Abstract
The ectoparasite Varroa (Acari: Varroidae) is considered to be the main pest of honey bees (Apis mellifera L.) in Nicaragua. The aim of this study was to determine morphotypes and mitochondrial haplotypes of the Varroa mites, related to infestation levels in A. mellifera hives in Nicaragua in a cross-sectional study (2013–2016). Samples were taken from 34 sentinel apiaries in five geographical zones; from 713 Varroa females collected during the study, 153 were selected for measurement of various morphometric characters for further classification into morphotypes. The mitochondrial haplotype was assigned to one of the two (Korean or Japanese), using the restriction by SacI of the PCR product of a fragment of the gene CO-I. Morphometric measurement and sequencing revealed the affiliation to the species Varroa destructor with a mean (± SD) body width of 1699.1 ± 60.2 µm and a body length of 1161.1 ± 34.9 µm. Body characters were significantly different among the 34 apiaries. Varroa destructor samples were classified into four morphotypes, with no significant differences in the geographical zones. As GAGCTC SacI enzyme cleavage sequences were not observed, all PCR products were identified as V. destructor Korean haplotype. The CO-I gene nucleotide sequences from two samples V. destructor showed both 100% similarity with the Korean haplotype and 99.8% similarity to the Japanese haplotype. Although the V. destructor mite was identified as a Korean haplotype, host-parasite association in 2 decades has led into a balance without entering into severe losses in the Nicaraguan apiculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ectoparasitic mites of the genus Varroa have become the most important pests in honeybees during the last 60 years, threatening worldwide the livelihoods of honey production and pollination-depending industries and the local economy of small-scale beekeeping (Delaplane and Hood 1999; Rosenkranz et al. 2010). This relevance is due to the damages the parasite can cause in the beehive, a decline in the honey bee population and thus a decrease in honey production or even the loss of entire hives (Rosenkranz et al. 2010; Le Conte et al. 2010). Various factors like genetic origin of the infested bees and their behavior (Rath 1999; Wilde et al. 2005; Harris 2008), environmental conditions (Le Conte and Navajas 2008), transmission of virus diseases (Chen and Siede 2007), and technical failure of handling the hives affect the reproductive success of the parasite.
Morphological variance and differences in behavior have led to the supposition that phenotypically similar mites have distinct population dynamics due to genetic characters (Rothenbuhler 1964; Rosenkranz and Engels 1994; Anderson and Fuchs 1998). According to Anderson and Trueman (2000), Varroa includes four species: Varroa jacobsoni Oudemans, Varroa underwoodi Delfinado-Baker & Aggarwal, Varroa rindereri De Guzman & Delfinado-Baker, and Varroa destructor Anderson & Trueman. Of these, V. destructor is present in Asia, Europe, Africa and America, parasitizing both Apis cerana (original host) and A. mellifera (new host) (Anderson and Trueman 2000; Anderson 2000).
Anderson and Trueman (2000) detected 18 haplotypes (mites with distinct mtDNA CO-I gene sequences) by sequence analysis of V. jacobsoni. The haplotypes were related to different countries in Asia and assigned to (i) Flores-Java clade with at least nine haplotypes, (ii) Japan/Thailand-Vietnam clade with at least six haplotypes, and (iii) three separate haplotypes. Morphometric comparison, mtDNA sequences and geographical distribution suggested the redefinition of the species V. jacobsoni, separating the Japan/Thailand-Vietnam clade and renaming the mites belonging to this genetic branch V. destructor, with six haplotypes. Varroa destructor has disseminated successfully into A. mellifera hives all over the world with only two haplotypes: the most common Korean/Russian haplotype infesting A. mellifera in Africa, Europe, the Middle East, Asia and the Americas and the less common Japan/Thailand haplotype infesting A. mellifera in Japan, Thailand and the Americas. New variants of the identified haplotypes are found since (Anderson and Trueman 2000; Solignac et al. 2005; Navajas et al. 2010).
Beekeeping in Nicaragua, as most of agricultural activities in Mesoamerica, is a small-scale business (Vandame and Palacio 2010). About 1500 beekeepers with approximately 2300 apiaries and 48,000 beehives are active in Nicaragua, the vast majority with less than 25 hives (Institute of Agricultural Protection and Health, pers. comm., March 18, 2020). Currently in Nicaragua there is no information about the systematic morphology and genetic variability of Varroa. The appearance of this mite was first mentioned in 1996 and the Ministry of Agriculture and Forestry started surveillance (OIE 1996). It was not until 2000 that three outbreaks were officially reported with no further information (OIE 2000). Two decades have passed and epidemiological surveillance reports a nationwide infestation of the apiaries and feral colonies. The aim of this study was to determine morphotypes and mitochondrial haplotypes of the Varroa mite, related to infestation levels in A. mellifera hives in Nicaragua.
Material and methods
Sample collection
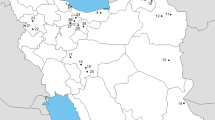
In a cross-sectional study (2013–2016) in areas with beekeeping activity in Nicaragua, samples were taken in five geographical zones in 34 sentinel honey bee apiaries, which are determined by the Institute of Agricultural Protection and Health (IPSA) for annual epidemiological surveillance; sentinel apiaries were identified because of an increased risk of contracting diseases such as proximity to borders and a high concentration of beekeepers, apiaries and hives. The locations of the five zones are: Pacific Lowlands with northwest (eight apiaries monitored) and southwest (two apiaries monitored) extension, Central Highlands (seven apiaries monitored), Northern Highlands (14 apiaries monitored), and South Caribbean Coast Autonomous Region (three apiaries monitored), covering 10 departments and 20 municipalities. According to Köppen Climatic Classification Systems, the Pacific Lowlands are drier and warmer, reaching 45 °C as a maximum temperature (elevation 0–290 m); Central and Northern Highlands have the lowest temperature, with records of minimum temperatures near to 14 °C (elevation 100–1450 m). South Caribbean Coast has high temperatures and high humidity during the entire year (elevation 10–170 m) (INETER 2020). Most of the apiculture activities are in the Pacific Lowlands (north and south extension), followed by the Central Highlands, Northern Highlands and the South Caribbean Coast. In the Northern Highlands, there is more diversity in fauna and flora according to geographical and climate conditions than in the Pacific Lowlands and the Central Highlands, therefore, more apiaries have been added to ensure diversity. In the South Caribbean Coast Autonomous Region, very few beekeepers have constant activities, because of rainy climate conditions, therefore only three apiaries were included.
Each selected apiary was monitored once during the investigation period. The samples were taken from three hives presenting bees with phoretic mites and every sample consisted of approximately 300 adult bees from three different frames of the breeding chamber. The bees were collected in labeled plastic containers (500 ml) with 150 ml of 97% ethanol for storage.
Following the shaking method (Jong et al. 1982), the plastic container was shaken to release the parasites from the bees. In the laboratory, plastic containers with the bees were shaken again and subsequently the content was emptied onto a white tray. The bees were individually checked and the mites that still adhered were removed onto the tray. Bees and mites in the sample were counted to calculate the infestation rate (IR).
Morphometric techniques
Of the 713 female Varroa collected during the study, 153 were selected for morphometric measurements, such as body width and length, length and width of the genital shield and of the anal shield, length of setae and chelicerae. The mites were processed in 0.9% NaCl and 5% KOH solutions, dried and then fixed with Canada Balsam (Millipore Sigma, USA) and measured with a Nikon Eclipse 55i microscope/Nikon digital sight DS-FA camera.
Genetic diagnosis and DNA extraction
From the saved samples, female Varroa were selected based on morphometric characteristics and geographic origin, to form 24 representative pools of five specimens each. The female mites in each pool were cut into small pieces with a scalpel and placed in a 2-ml vial, 200 μl of nuclease-free water was added and then macerated with a sterile mortar to obtain a pasty texture, 200 μl more of nuclease-free water was added. Of this mixture, 200 μl was placed in a new vial and then DNA extraction DNeasy Blood & Tissue Kit (Qiagen, Germany) was used, proceeding according to the manufacturer’s instructions.
Mitochondrial DNA amplification
The mitochondrial haplotype is assigned to one of the two V. destructor mtDNA types, using the restriction by SacI of the PCR product of a fragment of the gene CO-I (Anderson and Fuchs 1998). We performed conventional PCR with primers previously described for mtDNA amplification (Solignac et al. 2005): forward, 50-TACAAAGAGGGAAGAAGCAGCC-30; reverse, 50-GCCCCTATTCTTAATACATAGTGAAAATG-30. The PCR reaction was performed in a 50-µl volume, containing 2 µl of forward primer (10,000 nM), 2 µl of reverse primer (10,000 nM), 25 μl Master Mix 2X (Promega, USA), 16 µl of nuclease-free water and 5 µl of sample DNA. Amplification program was a first denaturation step at 94 °C for 4 min, followed by 35 cycles of 1 min denaturation at 95 °C, 1.30 min annealing at 52 °C and 1.30 min extension at 72 °C, after one step of 72 °C for 7 min and finally 4 °C for storage. The quality control was made adding a negative control from nuclease-free water to the extraction. PCR was processed in Laboratorio del Diagnóstico Molecular, del Centro Veterinario de Diagnóstico e Investigación (CEVEDI), Escuela de Ciencias Agrarias y Veterinarias (ECAV), Universidad Nacional Autónoma de Nicaragua-León (UNAN-León), using the 2720 Applied Biosystems Thermal Cycler.
Typing
Haplotypes were assigned using the restriction by SacI of the PCR product (376 pbp), the digestion mix contained 10 µl of PCR mix, 16 µl of nuclease-free water, 10 µl of 10 × Buffer SacI and 3 µl of SacI enzyme (Thermo Scientific, USA). The mix was incubated at 37 °C for 1 h, products were visualized on 1.3% agarose gel with ultraviolet light. Two fragments generated (128/124 and 252/256 bp) are considered as a Japanese haplotype, and a single fragment as a Korean haplotype (Solignac et al. 2005). Complete DNA of Escherichia coli ATCC 25922 added to SacI was used as a positive control for enzyme SacI digestion, whereas in the negative control no enzyme was applied.
Sequencing
Two PCR pools selected for their different morphometric characters and sampling zones, were sent to Centro de Investigación en Biología Celular y Molecular (CIBCM), Universidad de Costa Rica (UCR), and sequenced in both directions with the same primers used in conventional PCR. The obtained sequences were sent to the National Center for Biotechnology Information (NCBI), uploaded with access numbers MT328807 and MT328808 and then aligned using ClustalW v.1.6 software. The history of evolution was inferred using the neighbor-joining method (Saitou and Nei 1987) to compare with other sequences of GenBank; the analysis was conducted using MEGA X software (Tamura et al. 2013). In addition, the sequences were compared with other sequences around the world using NCBI BLAST database (Morgulis et al. 2008).
Statistical analysis
Continuous variables were evaluated using the Shapiro–Wilk test to determine their distribution. The results of the characters were submitted to statistical analysis as mean comparison for two parametric groups (Student’s t test). ANOVA with post-hoc Tukey honestly significant difference (HSD) test was realized for different morphometric characters and infestation rates, related to zones of sampling and the years during the study. For the identification of morphotypes of V. destructor, a hierarchical cluster analysis according to the Ward linkage method using Euclidean squared distance was applied; the conglomerates (morphotypes) were considered different if showing values > 5 in the Rescaled Distance Cluster on a standardized scale range to 25. A Fisher's exact test was applied to compare the frequency of morphotypes between 10 departments of the country and 4 years of sampling, and considered statistically significant at α = 0.05. Data management and analysis was performed using R v.3.4.1 statistical software (Foundation for Statistical Computing, Vienna, Austria).
Results
Infestation rate
The overall mean (± SD) infestation rate (IR) of the 34 apiaries during the study was 4.3 ± 3.9%. When comparing the IR of the five sampled zones, no significant differences were found between the years of sampling; the highest value was observed in 2015 with an IR of 5.3 ± 4.9%, whereas in 2013 it was only 2.7 ± 2.1%. The highest IR of the study was found in the Northern Highlands (6.6 ± 4.9%), and the lowest in the Central Highlands (2.4 ± 1.5%); however, no significant differences were observed among the sampling areas (Table 1).
Morphometric analysis
Of the 713 collected specimens, a subset of 204 was selected for morphometric diagnosis (six per apiary); after preparing and fixing them on microscope slides, 153 mites remained in body shape condition to be analyzed. In every fixed specimen, it was possible to determine width and length of the body (Fig. 1). Width and length of the genital shield could be measured in only 119 preparations, and width and length of the anal shield in only 117 preparations.
Morphometric measurement of Varroa found in Nicaraguan beehives revealed a mean (± SD) body width of 1699.1 ± 60.2 µm and a body length of 1161.1 ± 34.9 µm. The ratio of width-to-length was 1.46, emphasizing the typical ellipsoidal body shape of Varroa, wider than long (Table 2). According to the definition of Varroa taxon groups by Anderson and Trueman (2000), body width of 1708.9 ± 41.2 µm and length of 1167.3 ± 26.8 µm assign the mites to the species V. destructor. Varroa body size was significantly different among the 34 apiaries (ANOVA, body width: F33,119 = 1.89, p = 0.007; body length: F33,119 = 2.76, p < 0.001). A significant difference in width and length was only found between the four study years, not between the five study areas (Table 3).
Hierarchical conglomerate analysis showed that V. destructor samples were classified into four clusters based on body width, body length and mite width/length ratio (Table 4). Length and width of the genital shield, length and width of the anal shield, setae length and chelicerae length showed no resolution to differentiate between morphotypes. All 153 specimens of V. destructor were categorized into four morphotypes: 81 were identified as morphotype 1, 28 as morphotype 2, 39 as morphotype 3, and five specimens were identified as morphotype 4. Morphotype 3 was the widest (1751.1 ± 29.2 µm) and the longest (1202.8 ± 22.5 µm), whereas morphotype 1 had the largest body width-to-length ratio (1.49 ± 0.04). Using the three parameters described above, significant differences were observed between the identified morphotypes (Table 4).
Correlation analysis between body width and length clearly separated the four morphotypes of V. destructor (Fig. 2). The distribution of morphotypes by department did not show significant differences (Fisher's exact test: p = 0.19) (Fig. 3, Table 5), nor the distribution of morphotypes by sampling area (p = 0.48). In contrast, when comparing the distribution of the morphotypes among the sampling years, significant differences were found (p < 0.001); of the 39 mites collected in 2015, 29 specimens belonged to morphotype 3, whereas morphotype 4 was only determined in 2013 (Table 5).
Haplotype analysis
In the genetic determination of Varroa haplotypes, digestion of the SacI restriction enzyme was not observed in any of the PCR products, there was only a single band present (376 bp), and therefore the 24 pools were identified as V. destructor Korean haplotype. This result was confirmed for two pools in the sequencing, as GAGCTC SacI enzyme cleavage sequences were not observed (Table 6). The sequences BLAST search Query IDlcl|Query_63451 found more than 100 sequences in the database, with max score 355, total score 355, query cover 100% and per. ident 100%.
Phylogenetic analysis of 192 bp CO-I gene nucleotide sequences from two V. destructor pools analysed in this study (NCBI access numbers MT328807 and MT328808) showed 100% similarity with the Korean haplotype AF106899 uploaded to NCBI (Anderson and Trueman 2000); there was 99.5% similarity with a haplotype described in China in 2019 (MN551179.1) (Li et al. 2019), 99.8% similarity with a Japanese haplotype described in Japan (AF106897.1) (Anderson and Trueman 2000), and 97.3% similarity to V. jacobsoni (AF106907) (Anderson and Trueman 2000) (Fig. 4).
Phylogenetic analysis of geographic distribution CO-I gene nucleotide sequences of Varroa destructor haplotypes. The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree with the sum of branch length = 0.028 is shown. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The scale bar (0.002) indicates 0.2% divergence. This analysis involved 12 nucleotide sequences
Discussion
Varroa destructor infestation rate
In this study, only infested beehives were included, because of the purpose to study morphology and haplotypes of the mite. Nevertheless, the additional information about infestation levels can be used to open new pathways for investigation. Since 2000, infested apiaries are reported (OIE 2000), even though the first official national report about V. destructor in Nicaragua was in 1996 (then called V. jacobsoni). In 2008, varroosis was declared as a notifiable disease in domestic populations. Less than 10 years later, Varroa is found in apiaries all over the country, but has never led to a severe sanitary problem, primarily because of the absence of virus diseases. As Nicaragua has few modernizations in apiculture and poor specific investigation into this issue, it is not sure to what extent honey production is influenced by the high infestation levels or by an overall lack of managing procedures. Also, swarming tendencies can be due to infestation by Varroa (Fries et al. 2003). On the other hand, swarming is characteristic in Africanized bees, which are widespread in Nicaraguan beehives. For the first time wild swarms were observed in 1984 (Swezey 1986). In addition to the Varroa study, we conducted a morphometric and molecular characterization of the honey bee colonies to determine the level of Africanization, and we found similar results to Lobo in Costa Rica (Lobo Segura 2000), which sustains that in the Central American region there is a high level of Africanization in the species of A. mellifera.
In general, Varroa infestation during the study indicated a low level (IR = 4.3%) and without any significant difference between the five zones and the years of sampling, although the IR per apiary varied considerably. It is known that infestation dynamics can vary among seasons, food availability and even in small regions (Pinto et al. 2011). The annual surveillance of sentinel apiaries from Institute of Agricultural Protection and Health (IPSA 2009–2015) presented similar results with a general IR = 4.4% (Sandoval González 2020).
Reproductive ability and IR of V. destructor in tropical regions of the Americas show different impact levels depending on extrinsic factors like geographical variations, weather conditions and, in particular, the presence of Africanized honey bee colonies (Rosenkranz 1999; Carneiro et al. 2007). The influence of the high degree of Africanization in Nicaraguan honey bees is reflected in their excellent hygienic behaviour (C. Düttmann, pers. obs.) and, as a possible result, has led to a tolerance to mite infestation. This specific host-parasite association might be the principal reason for the low IR (Martin and Medina 2004; Calderón et al. 2010), and even more, the capacity to survive high IR and reduce the rate again without any control management of the beekeepers (DeJong and Soares 1997; Locke 2016). The Korean haplotype is known as more virulent, because of its higher reproductive potential (Garrido et al. 2003), nevertheless in Africanized honey bee colonies the presence of the Korean haplotype is not necessarily correlated with an increasing infestation rate (Pinto et al. 2015).
Morphometric analysis
As varroosis became a serious threat in some regions of the world, but not in others, it has been hypothesized that a species complex of the parasite existed with high host specificity, explaining the various effects in the beehives (Delfinado-Baker and Houck 1989). Based on the observation of differences in behaviour of the mites and in degrees of severity of colony infestation, morphometric characters of Varroa have been studied for improved understanding of their biology, systematics and geographic variation in the last 5 decades. First, morphological plasticity of Varroa was described in different biotypes in relation to geographical zones and specific bee hosts (Delfinado-Baker and Houck 1989). Then, the breakthrough came with the study by Anderson and Trueman (2000), and Varroa was classified into different species, by morphometric characters and genetics. In the last 2 decades, morphometric studies on the newly determined V. destructor in the Neotropical regions have found variation in the morphometric plasticity of the mites (Anderson and Trueman 2000; Maggi et al. 2009; Martínez et al. 2017; Loeza-Concha et al. 2018).
Anderson and Trueman (2000) described that no morphological characters of V. destructor (except body size) would distinguish mites belonging to one of the two main genetically distinct groups of mites, the Japanese and the Korean. In their study of V. destructor in Asia, the mean (± SD) body width was 1708.9 ± 41.2 µm and body length was 1167.3 ± 26.8 µm, similar to our findings of 1699.12 ± 60.17 and 1161.14 ± 34.93 µm, respectively (Table 2). Nevertheless, our morphotype 3 shows a bigger body of 1751.14 ± 29.18 µm wide and 1202.80 ± 22.46 µm long (Table 4). Although there was no significant difference in our sampling zones, we found a high diversity with representation of the different morphotypes in all zones, except for morphotype 4, which was only present in five specimens in the Northern Highland (3) and in the Northern Pacific Lowlands (2) (Table 5).
A possible explanation for the wide distribution of the various Varroa morphotypes in Nicaragua is the annual transhumance between different climate zones. Due to changes in the flowering of the most visited trees and plants of importance for local beekeeping, beekeepers move further transhumance distances, which leads to closer contact between the bee colonies from different regions, forming a new entrance into the transmission chain. Beekeepers from the Central Highland move their bees from their zone to the southern limit in a lowland at an altitude of 145 m above sea level, where the hives are located at the end of the rainy season. Due to an enormous flowering capacity in this area, several beekeepers from the Pacific Zone and the Northern Highlands also bring their beehives there. There is less transhumance activity from the Northern Highland to the Pacific zone (C. Düttmann, pers. obs.). Furthermore a biological corridor extends throughout Nicaragua, where a constant migration of feral bee colonies can be observed similar to the Pacific lowland of Costa Rica close to the Nicaraguan border (Lobo 1995). Those colonies are also a possible infestation source for productive beehives, as many beekeepers enlarge their colonies by trapping feral colonies.
In Argentina, three morphotypes in 12 regions were identified as a result of morphometric correlations between coexisting populations of V. destructor and A. mellifera (e.g., larger bees could support larger mites) (Maggi et al. 2009). The authors also discussed that if local and current conditions, for instance climatic conditions, would interfere in the morphometric characters, whereas the three morphotypes were present in all geographic locations, latitudinal effects would probably not be strong (Maggi et al. 2012b). This hypothesis matches with our results: in the tropical region of Nicaragua the climate is different from that of the eastern region of the Andes (Argentina), yet the morphotypes of mites were the same size. Another hypothesis discussed (Maggi et al. 2009, 2011, 2012b), i.e., the selection pressure on the mite exerted by acaricide treatment differentially influencing the appearance of morphotypes, cannot be confirmed for Nicaragua because acaricides are only sporadically used here. In addition, there is no state control program, so treatment is in the hands of the beekeepers. Often, treatment is performed only on seriously infested hives and not throughout the apiary. Mainly organic treatment methods are used, i.e., medications such as oxalic acid and essential oils. In relation to this, in Mexico an effect on morphometric plasticity was observed after the use of thymol (Loeza-Concha et al. 2018).
Phylogenetic (haplotype) analysis
Studies of mitochondrial DNA markers (mtDNA) have been used to identify V. destructor haplotypes that infest A. mellifera; however, only Korean and Japanese haplotypes have been reported to reproduce with success in A. mellifera colonies (Anderson and Trueman 2000). The Korean haplotype has spread worldwide in A. mellifera colonies, whereas the Japanese haplotype has a more restricted distribution and has only been reported in Japan, Thailand, and North and South America (Maggi et al. 2012a; Muntaabski et al. 2020).
PCR diagnosis and sequencing confirmed the Korean haplotype in the four Nicaraguan morphotypes of V. destructor. Phylogenetic analysis of CO-I sequences of V. destructor from Nicaragua revealed high similarity to Korean haplotype sequences throughout the world (Anderson and Trueman 2000). Although the Nicaraguan haplotype was less similar to the Japanese haplotype, a high degree of relationship was nonetheless achieved (Li et al. 2019). Further studies with consistent results described that the variation of the CO-I gene within the same Varroa species is less than 2% (Anderson and Trueman 2000). Therefore, these studies suggest that the use of the target marker CO-I in combination with other markers having high polymorphism such as NADH-dehydrogenase, may be useful to identify sub-haplotypes that may correspond to V. destructor morphotypes, environmental conditions, and genetic characters of A. mellifera (Muntaabski et al. 2020).
Although the Korean haplotype of V. destructor is the more virulent one, causing damage in bee hives worldwide (Maggi et al. 2012a; Giacobino et al. 2018), in Africanized honey bee colonies the host-parasite association has led to a balance and damage is much lower than in European honey bees (Garrido et al. 2003).
In order to increase the number of beehives in Nicaragua, colony multiplication is mainly done without selective breeding, by dividing the colonies, with or without introducing local queens. The capturing of feral colonies is also very common. As a consequence, it is almost certain that Africanized honey bees will be found in all apiaries, due to the mating advantage from the Africanized drones (Rinderer et al. 1985) and the shorter egg hatching period of Africanized honey bee queens (Harbo et al. 1981). Although the host-parasite association has only existed for 2 decades, probably a tolerance mechanism has evolved to maintain bee colonies in coexistence with the Varroa mite.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Anderson DL (2000) Variation in the parasitic bee mite Varroa jacobsoni Oud. Apidologie 31:281–292. https://doi.org/10.1051/apido:2000122
Anderson DL, Fuchs S (1998) Two genetically distinct populations of Varroa jacobsoni with contrasting reproductive abilities on Apis mellifera. J Apic Res 37:69–78. https://doi.org/10.1080/00218839.1998.11100957
Anderson DL, Trueman JW (2000) Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp Appl Acarol 24:165–189. https://doi.org/10.1023/a:1006456720416
Calderón RA, van Veen JW, Sommeijer MJ, Sanchez LA (2010) Reproductive biology of Varroa destructor in Africanized honey bees (Apis mellifera). Exp Appl Acarol 50:281–297. https://doi.org/10.1007/s10493-009-9325-4
Carneiro FE, Torres RR, Strapazzon R et al (2007) Changes in the reproductive ability of the mite Varroa destructor (Anderson e Trueman) in africanized honey bees (Apis mellifera L.) (Hymenoptera: Apidae) colonies in southern Brazil. Neotrop Entomol 36:949–952. https://doi.org/10.1590/S1519-566X2007000600018
Chen YP, Siede R (2007) Honey bee viruses. Adv Virus Res 70:33–80. https://doi.org/10.1016/S0065-3527(07)70002-7
DeJong D, Soares AEE (1997) An isolated population of Italian bees that has survived Varroa jacobsoni infestation without treatment for over 12 years. Am Bee J 137:742–745
Delaplane KS, Hood WM (1999) Economic threshold for Varroa jacobsoni Oud. in the southeastern USA. Apidologie 30:383–395. https://doi.org/10.1051/apido:19990504
Delfinado-Baker M, Houck MA (1989) Geographic variation in Varroa jacobsoni (Acari, Varroidae): application of multivariate morphometric techniques. Apidologie 20:345–358. https://doi.org/10.1051/apido:19890407
Fries I, Hansen H, Imdorf A, Rosenkranz P (2003) Swarming in honey bees (Apis mellifera) and Varroa destructor population development in Sweden. Apidologie 34:389–397. https://doi.org/10.1051/apido:2003032
Garrido C, Rosenkranz P, Paxton RJ, Gonçalves LS (2003) Temporal changes in Varroa destructor fertility and haplotype in Brazil. Apidologie 34:535–541. https://doi.org/10.1051/apido:2003041
Giacobino A, Pacini A, Molineri A et al (2018) Potential associations between the mite Varroa destructor and other stressors in honeybee colonies (Apis mellifera L.) in temperate and subtropical climate from Argentina. Prev Vet Med 159:143–152. https://doi.org/10.1016/j.prevetmed.2018.09.011
Harbo JR, Bolten AB, Rinderer TE, Collins AM (1981) Development periods for eggs of Africanized and European honeybees. J Apic Res 20:156–159. https://doi.org/10.1080/00218839.1981.11100490
Harris JW (2008) Effect of brood type on Varroa-Sensitive hygiene by worker honey bees (hymenoptera: apidae). Ann Entomol Soc Am 101:1137–1144. https://doi.org/10.1603/0013-8746-101.6.1137
INETER (2020) Instituto Nacional de Metereología. In: Dirección General de Meteorología. https://www.ineter.gob.ni/met.html
Jong D, Roma DDA, Gonçalves LS (1982) A comparative analysis of shaking solutions for the detection of Varroa jacobsoni on adult honeybees. Apidologie 13:297–306. https://doi.org/10.1051/apido:19820308
Le Conte Y, Ellis M, Ritter W (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41:353–363. https://doi.org/10.1051/apido/2010017
Le Conte Y, Navajas M (2008) Climate change: impact on honey bee populations and diseases. Rev Sci Tech Int Off Epizoot 27(485–497):499–510
Li W, Wang C, Huang ZY et al (2019) Reproduction of distinct Varroa destructor genotypes on honey bee worker brood. Insects 10:372. https://doi.org/10.3390/insects10110372
Lobo JA (1995) Morphometric isozymic and mitochondrial variability of Africanized honeybees in Costa Rica. Heredity 75:133–141. https://doi.org/10.1038/hdy.1995.116
Lobo Segura JA (2000) Highly polymorphic DNA markers in an Africanized honey bee population in Costa Rica. Genet Mol Biol 23:317–322. https://doi.org/10.1590/S1415-47572000000200013
Locke B (2016) Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 47:467–482. https://doi.org/10.1007/s13592-015-0412-8
Loeza-Concha H, Domínguez-Rebolledo Á, Escalera-Valente F et al (2018) Morphometric identification of Varroa destructor and its plasticity by the exposure to thymol. Abanico Vet 8:98–107
Maggi M, Medici S, Quintana S et al (2012a) Genetic structure of Varroa destructor populations infesting Apis mellifera colonies in Argentina. Exp Appl Acarol 56:309–318. https://doi.org/10.1007/s10493-012-9526-0
Maggi M, Peralta L, Ruffinengo S et al (2012b) Body size variability of Varroa destructor and its role in acaricide tolerance. Parasitol Res 110:2333–2340. https://doi.org/10.1007/s00436-011-2768-7
Maggi MD, Ruffinengo SR, Mendoza Y et al (2011) Susceptibility of Varroa destructor (Acari: Varroidae) to synthetic acaricides in Uruguay: Varroa mites’ potential to develop acaricide resistance. Parasitol Res 108:815–821. https://doi.org/10.1007/s00436-010-2122-5
Maggi MD, Sardella NH, Ruffinengo SR, Eguaras MJ (2009) Morphotypes of Varroa destructor collected in Apis mellifera colonies from different geographic locations of Argentina. Parasitol Res 105:1629. https://doi.org/10.1007/s00436-009-1605-8
Martin SJ, Medina LM (2004) Africanized honeybees have unique tolerance to Varroa mites. Trends Parasitol 20:112–114. https://doi.org/10.1016/j.pt.2004.01.001
Martinez P, Invernizzi C, Mendoza Y et al (2017) Morphometric correlation between Apis mellifera morphotypes (Hymenoptera) and Varroa destructor (Acari) from Uruguay. J Apic Res. https://doi.org/10.1080/00218839.2017.1287998
Morgulis A, Coulouris G, Raytselis Y et al (2008) Database indexing for production MegaBLAST searches. Bioinformatics 24:1757–1764. https://doi.org/10.1093/bioinformatics/btn322
Muntaabski I, Russo RM, Liendo MC et al (2020) Genetic variation and heteroplasmy of Varroa destructor inferred from ND4 mtDNA sequences. Parasitol Res 119:411–421. https://doi.org/10.1007/s00436-019-06591-5
Navajas M, Migeon A, Estrada-Peña A, et al (2010) Mites and ticks (Acari) Chapter 7.4
OIE (1996) HANDISTATUS II Annual animal disease status. http://web.oie.int/hs2/sit_mald_cont.asp?c_mald=134&c_cont=2&annee=2000. Accessed 10 Mar 2020
OIE (2000) HANDISTATUS II Annual animal disease status. http://web.oie.int/hs2/sit_mald_cont.asp?c_mald=134&c_cont=2&annee=1996. Accessed 10 Jan 2020
Pinto F, Puker A, Message D, Barreto L (2011) Varroa destructor in Juquitiba, Vale do Ribeira, Southeastern Brazil: seasonal effects on the infestation rate of ectoparasitic mites on honeybees. Sociobiology 57:511–518
Pinto FA, Puker A, Message D et al (2015) Infestation rate of the mite Varroa destructor in commercial apiaries of the Vale do Paraíba and Serra da Mantiqueira, southeastern Brazil. Arq Bras Med Veterinária E Zootec 67:631–635. https://doi.org/10.1590/1678-7264
Rath W (1999) Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie 30:97–110. https://doi.org/10.1051/apido:19990202
Rinderer TE, Hellmich RL, Danka RG, Collins AM (1985) Male reproductive parasitism: a factor in the africanization of European honey-bee populations. Science 228:1119–1121. https://doi.org/10.1126/science.228.4703.1119
Rosenkranz P (1999) Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. in South America. Apidologie 30:159–172
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103(Suppl 1):S96-119. https://doi.org/10.1016/j.jip.2009.07.016
Rosenkranz P, Engels W (1994) Genetic and environmental influences on the duration of preimaginal worker development in eastern (Apis cerana) and western (Apis mellifera) honey bees in relation to varroatosis. Rev Bras Genet 17:383–391
Rothenbuhler WC (1964) Behaviour genetics of nest cleaning in honey bees. I. Responses of four inbred lines to disease-killed brood. Anim Behav 12:578–583. https://doi.org/10.1016/0003-3472(64)90082-X
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sandoval González E (2020) Situación zoosanitaria de los niveles de infestación de varroosis en Apis mellifera en Nicaragua 2009–2015. Universidad Nacional Autónoma de Nicaragua, Leon
Solignac M, Cornuet J-M, Vautrin D et al (2005) The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honeybee (Apis mellifera), are two partly isolated clones. Proc Biol Sci 272:411–419. https://doi.org/10.1098/rspb.2004.2853
Swezey SL (1986) Africanized honey bees arrive in Nicaragua. J Apic Res 126:283–287
Tamura K, Stecher G, Peterson D et al (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Vandame R, Palacio MA (2010) Preserved honey bee health in Latin America: a fragile equilibrium due to low-intensity agriculture and beekeeping? Apidologie 41:243–255. https://doi.org/10.1051/apido/2010025
Wilde J, Fuchs S, Bratkowski J, Siuda M (2005) Distribution of Varroa destructor between swarms and colonies. J Apic Res 44:190–194. https://doi.org/10.1080/00218839.2005.11101177
Acknowledgements
The authors thank the beekeepers for their collaboration in the field work and for the permission to investigate on their beehives and Nicola J. Singh MAAT for her review and comments on the English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study has been performed in accordance with the standard procedures for the surveillance of emerging diseases in apiaries established by Organismo Internacional Regional de Sanidad Agropecuaria (OIRSA), and was previously approved by the Research Ethics Committee (ECAV, UNAN-León).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Düttmann, C., Flores, B., Sheleby-Elías, J. et al. Morphotype and haplotype identification of Varroa destructor (Acari: Varroidae), and its importance for apiculture in Nicaragua. Exp Appl Acarol 83, 527–544 (2021). https://doi.org/10.1007/s10493-021-00603-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-021-00603-9