C., Song, X. et al. Major ion chemistry, chemical weathering and CO2 consumption in the Songhua River basin, Northeast China. Environ Earth Sci 73, 7505–7516 (2015). https://doi.org/10.1007/s12665-014-3921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3921-2