Abstract
Exogenous application of methyl jasmonate (MeJA) could activate plant defense response against the two-spotted spider mite (TSSM), Tetranychus urticae Koch, in different plants. However, whether MeJA can also serve as an elicitor in cassava (Manihot esculenta Crantz) remains unknown. In this study, induced defense responses were investigated in TSSM-resistant cassava variety C1115 and TSSM-susceptible cassava variety KU50 when applied with MeJA. The performance of TSSM feeding on cassava plants that were pre-treated with various concentrations of MeJA was first evaluated. Subsequently, the activities of antioxidative enzymes (superoxide dismutase and catalase), detoxification enzymes (glutathione S-transferase, cytochrome P450 and carboxylesterase) and digestive enzymes (protease, amylase and invertase) in TSSM were analyzed at days 1, 2, 4 and 8 post-feeding. The results showed that MeJA treatment can induce cassava defense responses to TSSM in terms of reducing egg production and adult longevity as well as slowing development and prolonging the egg stage. Noticeably, C1115 exhibited stronger inhibition of TSSM development and reproduction than KU50. In addition, the activities of all the tested enzymes were induced in both C1115 and KU50, the most in C1115. We conclude that exogenous methyl jasmonate can induce cassava defense responses and enhance resistance to TSSM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cassava (Manihot esculenta Crantz) is the sixth most cultivated food crop for more than 800 million people in tropical and subtropical regions (Amelework and Bairu 2022; Otun et al. 2022; Sun et al. 2022). In South Africa, cassava is mainly cultivated for its large starchy roots and is the second most staple food crop after maize (Amelework and Bairu 2022; Rauwane et al. 2018). Though cassava is not served as staple food crop in China, it is recognized as energy crop for producing bioethanol (Jiang et al. 2019). In addition, cassava starch can be used in medicine, cosmetic formulations, paper, and beer industries (Edhirej et al. 2017; Panghal et al. 2021). In 2020, cassava planting area in China was 300 thousand hectares with a production of 4.87 million metric tons (FAOSTAT 2022). The cassava mite is great threat to cassava production. It has been reported that approximately 45 herbivorous mites may infest cassava plant in Americas, Africa and Asia (Zou et al. 2018), which cause enormous reduction of root yield, stem yield and leaf weight (Jiwuba et al. 2020; Ovalle et al. 2020). The two-spotted spider mite (TSSM), Tetranychus urticae Koch (Acari: Tetranychidae), is an important agricultural pest that can infect more than one thousand plant species (Grbić et al. 2011). TSSM can cause cassava leaves to form green and yellow spots and further reduce photosynthetic activity, in addition, the severe TSSM infestation may result in leaf defoliation, growth arrest and even death (Golan et al. 2021; Lu et al. 2021). Controlling TSSM with synthetic acaricides is restrained by the short life cycle, high fecundity, and the risk of resistance developments (Jakubowska et al. 2022; Namin et al. 2020). Hence, seeking for alternative control strategies is quite necessary.
Plant hormones play remarkable role in modulating multiple developmental processes and coping with abiotic and biotic stress. Jasmonates (JAs) such as jasmonic acid (JA), jasmonoyl-isoleucine (JA-Ile) and methyl jasmonate (MeJA) are important phytohormones, these chemicals can activate the JA-mediated signaling pathway and participate in plant defense (Dar et al. 2015; Ruan et al. 2019; Wang et al. 2021). MeJA is the volatile form of JA, since Farmer and Ryan (1990) reported that exogenous MeJA induced defense response in plants, application of MeJA on host plant to improve the resistance against insect herbivores had received extensive research attention. For example, foliar application of MeJA on rice caused higher mortality of second instar larva and adult of rice leaf folder Cnaphalocrocis medinalis (Senthil-Nathan 2019). Another study showed that MeJA treatment of young Norway spruce (Picea abies) plants reduced the number and size of feeding scars which were caused by pine weevils (Hylobius abietis) (Fedderwitz et al. 2016). Partial spray of MeJA on tomato and cabbage significantly reduced the larval growth performance of Spodoptera litura (Sripontan and Hwang 2016). Additionally, the feeding area of Monolepta hieroglyphica decreased by 17%–43% when fed on MeJA-treated Rosa rugosa leaves (Yan et al. 2021). Moreover, several studies indicated that the effect of exogenous MeJA on triggering plant defense was highly correlated to plant variety. After pre-treated by JAs, the defense responses were induced in both resistant and susceptible groundnut genotypes while they were exposed to Helicoverpa armigera (War et al. 2011a, 2011b, War and Sharma 2014) or S. litura (War et al. 2015), and the resistant ones presented better induction. On the contrary, other studies demonstrated that for certain plant species, the extent of MeJA-derived defense activation was not related to the resistance level, cases could be seen in the interactions between Norway spruce and pine weevil Hylobius abietis (Puentes et al. 2021), and apple tree and TSSM (Warabieda and Olszak 2010).
Nevertheless, how different cassava varieties react to TSSM while applying with MeJA remains unknown. In addition, in order to decipher how the MeJA-treated plants acquire better tolerance or resistance against insect pest, considerable efforts were focused on the defensive related physiological and biochemical alteration in plant, such as defense enzymes activities (Yan et al. 2021), secondary metabolites (Erbilgin et al. 2006; Sripontan and Hwang 2016), proteinase inhibitors content (Rohwer and Erwin 2010; Zhang et al. 2015) and volatile organic compounds (VOCs) which can attract natural enemies (Puentes et al. 2021). In comparison, limited attention was paid on exploring the internal change in insect pest. To fill this knowledge gap, in this study, a TSSM-resistant and a TSSM-susceptible cassava variety were pre-treated with different concentrations of MeJA, and the effects on development and reproduction, as well as activities of several enzyme systems including antioxidative enzymes, detoxification enzymes and digestive enzymes were evaluated. The purpose of the study was to clarify whether varietal differences of defense induction exist while cassava undergo MeJA treatment, and shed light on the possibility of MeJA application for TSSM management in cassava production.
Materials and methods
TSSM rearing and plant material cultivation
TSSM were reared on cowpea plants (Reke1#) in a growth chamber under a L16:D8 h photoperiod, 26 ± 2 °C and 65 ± 5% relative humidity. The TSSM-resistant cassava variety C1115 and the susceptible cassava variety KU50 were supplied by the National Cassava Germplasm Nursery of China, Chinese Academy of Tropical Agricultural Sciences (CATAS). Uniform cuttings of healthy cassava stems (20 cm in length) were planted into small plastic pots (18 cm width, 18 cm length, 20 cm height) with a 1:1 mixture of peat (Pindstrup Mosebrug A/S, Denmark) and vermiculite. Cassava seedlings were watered three times a week and cultured in greenhouse. Seedlings were grown for 90 days and then used for experiments.
MeJA treatment
The 1,089 μL of MeJA was fist dissolved in the mixture of 10 mL of ethanol and 0.5 mL of Tween-20, and then the mixture was diluted to 1 L of distilled water to prepare the 5.0 mM MeJA stock solution. Furthermore, the stock solution (5.0 mM) was diluted successively with distilled water containing 0.05% Tween-20 (vol/vol) to form the rest four MeJA solutions such as 1.0, 0.2, 0.04 and 0.008 mM (Bhavanam and Stout 2021). The control solution was distilled water containing 0.05% Tween-20 and 1% ethanol (vol/vol).
Cassava seedlings (90 days after planting, approximately 80–100 cm height) were sprayed with MeJA or control solution using a 100 mL pressurized hand sprayer until runoff. Every single sprayed cassava seedling was immediately covered with a sealed transparent plastic box (60 × 60 × 120 cm). After 6 h treatment, the sealed plastic box was removed and to allow the wetted leaves to dry for another 2 h. The plants treated with identical dose of MeJA were placed in separate growth chambers to minimize the interruption effects of volatile MeJA.
Bioassays with TSSM
To evaluate the effect of MeJA-treated cassava seedlings on population growth and fecundity of TSSM, six treatments were set up including five concentrations (5.0, 1.0, 0.2, 0.04 and 0.008 mM) of MeJA solution and control. Cassava seedlings were treated following the methods described above. After the cassava plant were pre-treated by MeJA for 8 h, three adult mites were carefully transferred to the leaf with a bristle brush. Once the egg hatched, only one newborn larva was remained on each leaf and the rest mites and eggs were all removed. Petiole of each inoculated leaf was smeared with lanolin to prevent mites from escaping. Each MeJA-treated concentration as well as control was performed on five cassava plants (5 replicates), in addition, six fully expanded leaves in the middle of each cassava plant (7th, 8th, 9th 10th 11th and 12th above from the bottom leaf) were selected for inoculating TSSM as described above, which was 30 leaves in total for each treatment (6 leaves × 5 cassava seedlings). The developmental duration (total pre-adult period) and adult longevity of TSSM were recorded. Additionally, the number of eggs was counted every 12 h to assess egg production. When the TSSM adult began to spawn, the first batch of freshly laid eggs (within 24 h) were transferred on to a small leaf disk (cut from cowpea Reke1# leaf with a hole pincher) and the egg stage (incubation period) were recorded.
Enzymatic activity assay
Cassava seedlings were pre-treated with 0.2 mM MeJA solution and control solution as described in Section “MeJA treatment”. Three fully expanded mature leaves from the middle of each cassava plant (10th 11th and 12th above from bottom leaf) were selected, and fifty adult TSSM were carefully transferred to one single leaf. Besides, petioles of inoculated leaves were smeared with lanolin to prevent mites from escaping. After the cassava plants were infested by TSSM for 1, 2, 4 and 8 days, all the inoculated adult mites were collected in 1.5-mL centrifuge tubes. Here, for each sampling time, mites from three cassava plants (150 mites from one plant represented one replicate) were considered as a treatment.
The TSSM sample was homogenized in 1 mL of 0.01 M of ice-cold PBS (pH 7.2–7.4) using a small handheld homogenizer. The homogenate was then centrifuged at 8000×g for 10 min at 4 °C. The supernatant was used directly as the crude extract for enzymatic determination. Activities of antioxidative enzymes (superoxide dismutase (SOD) and catalase (CAT)), detoxification enzymes (glutathione S-transferase (GSTs), cytochrome P450 (CYP450) and carboxylesterase (CarE)) and digestive enzymes (protease, amylase and invertase) were all determined according to the ELISA kit instructions (Shanghai Enzyme-linked Biotechnology Co. Ltd, Shanghai, China). The detection principle and operation method of each kit was similar, in which the Sandwich ELISA (monoclonal antibody of insect enzyme-tested aphid enzyme-horseradish peroxidase (HRP) labeled insect enzyme complex) was used. A 50 μL aliquot (10 μL of tested enzyme solution + 40 μL of supplied sample diluent) was transferred to the antibody-coated 96-well plate and the enzymatic activity was performed as described by the manufacturer's protocol: the plate was first incubated at 37 ℃ for 30 min, then the plate was washed by the washing solution for five times, after that, 50 μL of HRP-labeled antibody was added to the sample well, and then repeated the incubation and washing step. After washing, 100 μL of TMB (Tetramethylbenzidine) chromogen solution was added as substrate and incubated for another 10 min at 37 ℃. Finally, 100 μL of stop solution was added and incubated at 37 ℃ for 15 min, and the optical density (OD) value was measured using the Tecan Multimode Reader Platform (Spark M200, Grading, Austria) with the wavelength of 450 nm. The linear standard curve was established (using the standard solution within the kit, the series of standard activities of the standard solutions (U/L) were set as ‘X’, and the corresponding OD values were set as ‘Y’) in parallel with the tested samples. The activity of each enzyme was presented as U/L (calculated via the standard curve).
Statistical analysis
The data were analyzed using GraphPad Prism v.8.0 (GraphPad Software, San Diego, CA, USA) and IBM SPSS v.19.0 (IBM, Armonk, NY, USA) after checking for normality and homogeneity of variance. If necessary, data were transformed to normalize the error variances using log or square-root transformation. The effects of MeJA treatments on egg production, adult longevity, egg stage, and development duration were analyzed using one-way ANOVA. Statistical analysis of enzyme activity between treatment and control within the same day was based on Student’s t-test with the level of significance α = 0.05. To better interpret the effect of variety and MeJA application on the performance and enzyme activity of TSSM, a two-factor multivariate analysis of variance (MANOVA) was further conducted, with MeJA treatment and cassava variety as factors and egg production, adult longevity, development duration or egg stage as dependent response variables. For enzymatic data, variety and treatment time were set as factors and activities of antioxidative, detoxification or digestive enzymes as dependent response variables.
Results
Mite performance on MeJA-treated cassava seedlings
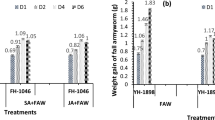
The egg productions of TSSM fed on MeJA-treated C1115 and KU50 cassava plants presented a decreasing trend when compared with their corresponding controls (Fig. 1A). In particular, the high concentrations (i.e., 0.2, 0.1 and 5 mM) (F5,30 = 21.30, P < 0.01) exhibited significantly stronger suppression effect on fecundity than the control and low concentrations and (i.e., 0.008 and 0.04 mM) (F5,30 = 43.79, P < 0.01). Moreover, the MANOVA on egg production indicated significant effects of MeJA treatment (F5,60 = 52.03) and variety (F1,60 = 46.09, both P < 0.01). The MeJA treatment × variety interaction effect was not significant (F5,60 = 0.30, P = 0.91) (Table 1). In addition, the fecundity reduction rates were ranged from 4.13 to 22.99% in C1115, which were higher than those of KU50 (from 2.91 to 21.81%), indicating that the TSSM-resistant variety C1115 showed better inhibition of egg production compared with the TSSM-susceptible variety KU50.
The adult longevity of TSSM fed on MeJA-treated C1115 and KU50 cassava plants also presented a decreasing trend when compared with their corresponding controls (Fig. 1B). Similarly, the high concentrations (i.e., 0.2, 0.1 and 5 mM) (F5,30 = 30.28, P < 0.01) could better shorten the adult longevity compared with the control and low concentrations (i.e., 0.008 and 0.04 mM) (F5,30 = 24.11, P < 0.01). MANOVA on longevity indicated significant effects of MeJA treatment (F5,60 = 51.82, P < 0.01), whereas the variety (F1,60 = 0.49, P = 0.49) as well as MeJA treatment × variety interaction (F5,60 = 1.79, P = 0.13) effects were not significant (Table 1). These results demonstrated that MeJA application may decrease the TSSM adult longevity, but would not differentiate the performance when fed on resistant or susceptible variety.
The development durations of TSSM fed on MeJA-treated C1115 (F5,30 = 5.37) and KU50 (F5,30 = 20.25, both P < 0.01) cassava plants were significantly prolonged when compared with their corresponding controls (Fig. 1C). MANOVA on development duration indicated significant effects of MeJA treatment (F5,60 = 14.48) and variety (F1,60 = 20.03, P < 0.01). The MeJA treatment × variety interaction effect was not significant (F5,60 = 0.28, P = 0.92) (Table 1). In addition, the duration increasing rates were ranged from 5.18 to 13.46% in C1115, which were higher than those of KU50 (from 4.86 to 10.52%), indicating that the TSSM-resistant variety C1115 slowed development more than the TSSM-susceptible variety KU50. Similar results were seen for the egg stage: significant effects of MeJA treatment (F5,60 = 68.63) and variety (F1,60 = 4.62, P = 0.036) were observed on egg stage, but their interaction effect was not significant (F5,60 = 0.23, P = 0.95) (Table 1). Additionally, MeJA treatment significantly prolonged the egg stage on both C1115 (F5,30 = 48.64) and KU50 (F5,30 = 26.54, both P < 0.01) and C1115 rendered better performance (Fig. 1D).
In summary, the higher concentration exhibited better performance in hindering the normal development and reproduction of TSSM, on the other hand, for either C1115 or KU50, 0.2, 1.0 and 5.0 mM of MeJA were the concentrations that significantly suppressed the fecundity and longevity, and significantly prolonged the development duration and egg stage, besides, there was no statistically difference among those three concentrations, therefore, we finally chose 0.2 mM as the optimal concentration for further enzymatic assay.
Activities of antioxidative enzymes in TSSM fed on MeJA-treated cassava seedlings
As Fig. 2 depicts, when fed on MeJA-treated C1115 or KU50, the activities of SOD (Fig. 2A) and CAT (Fig. 2B) in TSSM were all significantly increased (all P < 0.01) compared with their corresponding controls (enzyme activities from TSSM fed on MeJA-free plant at the same sampling time). More specifically, the activities of SOD in TSSM fed on KU50 and C1115 increased by 2.2-, 3.5-, 2.9- and 5.4-fold, and 2.4-, 5.0-, 9.6- and 5.2-fold, respectively, when compared with controls (corresponding to 1, 2, 4 and 8 days post treatment (dpt), respectively) (see the embedded panel in Fig. 2A), besides, the activities of CAT in TSSM fed on KU50 and C1115 increased by 17.3-, 9.1-, 7.9- and 16.3-fold, and 16.0-, 21.9-, 78.7- and 36.2-fold, respectively, when compared with controls (Fig. 2B). MANOVA on fold change of enzyme activity indicated significant effects of TSSM infestation time, variety and their interaction on SOD and CAT activity (Table 2).
Effects of methyl jasmonate (MeJA)-treated cassava seedlings on the antioxidative enzyme activities of two-spotted spider mite at 1, 2, 4, and 8 days after treatment. * and ** indicates significant differences between control and MeJA treatment at 0.05 and 0.01 levels, respectively. A SOD (superoxide dismutase); B CAT (catalase peroxidase). Embedded figures represent the fold change of enzyme activity for the two cassava varieties
Activities of detoxification enzymes in TSSM fed on MeJA-treated seedlings
As depicted in Fig. 3, when fed on MeJA-treated C1115 or KU50, the activities of CYP450 (Fig. 3A), GSTs (Fig. 3B) and CarE (Fig. 3C) in TSSM were all significantly increased (all P < 0.01) compared with their corresponding controls (enzyme activities from TSSM fed on MeJA-free plant at the same sampling time) except GSTs activity (KU50) at 1 dpt. More specifically, the activities of CYP450 in TSSM fed on KU50 and C1115 increased by 3.7-, 5.1-, 12.3- and 12.1-fold, and 13.2-, 6.4-, 24.6- and 71.3-fold, respectively, when compared with controls (corresponding to 1, 2, 4 and 8 dpt, respectively) (see the embedded panel in Fig. 3A). The activities of GSTs in TSSM fed on KU50 and C1115 increased by 1.2-, 1.7-, 1.9- and 1.6-fold, and 2.0-, 2.4-, 2.2- and 2.7-fold, respectively, when compared with controls (Fig. 3B), besides, the activities of CarE in TSSM fed on KU50 and C1115 increased by 4.8-, 3.3-, 9.4- and 5.7-fold, and 6.2-, 28.9-, 20.9- and 36.0-fold, respectively, when compared with controls (Fig. 3C). MANOVA on fold change of enzyme activity indicated significant effects of TSSM infestation time, variety and their interaction on CYP450, GSTs and CarE activity (Table 2).
Effects of methyl jasmonate (MeJA)-treated cassava seedlings on the detoxification enzyme activities of two-spotted spider mite at 1, 2, 4, and 8 days after treatment. * and ** indicates significant differences between control and MeJA treatment at 0.05 and 0.01 levels, respectively. A CYP450 (cytochrome P450); B GSTs (glutathione S-transferase); C CarE (carboxylesterase). Embedded figures represent the fold change of enzyme activity for the two cassava varieties
Activities of digestive enzymes in TSSM fed on MeJA-treated seedlings
As depicted in Fig. 4, when fed on MeJA-treated C1115 or KU50, the activities of AMY (Fig. 4A), PRO (Fig. 4B) and INV (Fig. 4C) in TSSM were all significantly upregulated (all P values < 0.01) compared with their corresponding controls (enzyme activities from TSSM fed on MeJA-free plant at the same sampling time) except INV activity (KU50) at 2 dpt. More specifically, the activities of AMY in TSSM fed on KU50 and C1115 increased by 2.6-, 1.9-, 1.7- and 2.6-fold, and 3.0-, 2.9-, 3.7- and 2.1-fold, respectively, when compared with controls (corresponding to 1, 2, 4 and 8 dpt, respectively) (see the embedded panel in Fig. 4A). The activities of PRO in TSSM fed on KU50 and C1115 increased by 1.9-, 2.4-, 2.4- and 2.1-fold, and 4.7-, 3.4-, 4.4- and 2.4-fold, respectively, when compared with controls (Fig. 4B), besides, the activities of INV in TSSM fed on KU50 and C1115 increased by 1.2-, 1.1-, 1.3- and 1.5-fold, and 1.4-, 1.5-, 1.9- and 3.2-fold, respectively, when compared with controls (Fig. 4C). MANOVA on fold change of enzyme activity indicated significant effects of TSSM infestation time, variety and their interaction on AMY, PRO and INV activity (Table 2).
Effects of methyl jasmonate (MeJA)-treated cassava seedlings on the digestive enzyme activities of two-spotted spider mite at 1, 2, 4, and 8 days after treatment. * and ** indicates significant differences between control and MeJA treatment at 0.05 and 0.01 levels, respectively. A AMY (amylase); B PRO (protease); C INV (invertase). Embedded figures represent the fold change of enzyme activity for the two cassava varieties
Discussion
MeJA application could activate plant defense response against TSSM, this phenomenon was demonstrated in several different plants (Montazersaheb et al. 2021; Rohwer and Erwin 2010; Warabieda and Olszak 2010). By contrary, whether MeJA also possesses identical effect on cassava remains unknown. In present study, both a TSSM-resistant variety C1115 and a TSSM-susceptible variety KU50 treated with MeJA presented negative effects on TSSM in terms of reducing egg production and adult longevity as well as prolonging development duration and egg stage. Our results are in agreement with a previous study demonstrating prolongation of protonymph, deutonymph and total pre-adult period of TSSM, while they fed on MeJA-treated kidney bean (Montazersaheb et al. 2021). Another study stated that MeJA application can reduce TSSM proliferation rate on impatiens and pansy (Rohwer and Erwin 2010). Moreover, there was a dose–effect relationship between herbivore performance and MeJA concentration, as the effect that different concentrations of MeJA posed distinct induced resistance to insect herbivores had been well documented (Puentes et al. 2021; Yang et al. 2013; Zhang et al. 2015). In this study, five MeJA concentrations (0.008, 0.04, 0.2, 1.0 and 5.0 mM) were tested, and the mites performed more poorly while fed on cassava plant that treated with high concentrations, i.e., 0.2, 1.0 and 5.0 mM, additionally, there was no statistically difference among these three concentrations. Furthermore, several studies indicated that improper use of MeJA could cause potential phytotoxicity to plants, i.e., negative impacts on plant growth and flowering, reducing yield (Bhavanam and Stout 2021; Boughton et al. 2006; Kraus and Stout 2019), taking the above factors into account, 0.2 mM was selected as the optimal concentration for the following enzyme assays.
Plants treated with MeJA will induce many defensive components, such as plant defensive proteins, protective enzymes and toxic secondary metabolites, which presents negative effect on the feeding, growth and development of insect herbivory (Puentes et al. 2021; Sripontan and Hwang 2016; Wei et al. 2021; Yan et al. 2021). Correspondingly, insect herbivory usually adjust their feeding habits (Cao et al. 2014), digestive physiology (War and Sharma 2014), host selection (Sanches et al. 2017; Slesak et al. 2001) and oviposition behavior (Disi et al. 2017) to protect them from stress damage. While applying MeJA as elicitor, previous studies basically focused on the enhanced defensive response in plant, or the visible performance change of insect pest, nevertheless, the internal biochemical processes in herbivory attracted less attention. Therefore, this study aims to the explore the activity alternations of antioxidative, detoxification and digestive enzymes in TSSM.
SOD, CAT and POD are important antioxidative enzymes in insects involved in protecting the organism against high concentrations of reactive oxygen species (ROS) (Zhang et al. 2014). SOD convert O2− into H2O2 and O2. CAT and POD are activated to catalyze the H2O2 into H2O and O2 (Felton and Summers 1995; Lushchak 2014; Lyakhovich et al. 2006). The present study demonstrated that TSSM fed on MeJA-treated C1115 and KU50 showed significant elevation of SOD and CAT activities than controls. Our results are in line with a previous study in which Clostera anachoreta larvae fed on MeJA-treated Populus showed increased SOD and CAT activities (Tianzi et al. 2018). The elevation in antioxidative enzyme activity suggested that feeding on MeJA-treated cassava produced excessive ROS in TSSM. The MeJA treatment activated the antioxidative enzymes of TSSM were responsible for detoxifying ROS and alleviating oxidative damage.
CYP450, GSTs, and CarE are important detoxication enzymes involved in metabolism of synthetic pesticides and plant allelochemicals via oxidation, reduction, hydrolysis, sequestration and conjugation (Després et al. 2007; Heidel-Fischer and Vogel 2015). In present study, the CYP450, GSTs and CarE activities in TSSM fed on MeJA-treated C1115 and KU50 were all up-regulated. These increased enzyme activities may attribute to xenobiotics defense and normal physiological function maintenance. These results are consistent with a previous study in which C. anachoreta larvae fed on MeJA-treated Populus showed increased GSTs and CarE activities (Tianzi et al. 2018). By contrast, the study conducted by Yang et al (2022) indicated that cotton bollworm feeding on JA-treated cotton plant presented lower GSTs, CYP450 and CarE activities compared with control. In addition, the activities of CarE and GSTs in adult Monolepta hieroglyphica were significantly inhibited when fed on MeJA-treated Rosa rugosa (Yan et al. 2021). We assumed the inhibition of detoxication enzyme activity might be due to the overwhelmed antioxidant system by the greater production of poisonous phytochemicals.
MeJA-mediated plant defense has been reported in many plant species involved in the induction activity of some anti-nutritional or anti-digestive proteins (Chen et al. 2005; Sun et al. 2011).The activity change of digestive enzymes in insect pest may reflect the palatability of the host plant. It is reported that digestive enzyme activities of Spodoptera littoralis on various mung bean cultivars may reveal the potential tolerance traits (Fard et al. 2022). Here, the activities of digestive enzymes in TSSM were also induced when fed on MeJA-treated C1115 and KU50. It seems likely that the TSSM increased digestive enzyme activities to cope with the alteration of nutrients contents and types.
The induction of plant defense by MeJA treatment exhibited a plant variety-dependent pattern. Studies illustrated that the MeJA-treated insect-resistant plant varieties performed greater defensive induction compared with the susceptible ones, examples can be related to the interactions between groundnut genotypes and moths (War et al. 2011a, b, 2015, War and Sharma 2014), in addition, the authors speculated that the stronger induction of defensive chemicals and proteins, for instances, secondary metabolites like flavonoid, tannins and phenols, protective enzymes such as peroxidase and polyphenol oxidase, and other defensive compounds such as malondialdehyde and hydrogen peroxide, were attributed to the better apply effect on the resistant plant (War and Sharma 2014; War et al. 2015). However, the studies conducted on apple tree (Warabieda and Olszak 2010) and spruce (Puentes et al. 2021) offered different results, in which the induced performance was basically equal for either resistant and susceptible plant varieties. These diverse results mentioned above suggest the necessity to reveal whether varietal-difference exists in cassava while applying MeJA. By employing two-factor MANOVA (including variety as a factor), the significant effect of variety on the MeJA apply effect was demonstrated. We found that the resistant variety C1115 can better inhibit the development and reproduction of TSSM, moreover, several defense-related enzymes were induced to significant higher level, in comparison with the susceptible variety KU50. In a recent study, five years of field experiments found that without applying any plant protection agents, C1115 can effectively reduce the TSSM population and leaf infestation, and maintain more than 60% of the yield, while KU50 suffered serious damage and yield loss (Liang et al. 2022). The present study provides promising prospect in combining used of resistant variety and plant elicitor to acquire better TSSM control effect. However, more effort should be devoted to verify this potential synergistic effect, besides, whether MeJA application may result in yield reduction should also be examined.
In conclusion, exogenous application of MeJA induced cassava defense response against TSSM, besides, the TSSM-resistant cassava variety C1115 possessed better capability in resistance strengthening than the TSSM-susceptible variety KU50. In addition, this study can elucidate the MeJA-mediated plant defense activation mechanism from physiological and biochemical perspectives of insect pest.
References
Amelework AB, Bairu MW (2022) Advances in genetic analysis and breeding of cassava (manihot esculenta crantz): a review. Plants 11:1617. https://doi.org/10.3390/plants11121617
Bhavanam S, Stout M (2021) Seed treatment with jasmonic acid and methyl jasmonate induces resistance to insects but reduces plant growth and yield in rice. Oryza Sativa. Front Plant Sci 12:691768. https://doi.org/10.3389/fpls.2021.691768
Boughton AJ, Hoover K, Felton GW (2006) Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol Exp Appl 120:175–188. https://doi.org/10.1111/j.1570-7458.2006.00443.x
Cao HH, Wang SH, Liu TX (2014) Jasmonate- and salicylate-induced defenses in wheat affect host preference and probing behavior but not performance of the grain aphid, Sitobion avenae. Insect Sci 21:47–55. https://doi.org/10.1111/1744-7917.12023
Chen H, Wilkerson CG, Kuchar JA, Phinney BS, Howe GA (2005) Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. P Natl Acad Sci 102:19237–19242. https://doi.org/10.1073/pnas.0509026102
Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H (2015) Jasmonates counter plant stress: a review. Environ Exp Bot 115:49–57. https://doi.org/10.1016/j.envexpbot.2015.02.010
Després L, David JP, Gallet C (2007) The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol Evol 22:298–307. https://doi.org/10.1016/j.tree.2007.02.010
Disi JO, Zebelo S, Ngumbi E, Fadamiro HY (2017) Cis-jasmone primes defense pathways in tomato via emission of volatile organic compounds and regulation of genes with consequences for Spodoptera exigua oviposition. Arthropod-Plant Inte 11:591–602. https://doi.org/10.1007/s11829-017-9503-y
Edhirej A, Sapuan SM, Jawaid M, Zahari NI (2017) Cassava: its polymer, fiber, composite, and application. Polym Composite 38:555–570. https://doi.org/10.1002/pc.23614
Erbilgin N, Krokene P, Christiansen E, Zeneli G, Gershenzon J (2006) Exogenous application of methyl jasmonate elicits defenses in norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus. Oecologia 148:426–436. https://doi.org/10.1007/s00442-006-0394-3
FAO (2022). https://www.fao.org/faostat/en/#search/Cassava. Accessed 26 June 2022
Fard SZ, Hemmati SA, Shishehbor P, Stelinski LL (2022) Growth, consumption and digestive enzyme activities of Spodoptera littoralis (Boisd) on various mung bean cultivars reveal potential tolerance traits. J Appl Entomol 9:1145–1154. https://doi.org/10.1111/jen.13055
Farmer EE, Ryan CA (1990) Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci 87:7713–7716. https://doi.org/10.1073/pnas.87.19.7713
Fedderwitz F, Nordlander G, Ninkovic V, Björklund N (2016) Effects of jasmonate-induced resistance in conifer plants on the feeding behaviour of a bark-chewing insect, Hylobius abietis. J Pest Sci 89:97–105. https://doi.org/10.1007/s10340-015-0684-9
Felton GW, Summers CB (1995) Antioxidant systems in insects. Arch Insect Biochem 29:187–197. https://doi.org/10.1002/arch.940290208
Golan K, Jurado IG, Kot I, Górska-Drabik E, Kmieć K, Łagowska B, Skwaryło-Bednarz B, Kopacki M, Jamiołkowska A (2021) Defense responses in the interactions between medicinal plants from lamiaceae family and the two-spotted spider mite Tetranychus urticae koch (acari: Tetranychidae). Agronomy 11:438. https://doi.org/10.3390/agronomy11030438
Grbić M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, Osborne EJ, Dermauw W, Thi Ngoc PC, Ortego F, Hernández-Crespo P, Diaz I, Martinez M, Navajas M, Sucena É, Magalhães S, Nagy L, Pace RM, Djuranović S, Smagghe G, Iga M, Christiaens O, Veenstra JA, Ewer J, Villalobos RM, Hutter JL, Hudson SD, Velez M, Yi SV, Zeng J, Pires-daSilva A, Roch F, Cazaux M, Navarro M, Zhurov V, Acevedo G, Bjelica A, Fawcett JA, Bonnet E, Martens C, Baele G, Wissler L, Sanchez-Rodriguez A, Tirry L, Blais C, Demeestere K, Henz SR, Gregory TR, Mathieu J, Verdon L, Farinelli L, Schmutz J, Lindquist E, Feyereisen R, Van de Peer Y (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492. https://doi.org/10.1038/nature10640
Heidel-Fischer HM, Vogel H (2015) Molecular mechanisms of insect adaptation to plant secondary compounds. Curr Opin Insect Sci 8:8–14. https://doi.org/10.1016/j.cois.2015.02.004
Jakubowska M, Dobosz R, Zawada D, Kowalska J (2022) A review of crop protection methods against the twospotted spider mite—Tetranychus urticae Koch (Acari: Tetranychidae)—with special reference to alternative methods. Agriculture 12:898. https://doi.org/10.3390/agriculture12070898
Jiang D, Hao MM, Fu JY, Tian GJ, Ding FY (2019) Estimating the potential of energy saving and carbon emission mitigation of cassava-based fuel ethanol using life cycle assessment coupled with a biogeochemical process model. Int J Biometeorol 63:701–710. https://doi.org/10.1007/s00484-017-1437-7
Jiwuba L, Danquah A, Asante I, Blay E, Onyeka J, Danquah E, Egesi C (2020) Genotype by environment interaction on resistance to cassava green mite associated traits and effects on yield performance of cassava genotypes in nigeria. Front Plant Sci 11:572200. https://doi.org/10.3389/fpls.2020.572200
Kraus EC, Stout MJ (2019) Seed treatment using methyl jasmonate induces resistance to rice water weevil but reduces plant growth in rice. PLoS ONE 14:e0222800. https://doi.org/10.1371/journal.pone.0222800
Liang X, Chen Q, Liu Y, Wu CL, Li KM, Wu MF, Yao XW, Qiao Y, Zhang Y, Geng Y (2022) Identification of cassava germplasms resistant to two-spotted spider mite in China: from greenhouse large-scale screening to field validation. Front Plant Sci 13:1054909. https://doi.org/10.3389/fpls.2022.1054909
Lu ZL, Gao YX, Zhang CH, Bao ZP, Wang WZ, Lin JB, Du FP (2021) Surface properties of Tetranychus urticae koch (Acari: Tetranychidae) and the effect of their infestation on the surface properties of kidney bean (Phaseolus vulgaris l.) hosts. Pest Manag Sci 77:5120–5128. https://doi.org/10.1002/ps.6551
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem-Biol Interact 224:164–175. https://doi.org/10.1016/j.cbi.2014.10.016
Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB (2006) Active defense under oxidative stress. The Antioxidant Responsive Element Biochemistry 71:962–974. https://doi.org/10.1134/S0006297906090033
Montazersaheb H, Zamani AA, Sharifi R, Darbemamieh M (2021) Effects of plant probiotic bacteria and herbivore-induced plant volatiles on life table parameters of Tetranychus urticae (acari: Tetranychidae) on kidney bean’s attached leaves. Int J Acarol 47:520–527. https://doi.org/10.1080/01647954.2021.1957012
Namin HH, Zhurov V, Spenler J, Grbić M, Grbić V, Scott IM (2020) Resistance to pyridaben in canadian greenhouse populations of two-spotted spider mites, Tetranychus urticae (koch). Pestic Biochem Phys 170:104677. https://doi.org/10.1016/j.pestbp.2020.104677
Otun S, Escrich A, Achilonu I, Rauwane M, Lerma-Escalera JA, Morones-Ramírez JR, Rios-Solis L (2022) The future of cassava in the era of biotechnology in southern africa. Crit Rev Biotechnol. https://doi.org/10.1080/07388551.2022.2048791
Ovalle TM, Vásquez-Ordóñez AA, Jimenez J, Parsa S, Cuellar WJ, Becerra Lopez-Lavalle LA (2020) A simple PCR-based method for the rapid and accurate identification of spider mites (tetranychidae) on cassava. Sci Rep-UK 10:19496. https://doi.org/10.1038/s41598-020-75743-w
Panghal A, Munezero C, Sharma P, Chhikara N (2021) Cassava toxicity, detoxification and its food applications: a review. Toxin Rev 40:1–16. https://doi.org/10.1080/15569543.2018.1560334
Puentes A, Zhao T, Lundborg L, Björklund N, Borg-Karlson A-K (2021) Variation in methyl jasmonate-induced defense among norway spruce clones and trade-offs in resistance against a fungal and an insect pest. Front Plant Sci 12:678959. https://doi.org/10.3389/fpls.2021.678959
Rauwane ME, Odeny DA, Millar I, Rey C, Rees J (2018) The early transcriptome response of cassava (Manihot esculenta crantz) to mealybug (Phenacoccus manihoti) feeding. PLoS ONE 13:e0202541. https://doi.org/10.1371/journal.pone.0202541
Rohwer CL, Erwin JE (2010) Spider mites (Tetranychus urticae) perform poorly on and disperse from plants exposed to methyl jasmonate. Entomol Exp Appl 137:143–152. https://doi.org/10.1111/j.1570-7458.2010.01043.x
Ruan J, Zhou Y, Zhou M, Yan J, Khurshid M, Weng W, Cheng J, Zhang K (2019) Jasmonic acid signaling pathway in plants. Int J Mol Sci 20:2479. https://doi.org/10.3390/ijms20102479
Sanches PA, Santos F, Peñaflor MFGV, Bento JMS (2017) Direct and indirect resistance of sugarcane to Diatraea saccharalis induced by jasmonic acid. B Entomol Res 107:828–838. https://doi.org/10.1017/S0007485317000372
Senthil-Nathan S (2019) Effect of methyl jasmonate (meja)-induced defenses in rice against the rice leaffolder Cnaphalocrocis medinalis (guenèe) (lepidoptera: Pyralidae). Pest Manag Sci 75:460–465. https://doi.org/10.1002/ps.5139
Slesak E, Slesak M, Gabrys B (2001) Effect of methyl jasmonate on hydroxamic acid content, protease activity, and bird cherry-oat aphid Rhopalosiphum padi (l.) probing behavior. J Chem Ecol 27:2529–2543. https://doi.org/10.1023/A:1013635717049
Sripontan Y, Hwang SY (2016) Jasmonate-induced defense in tomato and cabbage deterred Spodoptera litura (noctuidae) growth. J Asia-Pac Entomol 19:1125–1129. https://doi.org/10.1016/j.aspen.2016.10.004
Sun JQ, Jiang HL, Li CY (2011) Systemin/jasmonate-mediated systemic defense signaling in tomato. Mol Plant 4:607–615. https://doi.org/10.1093/mp/ssr008
Sun JL, Hui KD, Guo ZY, Li YZ, Fan XW (2022) Cellulose and lignin contents are negatively correlated with starch accumulation, and their correlation characteristics vary across cassava varieties. J Plant Growth Regul. https://doi.org/10.1007/s00344-022-10573-w
Tianzi G, Congcong Z, Changyu C, Hui L, Kairu H, Shuo T, Xudong Z, Dejun H (2018) Effects of exogenous methyl jasmonate-induced resistance in populus × euramericana ‘nanlin895’ on the performance and metabolic enzyme activities of clostera anachoreta. Arthropod-Plant Int 12:247–255. https://doi.org/10.1007/s11829-017-9564-y
Wang Y, Mostafa S, Zeng W, Jin B (2021) Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int J Mol Sci 22:8568. https://doi.org/10.3390/ijms22168568
War AR, Sharma HC (2014) Effect of jasmonic acid and salicylic acid induced resistance in groundnut on Helicoverpa armigera. Physiol Entomol 39:136–142. https://doi.org/10.1111/phen.12057
War AR, Paulraj MG, War MY, Ignacimuthu S (2011a) Herbivore- and elicitor- induced resistance in groundnut to Asian armyworm, Spodoptera litura (fab.) (Lepidoptera: Noctuidae). Plant Signal Behav 6:1769–1777. https://doi.org/10.4161/psb.6.11.17323
War AR, Paulraj MG, War MY, Ignacimuthu S (2011b) Jasmonic acid-mediated-induced resistance in groundnut (Arachis hypogaea l.) against Helicoverpa armigera (hubner) (lepidoptera: Noctuidae). J Plant Growth Regul 30:512–523. https://doi.org/10.1007/s00344-011-9213-0
War AR, Paulraj MG, Ignacimuthu S, Sharma HC (2015) Induced resistance to helicoverpa armigera through exogenous application of jasmonic acid and salicylic acid in groundnut, Arachis hypogaea. Pest Manag Sci 71:72–82. https://doi.org/10.1002/ps.3764
Warabieda W, Olszak R (2010) Effect of exogenous methyl jasmonate on numerical growth of the population of the two-spotted spider mite (Tetranychus urticae koch.) on strawberry plants and young apple trees. J Plant Protect Res 50:96–100. https://doi.org/10.2478/v10045-010-0089-y
Wei XQ, Vrieling K, Kim HK, Mulder PPJ, Klinkhamer PGL (2021) Application of methyl jasmonate and salicylic acid lead to contrasting effects on the plant’s metabolome and herbivory. Plant Sci 303:110784. https://doi.org/10.1016/j.plantsci.2020.110784
Yan JX, Tan Y, Lv YR, Wang F, Zhang YQ, Chi DF (2021) Effect of jasmonate treatments on leaves of Rosa rugosa ‘plena’ and detoxification enzymes and feeding of adult Monolepta hieroglyphica. J Forestry Res 32:1253–1261. https://doi.org/10.1007/s11676-020-01278-5
Yang SY, Wu HH, Xie JC, Rantala MJ (2013) Depressed performance and detoxification enzyme activities of Helicoverpa armigera fed with conventional cotton foliage subjected to methyl jasmonate exposure. Entomol Exp Appl 147:186–195. https://doi.org/10.1111/eea.12060
Yang SY, Cao Q, Peng KH, Xie JC (2022) Jasmonic acid-treated cotton plant leaves impair larvae growth performance, activities of detoxification enzymes, and insect humoral immunity of cotton bollworm. Neotrop Entomol 51:570–582. https://doi.org/10.1007/s13744-022-00970-x
Zhang K, Li ZB, Zhu SW, Weng QF (2014) 60co-γ irradiation affects the enzymatic antioxidant system of the citrus red mite Panonychus citri (Acari: Tetranychidae). Molecules 19:6382–6392. https://doi.org/10.3390/molecules19056382
Zhang YT, Zhang YL, Chen SX, Yin GH, Yang ZZ, Lee S, Liu CG, Zhao DD, Ma YK, Song FQ, Bennett JW, Yang FS (2015) Proteomics of methyl jasmonate induced defense response in maize leaves against Asian corn borer. BMC Genom 16:224. https://doi.org/10.1186/s12864-015-1363-1
Zou ZW, Xi JF, Liu G, Song SX, Xin TR, Xia B (2018) Effect of temperature on development and reproduction of the carmine spider mite, Tetranychus cinnabarinus (Acari: Tetranychiae), fed on cassava leaves. Exp Appl Acarol 74:383–394. https://doi.org/10.1007/s10493-018-0241-3
Acknowledgements
This research was jointly supported by the Hainan Major Science and Technology Project (No.ZDKJ202002), China Agriculture Research System (CARS-11-HNCQ), and NanFeng earmarked fund of Ministry of Agriculture and Rural Affairs of P.R.C (NFZX-2021).
Author information
Authors and Affiliations
Contributions
QC and YL conceived the study. YZ, MFW, XWY, YQ and XZ conducted the experiments. XL, CLW and XQL carried out the statistical analysis. YL wrote the draft manuscript. QC and YL participated in the editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Liu, Y., Liang, X. et al. Exogenous methyl jasmonate induced cassava defense response and enhanced resistance to Tetranychus urticae. Exp Appl Acarol 89, 45–60 (2023). https://doi.org/10.1007/s10493-022-00773-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00773-0