Abstract
Cassava storage roots contain large amounts of starch and low amounts of cellulose and lignin. However, the relationship between lignification with cellulose and starch accumulation during storage root development is not well understood. In the present study, the dynamic changes in starch, lignin, and cellulose contents as well as in root diameter, enzyme activities, and histochemical staining in the storage roots of six cassava varieties at different growth stages were assessed. The results revealed a negative correlation between the biosynthetic carbon allocation of starch and lignin (r = − 0.780, p < 0.0001) and that of starch and cellulose (r = − 0.873, p < 0.0001). Early and rapid formation of vascular tissue resulted in an increase in starch content in the six varieties and a transition of carbon flow from xylem development to starch formation at 100 days after planting was identified. Vascular vessels, cellulose, and starch exhibited a dynamic balance independent of cassava varieties. A more rapid decline in starch content was observed with increasing cellulose and lignin contents in high-starch varieties than that in low-starch varieties. This indicated that the optimal dynamic transition between structural and storage components during root development facilitates the formation of large amounts of starch in the parenchymal tissue. The fine regulation of lignification in cassava storage roots provides a potential strategy for breeding starch-rich cassava varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cassava, commonly known as manioc (Manihot esculenta Crantz), is the sixth most cultivated food crop worldwide, consumed by over 800 million people in tropical and subtropical regions (El-Sharkawy 2004; Maxmen 2019). Its storage roots contain large amounts of starch, which can be used for multiple purposes, such as the production of animal feed, medicine, cosmetic formulations, paper, degradable plastics, and biofuels (Jansson et al. 2009; Zhang et al. 2017). In China, cassava is an important energy crop for the production of fuel ethanol and the annual net energy surplus of cassava-based fuel ethanol is equivalent to 92.9 billion MJ (Jiang et al. 2019). Recently, increasing attention has been paid to the breeding of specific cassava varieties, such as those with edible fresh roots, enhanced nutrient composition, modified starch profiles, and improved processing properties (Zhang et al. 2017; Bull et al. 2018). However, to improve cassava yields and facilitate the breeding of starch-rich varieties, it is necessary to gain a better understanding of the genetic variations among the main starch-accumulating varieties.
In cassava, the storage roots originate from swelling of the primary roots via secondary growth during the early stage of development. In cassava storage roots, the transition of carbon flow from lignin and cellulose biosynthesis to starch formation commences when the roots receive swelling signals, for example, from phytohormones (6-benzylaminopurine and 1-naphthaleneacetic acid) and specific regulatory genes (Sojikul et al. 2015; Siebers et al. 2017). Numerous omics studies have revealed differences in the initiation of the formation of fibrous and storage roots in cassava based on transcriptome and proteome data and have enabled the identification of a series of candidate genes for storage root development, including MeWOX4.1, MeWOX4.2, MeKD83, MeKD82, MeKD106, MeKD154, and MeAGL20 (Sheffield et al. 2006; Sojikul et al. 2010, 2015; Siebers et al. 2017). However, the prolonged growth period of cassava and natural variations among its varieties hinder the characterization of starch formation and “omics” analyses during storage root development.
The storage root is the major harvestable part of cassava, with starch typically accounting for 16.6–34% of its fresh weight, depending on the cassava variety (Buddhakulsomsiri et al. 2018). The formation and expansion of storage roots in cassava are dependent on high-efficiency leaf photosynthesis, apoplastic phloem carbohydrate loading in the source leaves, unhindered stem transportation, and a symplastic phloem unloading system (Li et al. 2016; Mehdi et al. 2019; De Souza et al. 2020). Storage roots are primarily composed of three main parts: outer layer or phelloderm, parenchyma, and central vascular bundle. Lignin, cellulose, and hemicellulose are the major components of secondary cell walls, the deposition of which forms vascular tissues which transport water and nutrients. Although cassava storage roots have a high-starch content, their marginal lignin and cellulose contents, which contribute to the strength of their vascular bundles, reduce the fresh taste and processing performance of cassava storage roots. A recent study showed that the downregulation of fiber formation and lignification was associated with higher starch accumulation during storage root bulking in sweet potato (Singh et al. 2021). However, the relationship between starch accumulation and cell development in cassava storage roots has not been fully elucidated to date. The present study aimed to clarify the dynamic changes in starch, cellulose, and lignin formation during storage root development in six cassava varieties as well as to describe the coordination among these three components.
Materials and Methods
Experimental Design and Plot Management
Our field experiments were carried out at the Novel Station of Subtropical Agriculture in Guangxi, Guangxi University (107° 45′ E, 22° 9′ N, altitude 78 m) in the period from March 2019 to January 2020. The experimental site has a typical subtropical monsoon climate, with an annual rainfall of 1222 mm, mean temperature of 21.9 °C, and sunshine duration of 1550.5 h. Six farmer-preferred cassava varieties were used in this study: two landraces from China with a low-starch content (SC124 and SC16), one high-starch and one low-starch variety from Thailand (KU50 and R3, respectively), and one high-starch and one moderate-starch variety from Latin America (Arg7 and 16P, respectively). The year of their release in China and the main features of all varieties are listed in Table 1. The performances of the storage roots of these six varieties were evaluated via a completely randomized experimental design with four replicates. In each plot (36 m2), the cassava varieties were planted in four rows (row length of 10 m, row spacing of 1.2 m). Uniform cuttings of healthy cassava stems (20 cm in length) were used as seed stakes and a total of 8,333 cuttings per hectare were planted vertically on the ridges. Fertilizer was applied prior to planting (128 kg N, 120 kg P, and 15 kg K per hectare) and uniform storage root samples were harvested for physiological analyses (at 68, 100, 130, 160, 191, 211, and 245 days after planting [DAP]) using a destructive method.
Measurement of Storage Root Growth and Development
Assay of Starch and Soluble Sugar Contents in Storage Roots
The outer layer (peel) of fresh storage roots was carefully removed using a scalpel and an inner tissue (including the parenchyma and central vascular tissue) sample of 10 g was oven-dried at 65 °C to a constant weight. The dried tissues were powdered using a mortar and pestle and passed through a 60-mesh sieve (0.250-mm pore size). Subsequently, 20 mg of the sieved tissues were mixed with 8 mL of 2 M KOH and reacted for 20 min in an 85 °C water bath. After the reaction, the mix was immediately cooled to 25 °C, diluted to 25 mL with sterilized deionized water, and adjusted to pH 3.0 with 2 M HCl. The diluted substance (4 mL) was reacted with 1 mL of 1% KI–0.1% I2 solution for 15 min at 25 °C. The reaction solution was used to determine the starch content as described by Cheng et al. (2018).
A 20 mg sample of the sieved tissue was mixed with 5 mL of 80% ethanol to extract soluble sugars for 24 h at 25 °C and 100 μL of the extracted solution was transferred into a 5 mL centrifuge tube with 3 mL of an anthrone solution and then incubated in a boiling water bath for 15 min. The absorbance of the cooled reaction solution was measured at 620 nm. Soluble sugars were calculated using a standard curve, as described by Li (2005).
Determination of Cellulose and Lignin Contents in Storage Roots
A 0.5 g sample of the powdered dry tissue (S in the equation below), including the parenchyma and central vascular cylinder, was accurately weighed into a 100 mL flask, to which 50 mL of 2% cetyltrimethylammonium bromide (CTAB; 20 g CTAB dissolved in 1 L of sulfuric acid) and 1 mL of decahydronaphthalene was added. The solution was heated to boiling on an electric stove and allowed to boil for 60 min. Thereafter, the mixture was filtered repeatedly by suction filtration and rinsed with hot water (90–100 °C) until the pH of the filtrate was neutral. The residue was then decolorized with a small volume of acetone until it was colorless. The decolorized residue was dried at 65 °C for 12 h, cooled to room temperature (25 °C) in a desiccator, and weighed (W1). Acid-insoluble lignin and other ashes (W2) were then isolated using 72% sulfuric acid following the methods described by Van Soest (1963). The final cellulose content is determined using the following formula:
The lignin content in storage roots was determined using a modified Klason method, as previously described by Liu et al. (2018). The oven-dried samples (0.5 g) were added to a 10 mL tube with 5 mL of 72% H2SO4 at 25 °C for 2 h. The reaction solution was diluted in 3% H2SO4 with deionized water and then heated at 121 °C for 1 h in an autoclave (Hirayama, Hirayama Manufacturing Co., Japan). The reaction mixture was filtered, and the residue was dried at 65 °C for 12 h. The acid-insoluble lignin content was determined gravimetrically.
Enzyme Assays
After peeling off the outer layer, a freshly frozen storage root segment (0.2 g) was ground to a fine powder in liquid N2 using a mortar and pestle. A crude enzyme solution was prepared by homogenizing the powder in 5 mL of the assay buffer containing 10 mM of Tris–HCl (pH 8.0), 10 mM of MgCl2, 2 mM of ethylenediaminetetraacetic acid, 50 mM of β-mercaptoethanol, 0.05% of Triton X-100, and 5% of polyvinylpyrrolidone. The homogenate was centrifuged at 12,000×g for 5 min at 4 °C. In a microplate, a 25 μL aliquot of the obtained supernatant was mixed with 475 μL of the reaction buffer (containing 2.16 mL of H2O, 2.4 mL of the assay buffer, 720 μL of 20 mM NADP+, 240 μL of 50 mM uridine diphosphate glucose [UDPG], 240 μL of glucose-6-phosphate dehydrogenase [G6PDH], and 240 μL of phosphoglucomutase) for 10 s on a plate shaker. The microplate was then placed in a spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) to determine the absorbance at 340 nm at 30-s intervals. The activity of UDPG pyrophosphorylase (UGPase; EC 2.7.7.9) was calculated following the method described by Ménard et al. (2014).
Cinnamyl alcohol dehydrogenase (CAD) activity was determined based on the oxidation of coniferyl alcohol, as described by Wyrambik and Grisebach (1975), with slight modifications. In brief, freshly frozen samples (200 mg) were homogenized with 2 mL of the extraction buffer (pH 7.3) and the extract was centrifuged at 12,000×g for 5 min at 4 °C. A 200 μL aliquot of the obtained supernatant was mixed with 800 μL of the reaction buffer (100 μmol of coniferyl alcohol, 100 μmol of 50 mM NADP+, and 100 mM of Tris–HCl pH 8.9) and incubated for 10 min. The reaction was terminated by adding 100 μL of 6 M HCl, and the absorbance of the solution was measured at 340 nm to determine CAD activity.
Cinnamate 4-hydroxylase (C4H; E.C. 1.14.13.11) activity was determined as described by Kumar et al. (2013). Fresh root samples (0.2 g) were homogenized with a mortar and pestle in 2 mL of the extraction buffer containing 50 mM of Tris–HCL (pH 7.5), 15 mM of 2-mercaptoethanol, 4 mM of magnesium chloride, 5 mM of ascorbic acid, 1 mM of phenylmethanesulfonyl fluoride, 0.15% w/v of polyvinylpyrrolidone, 10% of glycerol, and 10 mM of leupeptin and the homogenate was centrifuged at 12,000×g for 10 min at 4 °C. The supernatants were used as a source of crude enzyme for the C4H assay. Each supernatant (400 μL) was mixed with 2.4 mL of the reaction solution (containing 10 μM of trans-cinnamic acid, 50 mM of Tris–HCl [pH 7.5], and 10 μM of NADPH]), the mixture was incubated at 28 °C for 30 min, and the reaction was terminated by adding 100 μL of 6 M HCl. The mixture was then centrifuged at 12,000×g for 10 min. Subsequently, the pH of the supernatant adjusted to pH 11.0 by adding 1.0 N of NaOH and its absorbance was recorded at 340 nm. All components without substrate were considered the control. One unit of C4H activity was expressed as an absorbance change of 0.01 per hour and the activity was expressed as U/g fresh weight/h.
Safranin O and Fast Green FCF Staining
The storage root samples were cut into pieces and fixed in formalin:acetic acid:alcohol, as described by Liu et al. (2018). The fixed tissues were sectioned to a thickness of 20 μm and affixed to glass slides for subsequent staining. To examine lignification, the sections were stained with 1% Safranin O solution for 12 h, after which the excess stain was rapidly washed off with distilled water. To observe starch accumulation, the tissue sections were counterstained with a conventional I2 (2%)–KI (1%) solution for 5 min following a previously described method (Li et al. 2016). Excess stain was removed by washing with distilled water. Fast Green FCF staining was also performed to visualize cellulose following the method described by Liu et al. (2018).
To assay the dynamic alteration in lignin during the development of storage roots, the samples were cross-sectioned into 5–7 mm-thick sheets using a sharp scalpel, stained 2% (w/v) phloroglucinol for 10 min and then soaked in 18% HCl for 5 min. The surfaces of the cross-sections were washed with distilled water to remove impurities, after which they were directly photographed using an EOS 750D camera (Canon, Tokyo, Japan).
Statistical Analysis
Differences in starch, lignin, cellulose, and soluble sugar contents, storage root diameter, and enzyme activities among the six cassava varieties at different growth stages were determined using analysis of variance (ANOVA). All data were evaluated using two-way ANOVA, and means were compared by Duncan’s multiple range tests at p = 0.05 using SPSS 13 (SPSS, Chicago, IL, USA). Data are shown as means ± standard deviations. The regression line of starch with lignin and cellulose contents was examined in EXCEL 2016 and a curve of starch accumulation was fitted to the logarithmic function [y = ɑ × ln(x)—ɓ] of the progression of the developmental period. Correlation analysis of starch accumulation with cellulose and lignin contents was performed using SPSS 13. Origin 9.5 (OriginLab, Northampton, MA, USA) was used for drawing the figures and performing principal component analysis (PCA).
Results
Starch Accumulation During Storage Root Development
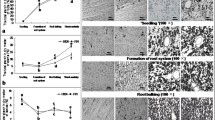
The storage root diameters of all six cassava varieties were greater than 0.5 cm at 68 DAP and at 100 DAP, and the roots of SC124 (3.12 cm), R3 (2.7 cm), and SC16 (3.5 cm) had diameters larger than those of 16P (1.84 cm), KU50 (2.59 cm), and Arg7 (1.71 cm; Fig. 1a), suggesting a rapid expansion rate of storage roots in the early development stages (from 68 to 100 DAP) of cassava. In the period from 100 to 191 DAP, the varieties SC124 and KU50 showed rapid storage root growth up to 6.95 cm and 7.26 cm, respectively. From 191 to 245 DAP, the storage root diameters in three varieties, namely, SC124, R3, and SC16, were observed to increase by 0.27, 0.19, and 0.55 cm. Conversely, the root diameter of Arg7, KU50, and 16P exhibited a high growth rate, increasing by 1.17, 0.82, and 0.89 cm, respectively, across 54 days (in the period from 191 to 245 DAP; Fig. 1a).
Changes in the diameter (a) and starch content (b) in the storage roots of the six cassava varieties during different growth stages. Different letters at each stage indicate significant differences according to Dunnett’s multiple comparison test (p < 0.05). Error bars represent the means ± standard deviations (n = 4)
Compared with the other varieties, SC124 showed the highest starch accumulation (238.3 mg g−1 of dry weight (DW) at 68 DAP), although in the period from 68 to 100 DAP, starch accumulation in this variety was slower and relatively delayed compared to that in the other varieties (16P, KU50, and Arg7). In Arg7, starch accumulation reached the maximum level at 211 DAP (586.1 mg g−1) and was maintained at this level until maturity (at 245 DAP). Compared with the other five varieties, R3 showed delayed starch accumulation at 130 DAP (336.5 mg g−1; Fig. 1b).
Changes in Polysaccharide Content During Storage Root Development
In all cassava varieties, the cellulose content in storage roots decreased as the plants grew (Fig. 2a). By 100 DAP, all the varieties showed substantial reduction in cellulose content (30.5–60.0%), except for R3, whereas a substantial reduction of 60% in R3 occurred at 130 DAP. In SC124, a substantial reduction in cellulose content by 38% and 57% was observed at 100 and 191 DAP, respectively, whereas the cellulose content in KU50 and Arg7 decreased at a steady rate after 100 DAP (Fig. 2a).
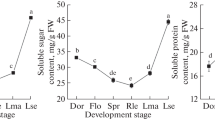
Changes in the formation of cellulose (a), lignin (b), and soluble sugar (c) in storage roots of the six cassava varieties at different developmental stages. Different letters at each stage indicate significant differences according to Dunnett’s multiple comparison test (p < 0.05). Error bars represent the means ± standard deviations (n = 4). DW dry weight
Throughout the growth period, the lignin content in SC124 and R3 was higher than that in KU50 and Arg7. It tended to decline from 149.4 to 2.7 mg g−1 DW over time in all varieties, with the largest reduction (75–91%) observed between 100 and 130 DAP (Fig. 2b). Upon the onset of low-temperature conditions, the increase in lignin content was observed at 191 DAP (Fig. S1), which suggests that starch accumulation was more rapid than lignin biosynthesis in storage roots in the period from 100 to 191 DAP (nearly mature period of storage roots) under suitable temperature conditions (> 20 °C).
Notable differences in the total soluble sugar content were observed among the six cassava varieties and it changed dynamically with the progression of the developmental period. Throughout the growth period, the sugar content was higher in KU50 and Arg7 than in SC124, except at 160 and 245 DAP. However, all varieties except for R3 showed an initial decrease in sugar content during the early stage of development as well as at 211 and 245 DAP (Fig. 2c); this was consistent with the sharp decrease in minimum temperature and maximum levels of temperature difference after 191 DAP (Fig. S1). In the period of 68–100 DAP, the sugar content in R3 increased, which may be related to the relatively slow conversion of sugar into starch (Figs. 1b and 2c).
Activity of Enzymes Involved in Cellulose and Lignin Biosynthesis
During the initial growth period, slight increases in the CAD activity of all six cassava varieties were noted and it subsequently began to decrease during the period of 160–211 DAP, except in R3 (Fig. 3a). The CAD activity in SC124 showed little change from 100 to 211 DAP, with the maximum activity with 43.5 U min−1 g−1 FW detected at m<aturity. Notably, the CAD activity in Arg7 was higher than that in SC124 during the entire growth period (Fig. 3a). The C4H activity exhibited a similar pattern to that of CAD, except for a marked increase in SC16 and KU50 at 160 DAP (Fig. 3b).
Changes in the activity of CAD (a), C4H (b), and UGPase (c) in storage roots of the six cassava varieties at different developmental stages. Different letters at each stage indicate significant differences according to Dunnett’s multiple comparison test (p < 0.05). Error bars represent the means ± standard deviations (n = 4). FW fresh weight, CAD cinnamyl alcohol dehydrogenase, C4H cinnamate 4-hydroxylase, UGPase UDP-glucose pyrophosphorylase
The activity of UGPase, which is required for cellulose biosynthesis, gradually decreased with the progression of cassava root growth and it was found to be higher in Arg7 and KU50 than in the other varieties (Fig. 3c).
Histochemical Staining of Cassava Roots
During cassava root development, the percentage of starch granules increased, whereas the cellulose content decreased in all six varieties. At the early stage of root development, the starch content was the highest in SC124 among all six varieties, whereas at maturity, the root systems of Arg7 and KU50 had a high-starch content (Fig. 4).
Results of the combined histochemical staining of starch, cellulose, and lignin. Combined staining was performed on transverse sections of storage roots of the six cassava varieties obtained at different stages of the growth cycle. Starch was stained brown with KI/I2 (indicated by solid arrowhead), cellulose was stained blue with Fast Green FCF (indicated by arrowhead), and xylem vessels (lignin) were stained red with Safranin O solution (indicated by solid arrow). Scale bar = 100 µm. DAP days after planting (Color figure online)
Lignin stains were more abundant in SC124 and R3 than in the other varieties at the early development stages of 68 and 100 DAP (Fig. 5), which was consistent with the results of the lignin assay (Fig. 2b). However, the phloroglucinol stain showed the tendency of increasing lignin content over time in all varieties, especially in 16P/SC16 at 160 and 191 DAP (Fig. 5). This lignin accumulation tendency seemed to be consistent with the C4H activity over time (Fig. 3b). However, no correlation was observed between lignin content and C4H activity (r = 0.023, p > 0.05).
Microscopic observation of lignification. Cassava storage roots at different stages of development were stained with phloroglucinol HCl to visualize lignin (scattered purple). The stained sections were observed under a Zeiss Axio Scan microscope (Carl Zeiss, Jena, Germany). Scale bar = 5 mm. Each image is one of the three replicates from each section. DAP days after planting
Model of Starch Accumulation in Storage Roots
The data obtained for starch accumulation in storage roots were fitted using a logarithmic function increase model, which showed a good fit for all varieties (R2 > 0.9; Fig. 6a). Notably, starch content in Arg7 showed a sharp increase with the progress of root growth, whereas that in SC124 showed a more gradual increase. In addition, starch accumulation in cassava storage roots was negatively correlated with lignin content (r = − 0.780, p < 0.0001) and cellulose content (r = − 0.873, p < 0.0001; Fig. 6b, c). Moreover, the equations for high-starch varieties indicated that their regression efficiency (slope) was greater than that for low-starch varieties (Fig. 6d).
Models of starch accumulation. a Correlation between starch content and starch accumulation (p < 0.01). b Correlation between starch and lignin contents (p < 0.0001). c Correlation between starch content and cellulose formation in the parenchymal tissues (p < 0.0001). d Correlation between cellulose and starch content (p < 0.0001). DW dry weight
PCA Results
In the present study, six cassava varieties and seven developmental phases were assessed and from 2019 to 2020, nine traits related to cassava root structural components (lignin and cellulose), starch accumulation (starch and total sugar contents), phenotype (peel thickness and parenchyma diameter), and key enzyme activities (UGPase, CAD, and C4H) were investigated. To determine the relationships among the trait variables underlying trait variations, PCA was performed for all nine traits. The results showed that the first principal component (PC1) explained 63.8% of the trait variance (Fig. 7a). Starch content, parenchyma diameter, and peel thickness showed high-positive loading on PC1 (0.31 to 0.38), whereas cellulose content, lignin content, and UGPase activity showed negative loading (− 0.34 to − 0.38; Fig. 7a, b). These results indicated that starch content and parenchyma diameter were distributed on the side of the scale opposite to that of cellulose and lignin contents in cassava varieties with comparable PC1 scores. These differences can be assumed to reflect a trade-off relationship between starch accumulation and structural components in the parenchyma tissue. Furthermore, PC2 explained 12.2% of the total variance, with a high loading for total sugar content (0.89; Fig. 7a), indicating that PC2 was representative of the dynamic changes in carbohydrates. PC2 also showed a moderately high loading (− 0.31) with respect to UGPase activity.
Results of the principal component analysis of cassava storage root characteristics. a Summary of the first three principal components (PC1 and PC2) for nine traits of the six cassava varieties over seven developmental stages. b Loading plot of PC1 and PC2. Starch content and structural components (cellulose and lignin) lie in opposite areas of the plot, indicating a trade-off relationship between the formation of structural components and starch accumulation for PC1, whereas PC2 represents total carbohydrates (total sugar content)
Discussion
During root development, the main function of xylem is transportation of water and nutrient components through the plant (Mehdi et al. 2019). However, the question of how the dynamic changes in lignification and cellulose content during cassava root swelling affect starch accumulation is yet to be answered. In the present study, cellulose and lignin contents were negatively correlated with starch accumulation and the correlation characteristics varied among the varieties.
Expansion Rate of Storage Roots, Lignification, and Cellulose Accumulation in Cassava
The proliferation and growth of cells involved in the process of storage root expansion provide a large space for the accumulation of starch and other compounds (Fan et al. 2019). Most of our current understanding of starch granule initiation and formation was obtained from studies on Arabidopsis and cereal species (Seung et al. 2017; Vandromme et al. 2019), whereas the processes occurring in storage roots are not well understood. The present study detected large natural variations in the percentage of lignification and cellulose content among six cassava varieties; these factors are known to influence starch formation during the early stage of storage root development. Storage roots of the varieties SC124 and R3, which are characterized by high lignin and cellulose contents, showed a rapid rate of expansion during early development, whereas the varieties with low lignin and cellulose content (KU50, Arg7, and 16P) had a notably slower expansion rate of early storage root bulking diameters. These results contradicted the findings showing that an increase in lignification and fiber formation inhibited storage root formation in sweet potato (Singh et al. 2019). However, in the present study, high-cellulose content in cassava roots was associated with rapid expansion of storage roots with prolonged developmental time (100 DAP). Conversely, the variety Arg7, which had the lowest lignin content among the six-examined varieties during the entire developmental period, had a smaller root diameter. In contrast to SC124, which exhibited an early increase in storage root diameter, KU50 showed a rapid increase in root bulking diameter in the period after 130 DAP. Storage root diameter is known to be one of the most important selection criteria for achieving a high-cassava fresh yield potential (Ntawuruhunga and Dixon 2010; Chipeta et al. 2016).
Negative Correlation of Starch Accumulation with Cellulose and Lignification During Cassava Storage Root Development
Previous studies have shown that manipulation of the percentages of lignin and cellulose deposition in poplar can improve its metabolic flexibility and provide a growth advantage (Hu et al. 1999). Recent studies have indicated that the decrease in lignin content resulted in an increase in ethanol production, up to 161% in poplar and an improvement in fruit quality traits by controlling stone cell formation in pear (De Meester et al. 2020; Zhang et al. 2021). The present study found that natural variation in lignin and cellulose contents was viable in the storage roots among the cassava varieties and noted a significant negative correlation between starch accumulation and lignification (r = − 0.780, p < 0.0001) as well as between starch accumulation and cellulose content (r = − 0.873, p < 0.0001) in the cassava varieties during storage root development. The development of an ideal vascular tissue (i.e., that with a high lignin content) can be achieved via a developmental transition through the formation of vascular cambial cells, which promote the formation of starch-accumulating parenchyma cells during the early stages of storage root growth (Firon et al. 2013; Singh et al. 2019); in the present study, this was observed in the cassava variety SC124, which is characterized by higher starch content and degree of lignification than those of the other examined varieties. However, lignification and cellulose content began to decrease with the increase in starch accumulation after the initiation of starch synthesis, which was consistent with the previous observations in sweet potato, thereby indicating that the downregulation of lignin biosynthesis and upregulation of starch biosynthesis occurred at the early stage of storage root formation (Firon et al. 2013). In addition, we found that from 68 to 130 DAP, the change in starch accumulation per unit of lignin was low in SC124 and R3 and high in KU50, Arg7, and 16P. There were substantial differences in the dynamic allocation of carbohydrates (photosynthates) to lignin and cellulose biosynthesis among the cassava varieties, which possibly influenced starch accumulation in storage roots. It has been demonstrated that photosynthesis and carbon allocation determine the plant carbon flow direction and the rate of starch synthesis in tuberous roots is strictly associated with the activities of key enzymes (Obata et al. 2020) but not with the net photosynthesis rate (De Souza and Long 2018). However, unless a specific variety with a higher photosynthesis rate was developed, the dynamic changes between starch accumulation and structural components in the storage roots of currently available cassava varieties will remain in a steady balance.
Model of High-Starch Accumulation in Cassava Storage Roots
To study the tendency of starch accumulation among the cassava varieties, we fitted several nonlinear regression equations to the data for starch content with regard to the stages of storage root development. We found that starch accumulation in cassava storage roots was well fitted to the logarithmic function [y = ɑ × ln(x) − ɓ]. However, the starch content during the early stage of storage root development was not predictive of the starch content at maturity, although the varieties with moderate-starch contents during the early stages of development were found to have the potential for a rapid increase in starch content. We observed an interesting phenomenon: the ɓ/ɑ ratio of the logarithmic function in the six varieties ranged from 3.0 to 3.7 and the varieties with the ɓ/ɑ value of approximately 3.3 (i.e., Arg7 and KU 50) showed a high rate of starch accumulation and a high final starch content in their storage roots. This seemed to be the optimal investment for maximal storage root growth, indicating higher allocation of carbon to starch formation during storage root development. In contrast, the varieties with ɓ/ɑ ≠ 3.3 (i.e., SC124 and R3) were characterized by a low rate of starch accumulation and a relatively low final starch content, which indicated a high investment into the biosynthesis of cellulose and lignin. The PCA results also indicated a high-positive loading of starch content on PC1, whereas lignin and cellulose contents showed a negative loading on PC1. In this regard, the fine regulation of carbon re-allocation in the inner tissues of storage roots remains to be elucidated using cassava genomic data and the CRISPR/Cas9 system.
Regulation of Enzymatic Activity
UGPase is a key enzyme catalyzing the production of UDPG, which is involved in the formation of sucrose and cell wall components. In non-photosynthetic tissues, cellulose synthase associated with sucrose synthase is membrane localized and preferentially channels energy toward cellulose synthesis using UDPG as a substrate (Amor et al. 1995). UGPase catalyzes the formation of UDPG from glucose-1-phosphate and UTP and it can also reversibly produce glucose-1-phosphate via by UDPG degradation. During the cassava storage root expansion stage, there is a reduction in the activity of UGPase, indicating that high-UGPase activity during the early stages of root development contributes to the formation of structural components (e.g., cellulose and β-glucan) via the production of UDPG. Consistently, the results of our PCA of phenotypic and biochemical parameters indicated that UGPase was plotted with cellulose and lignin contents.
Lignin biosynthesis is required for the activation of a series of enzymes, such as phenylalanine ammonia lyase, CAD, and 4-Coumarate: CoA ligase, C4H, and hydroxycinnamoyl CoA: shikimate hydroxycinnamoyl transferase. C4H catalyzes the p-hydroxylation of trans-cinnamic acid to form p-coumaric acid in the second step of the phenylpropanoid pathway and plays a rate-limiting role in carbon allocation (Davin et al. 2008). Results of lignin staining was consistent with C4H activity; however, no correlation was observed between lignin content and C4H enzyme activity over time, which was probably because the accumulation rate of lignin in tuberous roots was significantly lower than that of starch, which resulted in a relative decrease in lignin content per unit DW of the root system. Furthermore, it is known that CAD generally plays a positive role in the formation of lignin and is involved in species-dependent cross-talk with other metabolic pathways (Davin et al. 2008; Ma et al. 2018). In the present study, the activity of CAD and C4H was low and high during the early and late stage of storage root development, respectively, which was in contrast with the pattern of UGPase activity in cassava roots. These observations indicated that in addition to their important roles in lignin synthesis, C4H and CAD are also involved in other metabolic pathways.
An interesting phenomenon was observed in which a strong increase in soluble sugar content in storage roots appeared at 211 and 245 DAP, whereas starch accumulation plateaued at 191 DAP with only small increases thereafter, with the exception of Arg7. This could be attributed to the fact that low temperatures lead to a substantial increase in the sweetness of tuberous roots (Fig. S1), mostly by inducing the sugar metabolism pathway in tuberous roots through the regulation of key enzymes, such as sucrose synthase and invertase (Stein and Granot 2019; de Araújo et al. 2020). We also found that the accumulation of high levels of soluble sugars in cassava storage roots increased plant resistance to low temperatures at 211 and 245 DAP (December 2019 to January 2020, Fig. S1). Compared to the other varieties, Arg7 showed greater resistance to cold stress.
Conclusion
Our results revealed that the structural components of cassava storage roots (cellulose and lignin) were negatively correlated with starch accumulation. The dynamic changes in the ratio of carbohydrates (photosynthates) to lignin and cellulose varied among the cassava varieties. Our findings indicated that early development of lignin contributes to starch formation, but lower lignin content in the final (harvest) stage leads to an increase in starch content, that is, lower the structural component contents in cassava storage roots, higher the starch content at the final harvest stage. A potential strategy was proposed for optimizing the balance between storage root carbon allocation and starch accumulation through plant breeding.
Abbreviations
- DAP:
-
Days after planting
- CTAB:
-
Cetyltrimethylammonium bromide
- UDPG:
-
Uridine diphosphate glucose
- UGPase:
-
UDPG pyrophosphorylase
- CAD:
-
Cinnamyl alcohol dehydrogenase
- C4H:
-
Cinnamate 4-hydroxylase
- ANOVA:
-
Analysis of variance
- PCA:
-
Principal component analysis
References
Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92:9353–9357
Buddhakulsomsiri J, Parthanadee P, Pannakkong W (2018) Prediction models of starch content in fresh cassava roots for a tapioca starch manufacturer in Thailand. Comput Electron Agr 154:296–303
Bull SE, Seung D, Chanez C, Mehta D, Kuon JE, Truernit E, Hochmuth A, Zurkirchen I, Zeeman SC, Gruissem W, Vanderschuren H (2018) Accelerated ex situ breeding of GBSS- and PTST1-edited cassava for modified starch. Sci Adv 4:eaat6086
Cheng YE, Dong MY, Fan XW, Nong LL, Li YZ (2018) A study on cassava tolerance to and growth responses under salt stress. Environ Exp Bot 155:429–440
Chipeta MM, Shanahan P, Melis R, Sibiya J, Benesi IRM (2016) Early storage root bulking index and agronomic traits associated with early bulking in cassava. Field Crop Res 198:171–178
Davin LB, Jourdes M, Patte AM, Kim KK, Vassao DG, Lewis NG (2008) Dissection of lignin macromolecular configuration and assembly: comparison to related biochemical processes in allyl/propenyl phenol and lignan. Nat Prod Rep 25:1015–1090
de Araújo NO, Véras MLM, Santos MNS, de Araújo FF, Tello JPJ, Finger FL (2020) Sucrose degradation pathways in cold-induced sweetening and its impact on the non-enzymatic darkening in sweet potato root. Food Chem 312:125904
De Meester B, Madariaga Calderón B, de Vries L, Pollier J, Goeminne G, Van Doorsselaere J, Chen M, Ralph J, Vanholme R, Boerjan W (2020) Tailoring poplar lignin without yield penalty by combining a null and haploinsufficient CINNAMOYL-CoA REDUCTASE2 allele. Nat Commun 11:5020
De Souza AP, Long SP (2018) Toward improving photosynthesis in cassava: characterizing photosynthetic limitations in four current African cultivars. Food Energy Secur 7(2):e00130
De Souza AP, Wang Y, Orr DJ, Carmo-Silva E, Long SP (2020) Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light. New Phytol 225:2498–2512
El-Sharkawy MA (2004) Cassava biology and physiology. Plant Mol Biol 56:481–501
Fan C, Wang G, Wang Y, Zhang R, Wang Y, Feng S, Luo K, Peng L (2019) Sucrose synthase enhances hull size and grain weight by regulating cell division and starch accumulation in transgenic rice. Int J Mol Sci 20:4971
Firon N, LaBonte D, Villordon A, Kfir Y, Solis J, Lapis E, Perlman TS, Doron-Faigenboim A, Hetzroni A, Althan L, Nadir LA (2013) Transcriptional profiling of sweetpotato (Ipomoea batatas) roots indicates down-regulation of lignin biosynthesis and up-regulation of starch biosynthesis at an early stage of storage root formation. BMC Genomics 14:460
Hu WJ, Harding SA, Lung J, Popko JL, Ralph J, Stokke DD, Tsai CJ, Chiang VL (1999) Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat Biotechnol 17:808–812
Huang Q, Li J (2007) The collection, utilization and breeding of cassava germplasm in Guangxi. Trop Agric Guangxi 108:35–37 ((Chinese))
Jansson C, Westerbergh A, Zhang J, Hu X, Sun C (2009) Cassava, a potential biofuel crop in (the) People’s Republic of China. Appl Energ 86:S95–S99
Jiang D, Hao M, Fu J, Tian G, Ding F (2019) Estimating the potential of energy saving and carbon emission mitigation of cassava-based fuel ethanol using life cycle assessment coupled with a biogeochemical process model. Int J Biometeorol 63:701–710
Kumar S, Omer S, Patel K, Khan BM (2013) Cinnamate 4-Hydroxylase (C4H) genes from Leucaena leucocephala: a pulp yielding leguminous tree. Mol Biol Rep 40(2):1265–1274
Li HS (2005) Principles and techniques of plant physiological biochemical experiment. Higher Education Press, Beijing
Li YZ, Zhao JY, Wu SM, Fan XW, Luo XL, Chen BS (2016) Characters related to higher starch accumulation in cassava storage roots. Sci Rep-UK 6:19823
Liu N, Sun Y, Wang P, Duan H, Ge X, Li X, Pei Y, Li F, Hou Y (2018) Mutation of key amino acids in the polygalacturonase-inhibiting proteins CkPGIP1 and GhPGIP1 improves resistance to Verticillium wilt in cotton. Plant J 96:546–561
Ma D, Xu C, Alejos-Gonzalez F, Wang H, Yang J, Judd R, Xie DY (2018) Overexpression of artemisia annua cinnamyl alcohol dehydrogenase increases lignin and coumarin and reduces artemisinin and other sesquiterpenes. Front Plant Sci 19:9–828
Maxmen A (2019) How African scientists are improving cassava to help feed the world. Nature 565:144–147
Mehdi R, Lamm CE, Anjanappa RB, Müdsam C, Saeed M, Klima J, Kraner ME, Ludewig F, Knoblauch M, Gruissem W, Sonnewald U, Zierer W (2019) Symplasmic phloem unloading and radial post-phloem transport via vascular rays in tuberous roots of Manihot Esculenta. J Exp Bot 70:5559–5573
Ménard G, Biais B, Prodhomme D, Ballias P, Gibon Y (2014) Analysis of Enzyme Activities. In: Dieuaide-Noubhani M, Alonso AP, (eds.), Plant metabolic flux analysis: methods and protocols, methods in molecular biology. Springer Science Business Media New York, pp 249–259
Ntawuruhunga P, Dixon AGO (2010) Quantitative variation and interrelationship between factors influencing cassava yield. J Appl Biosci 26:1594–1602
Obata T, Klemens PAW, Rosado-Souza L, Schlereth A, Gisel A, Stavolone L, Zierer W, Morales N, Mueller LA, Zeeman SC, Ludewig F, Stitt M, Sonnewald U, Neuhaus HE, Fernie AR (2020) Metabolic profiles of six African cultivars of cassava (Manihot esculenta Crantz) highlight bottlenecks of root yield. Plant J 102(6):1202–1219
Qin QY, Lu C, Chen X, Sun YF, Wang WQ (2016) Characterization of biomass and expression of gene involved in starch accumulation among. J China Agr Univ 21:42–50 ((Chinese))
Seung D, Boudet J, Monroe J, Schreier TB, David LC, Abt M, Lu KJ, Zanella M, Zeeman SC (2017) Homologs of protein targeting to starch control starch granule initiation in Arabidopsis leaves. Plant Cell 29:1657–1677
Sheffield J, Taylor N, Fauquet C, Chen S (2006) The cassava (Manihot esculenta Crantz) root proteome: protein identification and differential expression. Proteomics 6:1588–1598
Siebers T, Catarino B, Agusti J (2017) Identification and expression analyses of new potential regulators of xylem development and cambium activity in cassava (Manihot esculenta). Planta 245:539–548
Singh V, Sergeeva L, Ligterink W, Aloni R, Zemach H, Doron-Faigenboim A, Yang J, Zhang P, Shabtai S, Firon N (2019) Gibberellin promotes sweetpotato root vascular lignification and reduces storage-root formation. Front Plant Sci 10:1320
Singh V, Zemach H, Shabtai S, Shabtai S, Aloni R, Yang J, Zhang P, Sergeeva L, Ligterink W, Firon N (2021) Proximal and distal parts of sweetpotato adventitious roots display differences in root architecture, lignin, and starch metabolism and their developmental fates. Front Plant Sci 11:2161
Sojikul P, Kongsawadworakul P, Viboonjun U, Thaiprasit J, Intawong B, Narangajavana J, Svasti MR (2010) AFLP-based transcript profiling for cassava genome-wide expression analysis in the onset of storage root formation. Physiol Plant 140:189–198
Sojikul P, Saithong T, Kalapanulak S, Pisuttinusart N, Limsirichaikul S, Tanaka M, Utsumi Y, Sakurai T, Seki M, Narangajavana J (2015) Genome-wide analysis reveals phytohormone action during cassava storage root initiation. Plant Mol Biol 88:531–543
Sriroth K, Santisopasri V, Petchalanuwat C, Kurotjanawong K, Piyachomkwan K, Oates CG (1999) Cassava starch granule structure–function properties: influence of time and conditions at harvest on four cultivars of cassava starch. Carbohyd Polym 38:161–170
Stein O, Granot D (2019) An overview of sucrose synthases in plants. Front Plant Sci 10:95
Van Soest PJ (1963) Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. AOAC 46:829–835
Vandromme C, Spriet C, Dauvillée D, Courseaux A, Putaux JL, Wychowski A, Krzewinski F, Facon M, D’Hulst C, Wattebled F (2019) PII1: a protein involved in starch initiation that determines granule number and size in Arabidopsis chloroplast. New Phytol 221:356–370
Wyrambik D, Grisebach H (1975) Purification and properties of isoenzymes of cinnamyl-alcohol dehydrogenase from soybean-cell-suspension cultures. Eur J Biochem 59:9–15
Ye JQ, Zhang J, Xiao XH, Wu CY, Xue MF, Li KM (2017) Bring characteristics evaluation of 54 cassava germplasm from Columbia. J Centr China Normal Uni (nature Sci) 51:809–816 ((Chinese))
Zhang P, Ma Q, Naconsie M, Wu X, Zhou W (2017) Advances in genetic modification of cassava. https://doi.org/10.19103/AS.2016.0014.17
Zhang MY, Xue C, Hu H et al (2021) Genome-wide association studies provide insights into the genetic determination of fruit traits of pear. Nat Commun 12:1144
Acknowledgements
We are grateful to Professor Wen-Quan Wang of the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, for kindly providing the cassava varieties. This research was supported by the National Natural Science Foundation of China (32160429), the Guangxi Natural Science Foundation (2021GXNSFDA196009, 2015GXNSFAA139078), and the State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources (SKLCUSA-a202003).
Author information
Authors and Affiliations
Contributions
FXW conceived and supervised the study and revised the manuscript. SJL performed all experiments and prepared the manuscript. HKD and GZY performed the field experiments. LYZ supervised the project and analyzed the PCA data. All authors have reviewed the final manuscript.
Corresponding author
Additional information
Handling Author: Heather Nonhebel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Sun, J., Hui, K., Guo, Z. et al. Cellulose and Lignin Contents are Negatively Correlated with Starch Accumulation, and Their Correlation Characteristics Vary Across Cassava Varieties. J Plant Growth Regul 42, 658–669 (2023). https://doi.org/10.1007/s00344-022-10573-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10573-w