Abstract
Arthropods, especially ixodid ticks, have been incriminated in the epidemiology of Spotted Fever Group rickettsioses globally leading to an increasing spectrum of emerging and re-emerging zoonoses with attendant consequences on trade and tourism. The objective of this study was to determine the role of ixodid ticks infesting small ruminants in Plateau State, Nigeria, in the epidemiology of Spotted Fever Group Rickettsiae (SFGR) in the study area. DNA from 130 out of 323 ixodid ticks collected from 179 goats and 121 sheep owned by agro-pastoralists in Plateau State were screened for the evidence of SFGR by molecular methods. Six tick species from four genera were identified: Amblyomma, Hyalomma, Rhipicephalus (Boophilus) and Rhipicephalus. Rhipicephalus sanguineus sensu lato (s.l.) was the predominant (54.5%) species among collected ticks. Tick infestation was significantly associated with the species of small ruminants, the sex of the animals and the sampling locations except for Jos South. Conventional PCR targeting the 381 bp of the citrate synthase (gltA) and 820 bp of the outer membrane protein B (ompB) genes detected DNA of SFGR in nine and eight samples, respectively. Sequence analysis revealed that five sequences obtained from Amblyomma variegatum were 99–100% identical to Rickettsia africae and three sequences from Rh. sanguineus (s.l.) were 100% identical to Rickettsia massiliae reported from Spain. To our knowledge, this is the first report of the detection of R. africae DNA in Am. variegatum collected from small ruminants in Plateau State. Ixodid ticks infesting small ruminants in Plateau state harbor DNA of SFGR with potential veterinary and public health implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A significant number of Western African countries, particularly those in the northern half of the region, depend on livestock as a source of generating substantial revenue and ensuring livelihoods of the populace (Molina-Flores et al. 2020). In Nigeria, most of the farmer population are rural or peri-urban dwellers, engaged in integrated crop and livestock farming. Nigeria is reputed to have the largest population of small ruminants in Africa comprising of 73.8 million goats and 42.1 million sheep, mostly owned by smallholder farmers and serve as important source of income for the family upkeep (CSIRO 2021). As at 2016, the gross production value of sheep and goats in Nigeria was estimated at US$ 73.4 and 373.1 million, respectively (CSIRO 2021). Therefore, sheep and goats play significant roles in the socio-economic well-being of Nigerians, generating employment for women and youths with about 13 million people engaged in the small ruminant value chain (Ayoade 2000; Lawal-Adebowale 2012; CSIRO 2021). The production system being practiced is either extensive or semi-intensive, where the animals are allowed to roam freely and fend for themselves or receive supplementary feeding at some point in the day depending on the season. This practice exposes the animals to many pathogens and disease vectors, such as ticks. More so, the animals are usually housed in pens located in or around the residence increasing the possibility of the transfer of diseases or vectors between humans and the animals.
Ticks have been reckoned the second most important disease vectors after mosquitoes globally (Estrada-Pena and Jongejan 1999; de la Fuente et al. 2008). Some tick species have been reported to aggressively attack humans (Walker et al. 2003; Okoli et al. 2006). Therefore, where the conditions for the transfer of ticks between humans and animals are favorable, the risks of infestation and transmission of zoonotic disease is predictably high. Studies in Nigeria have reported diversity of ticks infesting animals, some of them harboring pathogens of zoonotic importance, including Rickettsia species (Ogo et al. 2012; Kamani et al. 2015, 2018; Lorusso et al. 2013a, b, 2016; Onyiche et al. 2020; Mamman et al. 2021).
Members of the genus Rickettsia are obligate intracellular, Gram-negative bacteria that infect a wide range of vertebrate hosts and arthropods, especially hard ticks are reputed as their vectors or reservoirs (Roux and Raoult 2000). Available data suggest that domestic livestock may play a role in the maintenance of human pathogenic rickettsiae (Kelly et al. 1991). A number of the SFGR have been incriminated in causing human diseases globally and are considered as threat to international travelers with negative impact on trade and tourism (Jensenius et al. 2006). The symptoms of the conditions in humans may include high fever, headache, rash and occasional eschar formation at the site of the tick bite, with the potential for a fatal outcome (Parola and Raoult 2001; Parola et al. 2005; Rashid et al. 2008). These symptoms mimic other febrile conditions such as malaria and typhoid fever in tropical countries and may complicate treatment outcome due to the possibilities of misdiagnosis.
In Nigeria, there is reported evidence of antibodies to SFGR among small ruminants (Nnabuife et al. 2021), and the detection of the DNA of R. africae, Rickettsia aeschlimannii, Rickettsia conorii and R. massilliae in ixodid ticks collected from animals and vegetation (Ogo et al. 2012; Reye et al. 2012; Lorusso et al. 2013a, b, 2016; Kamani et al. 2015, 2018). However, reports on the molecular detection of zoonotic rickettsiae in ticks collected from small ruminants are scanty and far apart. Therefore, this study aimed to use PCR and sequencing to detect and characterize SFGR in ticks collected from sheep and goats in Plateau State, Nigeria.
Materials and methods
Animal ethics
The approval for the study was granted by the Institutional Animal Care and Use Committee (IACUC), National Veterinary Research Institute (NVRI), Vom, Plateau State, Nigeria, No. AEC/02/62/18. In addition, informed consent was obtained from animal owners before the animals were sampled.
Study areas
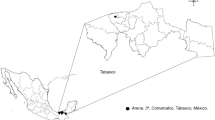
The study was carried out from September to November 2018, in farming communities located in Jos-South, Kanke and Shendam Local Government Areas (LGAs) of Plateau State, Nigeria (Fig. 1). Plateau State occupies an area of approximately 26,899 km2 in the central part of Nigeria. The state has an estimated population of about 3.2 million inhabitants (NPC 2006). The majority of the population are rural based and are engaged in integrated crop-livestock farming as their main occupation. The state is located on an altitude of about 1238 m above sea level and experiences a near temperate climate, which is favorable for crop and livestock production.
Study design
Three LGAs, each representing the northern, central and southern senatorial zones of Plateau State were randomly selected (Fig. 1). In each of the selected LGAs, farming communities were visited and farmers were recruited to the study through the snowballing technique. In all, 15 farming communities, Jos South (n = 4), Kanke (n = 6) and Shendam (n = 5) were selected for tick sampling. Consenting farmers were given an open-ended structured questionnaire in order to obtain data on their livestock farming activities.
Tick sampling
Small ruminants (121 sheep and 179 goats) were randomly selected from the 1174 (473 sheep, 701 goats) small ruminant holdings from 15 farming communities in three LGAs of Plateau state and examined for tick infestation. Animals to be examined were properly restrained and the entire body was examined for the presence of ticks. Ticks found on the body were removed using forceps and placed in coded vials containing 70% ethanol. Data on the location, age and sex of the animal were also recorded.
Ticks collected were transported to the Parasitology Division, National Veterinary Research Institute (NVRI), Vom, Plateau State, Nigeria, for identification and molecular detection of SFGR. In the laboratory, all collected ticks were examined under a stereomicroscope and identified to the genus and species level following the morphological keys by Walker et al. (2003). Thereafter, ticks were sorted according to genus/species, counted and stored in coded vials.
DNA extraction from ticks
One hundred and thirty ticks were selected based on species and the host for DNA extraction. DNA was extracted from adult Amblyomma variegatum, Hyalomma truncatum, Rhipicephalus sanguineus (s.l.), Rhipicephalus evertsi evertsi, Rhipicephalus muhsamae and Rhipicephalus (Boophilus) decoloratus. The ticks were individually washed in three changes of sterile phosphate buffered saline (PBS), and cut into small pieces using a sterile razor blade before homogenization in 1.5-ml Eppendorf tubes each containing 50 µL PBS. Genomic DNA was extracted using the QIAamp DNA Tissue Extraction Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA from each tick was eluted in 200 µL of elution buffer and stored at −20 °C until used for PCR.
Identification of ticks
Ticks were initially identified using morphological keys according to Walker et al. (2003). To confirm the morphological identification, a subset of ticks from each species were subjected to PCR for the amplification of the 16S rRNA and the cytochrome oxidase 1 (cox 1) genes using published primers (Folmer et al. 1994; Mangold et al. 1998) under the conditions listed in Table 1.
Detection of DNA of SFGR in ticks by Conventional PCR
An initial screening of all 130 tick DNA samples for the presence of the SFGR was conducted by subjecting them to conventional PCR to amplify the 381 bp gltA gene of Rickettsia species. Samples that were gltA-positive were further subjected to conventional PCR targeting the 820 ompB gene. PCR primers and amplification conditions were sourced from the literature and are presented in Table 1. The reaction was conducted in a 20 µL total volume consisting of 4 µL of 5X FIREPol Master Mix (Solis BioDyne, Estonia), 0.5 µL of each primer, 5 µL DNA template and 10 µL of nuclease free water (BioConcept, Switzerland). The amplification program consisted of: initial denaturation at 95 °C for 5 min followed by denaturation at 95 °C for 30 s (annealing and number of cycles as presented in Table 1), elongation at 72 °C for 1 min and a final elongation at 72 °C for 10 min. Amplification was conducted on a GenAMP 7400 (Applied Biosystems, Foster City, CA, USA) in the Biotechnology Division, NVRI, Vom, Nigeria. The PCR products were electrophoresed on 1.5% agarose gels in Tri-acetate-ethylene diamine tetra-acetic acid (TAE) buffer stained with ethidium bromide. The gels were visualized under Ultra Violet (UV) light in comparison to a 100 bp DNA ladder. Positive amplicons were sequenced in both directions at a commercial facility (Macrogen Europe, The Netherlands) using the PCR primers.
Nucleotide sequences obtained in this study were edited manually using the Bioedit Software (Hall 1999) and checked for similarities with sequences in the GenBank using the BLASTn algorithm hosted by NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences obtained in this study were deposited in GenBank under the following accession numbers: [ticks] 16S rRNA: R. sanguineus and A. variegatum, OM348705–OM348714; Cox 1: OM349598–OM349603; [Rickettsia spp.] gltA: R. africae, OM393698, OM393700 and OM393702; R. massiliae, OM393702–OM393704; ompB: R. africae, OM393706–OM393710; R. massiliae, OM393705.
Data analysis
The prevalence of ticks was calculated as percentage of animals infested over the total number examined. The association between tick infestation and the variables: type of small ruminant (sheep or goats), sex of animals and the sample location were analyzed. Univariate analysis was performed for each risk factor using the χ2 test. The analysis was performed using the R Statistical Software (R Core Team 2009). The level of significance was set at α = 0.05.
Results
Ticks identified from sheep and goats
In this study, a total of 300 small ruminants (121 sheep, 179 goats) were examined for tick infestation. Overall, 139 out of the 300 (46.3%) animals examined were infested with ixodid ticks. Higher prevalence of tick infestation in sheep than goats was recorded in Kanke and Shendam but not in Jos South LGA (Table 2). Similarly, there was an association between the sex of the sheep and goats examined and tick infestation (Table 2). The morphological identification of ticks was confirmed by PCR and sequencing (Fig. S2). Furthermore, the 16S rRNA and cox 1 nucleotide sequence generated in this study were 99–100% identical to the sequences of the respective tick species in GenBank. Overall, Rh. sanguineus (s.l.) was the most prevalent (54.5%) tick species identified in this study both on sheep (n = 106, 32.8%) and goats (n = 70, 21.7%). Rh. evertsi evertsi, Rh. (B.) decoloratus and Am. variegatum recorded prevalence of 17.3, 9.9 and 9.6%, respectively (Table 3).
Detection of DNA of SFGR in ticks by conventional PCR
Genomic DNA was extracted from 130 ixodid ticks including Rh. sanguineus (s.l.) (n = 70), Am. variegatum (n = 16), Rh. evertsi evertsi (n = 10), Rh. (B.) decoloratus (n = 28), Rh. muhsamae (n = 4) and Hy. truncatum (n = 2) and used for this study. The DNA of SFGR was detected in nine out of the 130 (6.9%) samples examined targeting the gltA gene, whereas eight out of the nine gltA-positive samples were further amplified using the ompB gene (Table 4). The positive samples included five Am. variegatum (three females and two males), and four Rh. sanguineus (s.l.) (two females, one male and one larva). None of the other tick species examined in this was positive for SFGR DNA (Table 4). Five of the ompB positive ticks were from sheep and three from goats. Six positive samples were from Kanke, two from Jos South and one from Shendam LGA. The DNA of R. africae was detected in four Am. variegatum, three being adult females and one a male. All of them were collected from sheep in Kanke LGA. Interestingly, DNA of R. africae was detected in Rh. sanguineus (s.l.) larva removed from a goat in Shendam LGA. Rickettsia massiliae DNA was detected in one male, one female and a larva of Rh. sanguineus (s.l.) collected from sheep in Jos south and Kanke LGAs. The DNA of SFGR was not detected in any of the Hy. truncatum, Rh. muhsamae, Rh. (B.) decoloratus and Rh. evertsi evertsi tested in this study (Table 4).
The SFGR gltA nucleotide sequences detected in this study were 99% identical to each other and 99.9–100% identical to sequences of several SFGR in GenBank (Table 4, Fig. 2). Three each of the ompB sequences generated in this study were 99–100% identical to sequences of R. massiliae and R. africae in GenBank. One of the gltA sequence in this study was 99.9% identical to the sequence of R. parkeri in GenBank; however, the ompB sequence did not yield any significant result on BLASTn, thus the gltA result was not considered further.
The evolutionary history of the SFGR gltA nucleotide sequences was inferred using the Neighbor-Joining method (Saitou and Nei 1987). Percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. This analysis involved 21 nucleotide sequences. There were in total 1806 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (Kumar et al. 2018)
Discussion
Ixodid ticks play a major role in the transmission of several pathogens including members of the SFGR to both humans and animals. This is particularly common where the livestock production system predisposes the animals to high tick infestation and equally bring them in close contact with their owners as is the case in this study. Available records indicated the diversity of ixodid ticks infesting livestock in Nigeria (Dipeolu 1975; Lorusso et al. 2013a; Mamman et al. 2021). Furthermore, these ticks have been shown to harbor several pathogenic organisms including emerging and re-emerging pathogens such as the SFGR underscoring the public health implications (Reye et al. 2012; Ogo et al. 2012; Lorusso et al. 2013b, 2016; Kamani et al. 2015, 2018; Onyiche et al. 2020).
This study recorded an overall prevalence of 46.3% tick infestation in the 300 small ruminants examined. The reported prevalence in this study is similar to the 45.95% reported in sheep and goats in Enugu state. However, the pattern of infestation differed between the two studies. Higher prevalence of 61.7% was recorded in goats compared to 18.5% recorded in sheep (Ugochukwu and Apeh 1985), in contrast to the 66.9% in sheep and 32.4% in goats in this study. Elsewhere, lower tick infestation prevalence of 26.9% was reported in Sokoto state with 23.1 and 3.8% prevalence in sheep and goats, respectively (Alayande et al. 2016), whereas higher prevalence of 76.4% was reported in Oyo state with 80.3 and 67.3% of goats and sheep, respectively, being infested (Ameen et al. 2014). Despite the wide variation in tick infestation prevalence, the tick species diversity was similar, with the members of Amblyomma and Rhipicephalus genera being dominant. Interestingly, members of these two tick genera have been implicated in the epidemiology of SFGR in Nigeria (Ogo et al. 2012, Reye et al. 2012; Lorusso et al. 2013b, 2016; Kamani et al. 2018). The DNA of R. africae has been amplified in Am. variegatum in Nigeria (Ogo et al. 2012; Lorusso et al. 2013b). Furthermore, the DNA of R. massilae was detected in Rh. sanguineus (s.l.) tick collected from cattle in Nigeria (Lorusso et al. 2013b, 2016). In addition, the DNA of R. conorii and a yet unidentified variant of Rickettsia spp. was amplified from Rh. sanguineus (s.l.) ticks collected from pre-domestic rodents in Nigeria (Kamani et al. 2018). Therefore, the detection of the DNA of R. africae and R. massiliae in Am. variegatum and Rh. sanguineus (s.l.) collected from sheep and goats in this study further attests to the role of these ticks in the epidemiology of SFGR infection in small ruminants in Nigeria. Our previous study findings (Nnabuife et al. 2021) that demonstrated rickettsioses in small ruminants in Nigeria further affirm the above argument. Our findings in this study are similar to the results of a study in Senegal (Mediannikov et al. 2010) that revealed molecular evidences of ixodid tick infection with several members of the SFGR. Amblyomma variegatum is the putative vector of R. africae (Kelly et al. 1996), and a high prevalence of up to 100% R. africae DNA detected in this tick species in Guinea was attributed to the extreme fitness of R. africae to this vector (Mediannikov et al. 2012). Amblyomma variegatum are common in most of sub-Saharan Africa and are reputed as being aggressive and frequently bite humans (Nduaka and Ikeme 1973; Walker et al. 2003).
The two Rickettsia spp. (R. africae and R. massiliae) detected in ticks collected from sheep and goats in this study are zoonotic. Rickettsia africae is the causative agent of African tick-bite fever (ATBF) in humans (Socolovschi et al. 2009). Most cases of African tick bite fever have a benign and self-limiting course, which is characterized by fever, headache, nuchal myalgia and inoculation eschar with regional lymphadenitis (Raoult et al. 2001). Rickettsia massiliae has been repeatedly detected in Rhipicephalus sp. ticks in Africa (Mediannikov et al. 2010; Lorusso et al. 2016) and was first linked to human infection in 2005 (Vitale et al. 2005). Interestingly, we detected the DNA of R. massiliae in a larva of Rh. sanguineus (s.l.) collected from a goat in Shendam, Nigeria. This suggests the transovarian transmission of R. massiliae by the brown dog tick (Matsumoto et al. 2005). Similarly, the transovarial and transstadial transmission of R. africae in Am. variegatum ticks have been reported (Socolovschi et al. 2009), demonstrating their vector competence and their role in perpetuating SFGR in the ecosystem. The non-detection of SFGR DNA in Rh. evertsi evertsi, Rh. (B.) decoloratus, Rh. muhsamae and Hy. truncatum in this study might be due to the small sample numbers tested. This is in accord with the low prevalence reported in these tick species (Mediannikov et al. 2010).
The results from this study are in accord with reports of earlier studies on ticks from vegetation and other mammals and thus, further reinforce the potential roles of ixodid ticks in the epidemiology of SFGR in Nigeria (Ogo et al. 2012; Reye et al. 2012; Lorusso et al. 2013b, 2016; Kamani et al. 2018). The detection of SFGR DNA in Am. variegatum and Rh. sanguineus (s.l.) from sheep and goats in this study was not surprising considering that both ticks utilize different animal hosts during their developmental stages. Therefore, any of the adult ticks removed form sheep or goat might have fed on other animal or man during one of the developmental stages. This is particularly worrying as the traditional practice of small ruminant production among the agro-pastoralist in the study areas encourages close contact between the animals and man, hence the high possibility of the transfer of ticks and their associated pathogens between them as well. Additionally, there are many dogs in the study areas as most of the farmers keep dogs either for shepherding, hunting or as pets. Dog meat is also consumed by humans as a source of protein in most of the study areas. This may explain the high infestation of the sheep and goats by the brown dog tick in this study. Studies have shown that although, dogs are the preferred host of Rh. sanguineus, this tick can infest and adapt to a wide variety of hosts and ecological conditions, earning it the appellation ‘catholic tick’ (Dantas-Torres 2010). Therefore, based on the results from previous studies (Reye et al. 2012; Ogo et al. 2012; Lorusso et al. 2013b, 2016; Kamani et al. 2015, 2018; Onyiche et al. 2020) and the findings from this study, it should be noted that SFGR are prevalent and widespread in ticks in Nigeria and should be accorded priority among pathogens of public health importance.
In conclusion, this study reports the detection of the DNA of R. africae and R. massiliae in Am. variegatum and Rh. sanguineus (s.l.) ticks collected from small ruminants in Plateau State, Nigeria. This finding adds to the list of hosts of ixodid ticks positive for SFGR in Nigeria. To the best of our knowledge, this is the first detection of R. africae in Am. variegatum collected from small ruminants in Nigeria. Owing to close contact of human and livestock, and also the non-selective biting nature of ticks that facilitates human exposure to ticks further evokes the importance of SFGR agents in public health perspectives.
References
Alayande MO, Mayaki AM, Lawal MD, Abubakat A, Kassu M, Talabi AO (2016) Pattern of ticks and lice infestation on small ruminants in Sokoto, Sokoto State. Nigerian J Anim Sci 1:183–189
Ameen SA, Odetokun IA, Ghali-Muhammed LI, Azeez OM, Raji LO, Kolapo TU (2014) Status of ticks infestation in ruminant animals in Ogbomoso area of Oyo State, Nigeria. J Environ Issues Agric Dev Countr 6(2 & 3):48–53
Ayoade JA (2000) Problems and prospect of small ruminant production in Benue State. In: Proceedings of the annual conference of nigeria society for animal production. Michael Okpala University of Agriculture Umudike, 19–23 March 2000, pp 150–151
Commonwealth Scientific and Industrial Research Organization (CSIRO) (2015–2020) Small ruminant production in Nigeria. https://research.csiro.au/livegaps/. Accessed 13 Jan 2022
Dantas-Torres F (2010) Biology and ecology of the brown dog tick, Rhipicephalus Sanguineus. Parasites Vectors 3:26
de la Fuente J, Estrada-Pena A, Venzal JM, Kocan KM, Sonenshine DE (2008) Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci 13:6938–6946
Dipeolu OO (1975) The incidence of ticks of Boophilus species on cattle, sheep and goats in Nigeria. Trop Ani Hlth Prod 7:35–39
Estrada-Pena A, Jongejan F (1999) Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol 23:685–715
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Marine Bio Biotech 3(5):294–299
Hall TA (1999) Bioedit: a user-friendly biological sequence alignment editor and analysis program for Window 94/98/NT. Nucleic Acids Sympos Ser 41:95–98. http://tools.immuneepitope.org/bcell/
Jensenius M, Parola P, Raoult D (2006) Threats to international travellers posed by tick-borne diseases. Travel Med Infect Dis 4:4–13
Kamani J, Baneth G, Apanaskevich DA, Mumcuoglu KY, Harrus S (2015) Molecular detection of Rickettsia aeschlimannii in Hyalomma spp. ticks from camels (Camelus dromedarius) in Nigeria, West Africa. Med Vet Entomol 29:205–209. https://doi.org/10.1111/mve.12094
Kamani J, Baneth G, Gutiérrez R, Nachum-Biala Y, Mumcuoglu KY, Harrus S (2018) Coxiella burnetii and Rickettsia conorii: two zoonotic pathogens in peridomestic rodents and their ectoparasites in Nigeria. Ttbds 9:86–92
Kelly PJ, Mason PR, Manning T, Slater S (1991) Role of cattle in the epidemiology of tick-bite fever in Zimbabwe. J Clin Microbiol 29:256–259
Kelly PJ, Beati L, Mason PR, Matthewman LA, Roux V, Raoult D (1996) Rickettsia africae sp. nov., the etiological agent of African tick bite fever. Int J Syst Bacteriol 46:611–614
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Bio Evol 35:1547–1549
Lawal-Adebowale OA (2012) Dynamics of ruminant livestock management in the context of the Nigerian agricultural system. In: Javed K (ed) Livestock production. IntechOpen, London. https://doi.org/10.5772/52923
Lorusso V, Picozzi K, de Bronsvoort BM, Majekodunmi A, Dongkum C, Balak G, Igweh A, Welburn SC (2013a) Ixodid ticks of traditionally managed cattle in Central Nigeria: where Rhipicephalus (Boophilus) microplus does not dare (yet?). Parasites Vectors 6:171
Lorusso V, Gruszka KA, Majekodunmi A, Igweh A, Welburn SC, Picozzi K (2013b) Rickettsia africae in Amblyomma variegatum ticks, Uganda and Nigeria. Emerg Infect Dis 19:1705–1707
Lorusso V, Wijnveld M, Majekodunmi AO, Dongkum C, Fajinmi A, Dogo AG, Thrusfield M, Mugenyi A, Vaumourin E, Igweh AC, Jongejan F, Welburn SC, Picozzi K (2016) Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasites Vectors 9:217. https://doi.org/10.1186/s13071-016-1504-7
Mamman AH, Lorusso V, Adam BM, Dogo AG, Bown KJ, Birtles RJ (2021) First report of Theileria annulata in Nigeria: Findings from cattle ticks in Zamfara and Sokoto States. Parasite Vectors 14:242. https://doi.org/10.1186/s13071-021-04731-4
Mangold AJ, Bargues MD, Mas-Coma S (1998) Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol Res 84:478–484
Marquez FJ, Muniain MA, Soriguer RC, Izquierdo G, Rodriguez-Bano J, Borobio MV (1998) Genotypic identification of an undescribed spotted fever group rickettsia in Ixodes ricinus from southwestern Spain. Am J Trop Med Hyg 58(5):570–577. https://doi.org/10.4269/ajtmh.1998.58.570
Matsumoto K, Ogawa M, Brougui P, Raoult D, Parola P (2005) Transmission of Rickettsia massiliae in the tick, Rhipicephalus turanicus. Med Vet Entomol 19:263–270
Mediannikov O, Diatta G, Fenollar F, Sokhna C, Trape JF, Raoult D (2010) Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis 4(9):e821. https://doi.org/10.1371/journal.pntd.0000821PLoS
Mediannikov O, Diatta G, Zolia Y, Balde MC, Kohar H, Trape J-F, Raoult D (2012) Tick-borne rickettsiae in Guinea and Liberia. Ttbds 3:43–44. https://doi.org/10.1016/j.ttbdis.2011.08.002
Molina-Flores B, Manzano-Baena P, Coulibaly MD (2020) The role of livestock in food security, poverty reduction and wealth creation in West Africa. Accra. FAO, Rome. Accessed 20 Nov 2021
National Population Commission (2006). Plateau State. http://www.population.gov.ng. Accessed 6 May 2022
Nduaka O, Ikeme MM (1973) Human skin lesions in East Central State, Nigeria, due to the larvae of Amblyomma variegatum (Fabricius, 1794). Niger Med J 3:140–143
Nnabuife HE, Matur B, Ogo NI, Goselle O, Dakul A, Egbuji A, Tekki IS, Kamani J (2021) Seroprevalence of Spotted Fever Group Rickettsiae in extensively managed sheep and goats in Nigeria, West Africa. Trop Ani Health Prod 53:425
Ogo NI, Fernández de Mera GI, Galindo RC, Okubanjo OO, Inuwa HM, Agbede RIS, Torina A, Alongi A, Vicente J, Gortázar C, de la Fuente J (2012) Molecular identification of tick-borne pathogens in Nigerian ticks. Vet Parasitol 187:572–577
Okoli IC, Okoli CG, Opara M (2006) Environmental and multi-host infestation of the brown dog tick, Rhipicephalus sanguineus in Owerri, South-East Nigeria—a case report. Vet Arhiv 76:93–100
Onyiche TE, Răileanu C, Tauchmann O, Fischer S, Vasić A, Schäfer M, Biu AA, Ogo NI, Thekisoe O, Silaghi C (2020) Prevalence and molecular characterization of ticks and tick-borne pathogens of one-humped camels (Camelus dromedarius) in Nigeria. Parasites Vectors 13:428. https://doi.org/10.1186/s13071-020-04272-2
Parola P, Raoult D (2001) Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin Infect Dis 32:897–928
Parola P, Paddock CD, Raoult D (2005) Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 18:719–756
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Raoult D, Fournier PE, Fenollar F, Jensenius M, Prioe T, de Pina JJ, Caruso G, Jones N, Laferl H, Rosenblatt JE, Marrie TJ (2001) Rickettsia africae, a Tick-Borne pathogen in travelers to sub-Saharan Africa. N Engl J Med 344(20):1504–1510. https://doi.org/10.1056/NEJM200105173442003
Rashid R, Pasqualotto AC, Denning DW (2008) A case of spotted fever group rickettsiosis imported into the United Kingdom and treated with ciprofloxacin: a case report. J Med Case Reports 2:98. https://doi.org/10.1186/1752-1947-2098
Reye AL, Arinola GO, Hübschen JM, Muller CP (2012) Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl Environ Microbiol 78(8):2562–2568
Roux V, Raoult D (2000) Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol 50:1449–1455
Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Socolovschi C, Huynh TP, Davoust B, Gomez J, Raoult D, Parola P (2009) Transovarial and trans-stadial transmission of Rickettsiae africae in Amblyomma variegatum ticks. Clin Microbiol Infect 15(Suppl. 2):317–318
Ugochukwu EI, Apeh AO (1985) Prevalence of ectoparasites of small ruminants in Nsukka, Nigeria. Int J Zoon 12(4):313–317
Vitale G, Mansueto S, Rolain JM, Raoult D (2005) Rickettsia massiliae human isolation. Emerg Infect Dis 12:174–175
Walker AR, Bouattour A, Camicas JL, Estrada-Pena A, Horak IG, Latif AA, Pegram RG, Preston PM (2003) Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports, Edinburgh
Acknowledgements
The authors are grateful to Mike Shands of the School of Geographical and Earth Sciences, University of Glasgow, Glasgow, UK for providing Fig. 1. The contributions of Mr. Bobmanuel Echeonwu and Drs. TM Joannis, LD. Pam, A. Benshak, S. Makama & A. Egbuji (NVRI, Vom), Anyaeji Nnabuenyi N (USA), Dr. Emmanuel Nonso Udebunu (Australia) of Our Medical Home 1/9 Hollinsworth Rd, Marsden Park, NSW 2765, Australia for his contribution to this work.
Author information
Authors and Affiliations
Contributions
NHE, MB, OIN and GO. conceived and designed the study. NHE, OIN and JK. conducted sampling.SI, MN, OE and CN conducted laboratory analyses. NHE, JK and MN conducted data analysis. NHE, MB, OIN and KJ. Wrote the draft manuscript. All authors read and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

10493_2022_769_MOESM1_ESM.jpg
Figure S1 Gel electrophoresis of amplification of partial sequence of ompB gene of SFRG (product size ≈820 bp) from ixodid ticks of small ruminants in Nigeria. (M): molecular weight marker = 100 bp; positive samples in lanes 2, 3, 4, 6, 7, 8, 9 and negative samples in lanes 1, 5, 10. Positive control = 12. Non-template control = 13 (JPG 201 KB)

10493_2022_769_MOESM2_ESM.jpg
Figure S2: Gel electrophoresis of amplification of partial sequence of 16S rRNA gene (≈420 bp) of ixodid ticks from small ruminants in Nigeria. (M): molecular weight marker = 100 bp; lane 16-positive control; lane 17-NTC. All the samples were amplified at varying intensities except lane 13 where there was no amplification (JPG 205 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nnabuife, H.E., Matur, B., Ogo, N.I. et al. Rickettsia africae and Rickettsia massiliae in ixodid ticks infesting small ruminants in agro-pastoral settlements in Plateau State, Nigeria. Exp Appl Acarol 89, 117–130 (2023). https://doi.org/10.1007/s10493-022-00769-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00769-w