Abstract
The cassava green mites Mononychellus tanajoa and M. mcgregori are highly invasive species that rank among the most serious pests of cassava globally. To guide the development of appropriate risk mitigation measures preventing their introduction and spread, this article estimates their potential geographic distribution using the maximum entropy approach to distribution modeling. We compiled 1,232 occurrence records for M. tanajoa and 99 for M. mcgregori, and relied on the WorldClim climate database as a source of environmental predictors. To mitigate the potential impact of uneven sampling efforts, we applied a distance correction filter resulting in 429 occurrence records for M. tanajoa and 55 for M. mcgregori. To test for environmental biases in our occurrence data, we developed models trained and tested with records from different continents, before developing the definitive models using the full record sets. The geographically-structured models revealed good cross-validation for M. tanajoa but not for M. mcgregori, likely reflecting a subtropical bias in M. mcgregori’s invasive range in Asia. The definitive models exhibited very good performance and predicted different potential distribution patterns for the two species. Relative to M. tanajoa, M. mcgregori seems better adapted to survive in locations lacking a pronounced dry season, for example across equatorial climates. Our results should help decision-makers assess the site-specific risk of cassava green mite establishment, and develop proportional risk mitigation measures to prevent their introduction and spread. These results should be particularly timely to help address the recent detection of M. mcgregori in Southeast Asia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 800 million people in the tropics depend on cassava (Manihot esculenta) as a source of food and income (Lebot 2009). It is widely distributed in regions at latitudes between 30°S and 30°N, and from sea level up to 1,800 m above sea level (Ceballos et al. 2007; Fig. 1). Cassava production, however, can be severely limited by a complex of arthropod pests (Bellotti and van Schoonhoven 1978; Bellotti et al. 1999). Top among these pests are a few Neotropical mite species of the genus Mononychellus, commonly known as the “cassava green mites” (Bellotti et al. 2012). The most notorious species is M. tanajoa, whose accidental introduction into Africa in the 1970s reduced cassava yields by up to 80 % (Yaninek 1988; Yaninek and Herren 1988). Although largely understudied, M. mcgregori follows in importance. This species was first detected in China in 2008 (Lu et al. 2014a), and shortly thereafter begun causing yield losses reaching up to 60 % (Chen et al. 2010: cited in Lu et al. 2014a). A year later, M. mcgregori was reported in Vietnam and Cambodia (Bellotti et al. 2012; Vásquez-Ordóñez and Parsa 2014), raising concerns over its potential spread throughout the region.
Global distribution of cassava, adapted from Ceballos et al. (2007)
Cassava green mites feed only on cassava (Bellotti et al. 2012). They are most abundant at the top of the canopy, from the shoot tip to the youngest unfolded leaves (Bellotti and van Schoonhoven 1978). Their feeding kills leaf cells and reduces photosynthesis, interfering with normal leaf development (Yaninek and Herren 1988). Under field conditions in the tropics, cassava green mites have overlapping generations, each completed in less than 1 month, and are most abundant during dry seasons and at the beginning of the rain season (Bellotti and van Schoonhoven 1978). Early rains cause a flush of new leaf growth that promotes their rapid population growth (Yaninek et al. 1989). Continued rains eventually help suppress them to sub-economic levels through a combination of plant compensation and rainfall mortality (Yaninek et al. 1989). Green mites can also be suppressed to sub-economic levels by phytoseiid mites (Bellotti et al. 2012), which have been successfully deployed in classical biological control against M. tanajoa in Africa (Yaninek and Hanna 2003). A similar effort has been advocated to control M. mcgregori in Asia (Bellotti et al. 2012), but its potential remains to be investigated.
Pest risk maps, based on models estimating climatic suitability for a species, are important decision-support tools for the management of invasive pests (Venette et al. 2010). They can be based on two complementary approaches: (1) the mechanistic or deductive approach, which relies on the species’ physiological data (Kearney and Porter 2009); and (2) the correlative or inductive approach, which relies on the species’ occurrence data (Elith and Leathwick 2009). When a pest’s biology is still poorly known, correlative models provide the most rapid and effective means to develop risk maps (Venette et al. 2010).
This article responds to the need to better assess and address the risk of invasive cassava green mites, emphasizing the invasion of M. mcgregori in Asia. Our principal objective was to develop correlative models predicting their potential geographic distribution, therefore guiding site-specific risk mitigation strategies. The resulting risk maps could also be used to identify exploration sites for natural enemies with a high probability of establishment in the affected locations.
Materials and methods
Occurrence data
We compiled occurrence data (i.e. presence-only) from three sources. Native distribution records for both species originated from a database submitted by the International Center for Tropical Agriculture (CIAT, its Spanish acronym) to the Global Biodiversity Information Facility (GBIF; Vásquez-Ordóñez and Parsa 2014). Because this source covers areas where the species co-occur, and may be misidentified without proper mounting, we only extracted specimen-based records from it. Exotic distribution records of M. tanajoa in Africa originated from a database compiled by the International Institute of Tropical Agriculture (IITA), as part of the monitoring efforts of their Africa-wide Biological Control Programme (ABCP; Yaninek 1988; Yaninek and Herren 1988). Exotic distribution records of M. mcgregori in Asia originated partly from specimen-based records submitted to GBIF (Vásquez-Ordóñez and Parsa 2014) and partly from published records reporting its invasion in Hainan, China (Lu et al. 2012). This last source originally misreported the species as M. tanajoa, subsequently correcting its identification after submitting samples for verification to CIAT’s Arthropod Reference Collection. Subsequent publications by the authors report the species as M. mcgregori (e.g., Lu et al. 2014a). To mitigate the impact of uneven sampling in our occurrence data (see Reddy and Davalos 2003; Peterson et al. 2014; Radosavljevic and Anderson 2014), we applied a distance correction by taking only one point within a radius of 5 km for the Andean region, 10 km for the African highlands, 12 km for the Caribbean, and 25 km for the African lowlands. During exploratory analyses, we found that these distances resulted in a sampling intensity proportional to the region’s environmental heterogeneity, without unduly reducing our record set. Distance correction resulted in 429 spatially-filtered occurrence records for M. tanajoa and 55 for M. mcgregori.
Environmental data
Our source of environmental data for species distribution modeling was the WorldClim database (Hijmans et al. 2005), from which we obtained 19 bioclimatic predictor layers summarizing annual trends, seasonality and extremes in temperature and precipitation at a spatial resolution of 30 arc-seconds (i.e. 1 km2). As advocated by Peterson et al. (2007), we first extracted all 19 bioclimatic predictors for our occurrence records and examined their pairwise correlations to select a subset of uncorrelated predictors (Pearson’s |r| < 0.70). To facilitate model interpretation, our selection favored variables capturing annual trends over quarterly or monthly trends. We therefore selected: (1) Annual Mean Temperature (Bio01), (2) Mean Diurnal Range (Bio02), (3) Isothermality (Bio03), (4) Temperature Seasonality (Bio04), (5) Annual Precipitation (Bio12), and (6) Precipitation Seasonality (Bio15).
Distribution modeling
Our species distribution modeling relied on the maximum entropy approach implemented in Maxent (version 3.3.3 k; Elith et al. 2011; Phillips et al. 2004, 2006), one of the best performing methods to model presence-only occurrence data (Elith et al. 2006). The uncorrelated bioclimatic variables were used as environmental layers to predict species occurrences. Following general recommendations for background selection (Barve et al. 2011; Radosavljevic and Anderson 2014), the study area was delimited by the tropical region of the continent(s) containing the training records, between the latitudes 39°S and 31°N. The models for both species were run selecting the auto features, logistic output and random seed, and the maximum number of background points maintained at 10,000. To ensure model convergence, we increased the maximum iterations to 5,000, maintaining the convergence threshold at 0.00001. To maximize model sensitivity, we explored a range of regularization coefficients (1–4; Merow et al. 2013; Radosavljevic and Anderson 2014), arriving at a value of 1 for M. tanajoa and 3 for M. mcgregori. The Jackknife test was performed to estimate the importance of the environmental predictors. We developed geographically-structured models trained and tested with records from different continents, a practice recommended for invasive species risk assessments (Jiménez-Valverde et al. 2011). The definitive models were based on the full datasets. To assess model performance, we used the Area Under the Curve (AUC), following the guidelines of Baldwin (2009): AUC > 0.9: “very good,” 0.9 > AUC > 0.7: “good”, AUC < 0.7: “uninformative.”
Results
A total of 1,232 occurrence records for M. tanajoa and 99 for M. mcgregori were compiled. Out of these, only 617 M. tanajoa records and 86 M. mcgregori records were spatially unique. Their distribution shows some level of geographic overlap within their native range in South America (Fig. 2). Outside this range, M. tanajoa occurrence is restricted to Africa and M. mcgregori to Southeast Asia (Fig. 2).
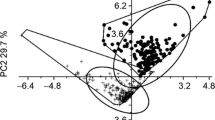
An examination of the Pearson’s correlation matrix for the bioclimatic variables revealed significant multicolinearity (|r| < 0.70), leading to the selection of Bio01–Bio04, Bio12 and Bio15 as environmental predictors for our models. Bio01 was correlated with Max Temperature of Warmest Month (Bio05), Min Temperature of Coldest Month (Bio06), Mean Temperature of Wettest Quarter (Bio08), Mean Temperature of Driest Quarter (Bio09), Mean Temperature of Warmest Quarter (Bio10), and Mean Temperature of Coldest Quarter (Bio11). Bio02 was correlated with Temperature Annual Range (Bio07). Bio03 and Bio04 were not correlated with any other bioclimatic variable. Bio12 was correlated with Precipitation of Wettest Month (Bio13), Precipitation of Wettest Quarter (Bio16), Precipitation of Warmest Quarter (Bio18), and Precipitation of Coldest Quarter (Bio19). Finally, Bio15 was only correlated with Precipitation of Driest Quarter (Bio17). Boxplots showing the ranges for these uncorrelated environmental predictors for both species and across continents are presented in Fig. 3.
Box plots of bioclimatic predictor variables used to model the spatial distribution of Mononychellus tanajoa and M. mcgregori. Bio01 Annual mean temperature, Bio02 mean diurnal range, Bio03 isothermality, Bio04 temperature seasonality, Bio12 annual precipitation, Bio15 precipitation seasonality. Temperatures are in °C and precipitation in mm
The results from our geographically-structured model evaluations are presented in Table 1. AUCs reveal “good” cross-validation for M. tanajoa but not for M. mcgregori, suggesting M. mcgregori’s invasive occurrence falls outside its previously-known climatic range. This inference is supported by the boxplots in Fig. 3, particularly for Bio03 and Bio04. For both species, predictions were “very good” within the training region.
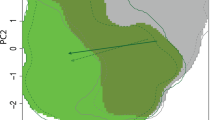
Predicted distributions based on full datasets are presented in Fig. 4. The AUC was 0.90 for M. tanajoa and 0.91 for M. mcgregori, indicating “very good” model performance for both species. M. tanajoa’s distribution was best explained by Bio04 (32.6 % contribution), followed by Bio12 (31.2 % contribution) and Bio02 (24.7 % contribution). In turn, M. mcgregori’s distribution was best explained by Bio03 (36.4 % contribution), followed by Bio04 (31.2 % contribution) and Bio02 (12.9 % contribution). Jackknife tests for variable importance are presented in Fig. 5. Both analyses of variable contributions and variable importance suggest precipitation has a greater impact on M. tanajoa relative to M. mcgregori. This difference is also reflected in the predicted distribution map (Fig. 4). For example, the Congo Basin, an area where high rainfall limits the establishment of the cassava mealybug Phenacoccus manihoti (Parsa et al. 2012), was rendered suitable for M. mcgregori but not for M. tanajoa (Fig. 4). The same is true for areas around the equator in Southeast Asia.
Predicted distribution maps for Mononychellus tanajoa (a, c) and M. mcgregori (b, d). Predictions for M. tanajoa are based on 429 spatially-filtered occurrence records from South America and Africa. Predictions for M. mcgregori are based on 55 spatially-filtered occurrence records from South America and Asia
Discussion
Our main objective was to predict the potential distribution of M. tanajoa and M. mcgregori in order to guide the development of appropriate risk mitigation measures. These measures could include the passage of phytosanitary regulations, the establishment of pest-surveillance networks, and the development of emergency response plans to address their potential incursion (Venette et al. 2010). Our predictions should therefore be most valuable for major cassava growing regions that are highly suitable for the species, but where the species are still absent. In Southeast Asia, for example, these locations include Thailand and Vietnam for M. tanajoa and Indonesia for M. mcgregori. These three countries alone accounted for over 70 % of the 4.2 million hectares of cassava produced in the region in 2013 (FAOSTAT 2014).
Given the magnitude and spatial coverage of our database, we suspect the risk maps presented here represent the best approximation to M. tanajoa and M. mcgregori’s potential distribution available to date. A previous effort to model M. tanajoa relied on only 215 occurrence records (Herrera Campo et al. 2011), and generated broadly similar predictions to ours, albeit rendering high-rainfall locations more suitable for the species than our model. Our predictions, based on 429 spatially-filtered records, rendered the same locations relatively unsuitable, but are more consistent with previous research demonstrating rainfall is a primary mortality factor limiting M. tanajoa populations (Gutierrez et al. 1988; Yaninek et al. 1989). Previous models of M. mcgregori may be less reliable, as they utilized M. tanajoa and M. mcgregori occurrence records jointly as data inputs (Lu et al. 2012, 2014b), potentially biasing the latter’s predicted distribution.
Our geographically-structured models suggest the invasive range of M. mcgregori shares little climatic similarity with its known native range in the Americas. The differences are particularly acute with respect to isothermality and temperature seasonality, likely reflecting a tropical bias in our native distribution records and a subtropical bias in our invasive distribution records. As indicated by AUC of the definitive M. mcgregori model, however, the biases were corrected by deploying the full occurrence dataset.
Our results suggest that unlike M. tanajoa, M. mcgregori typically occurs in locations with no pronounced dry season. Its ability to survive in those locations, however, does not necessarily imply an ability to reach economic status. It is generally believed that cassava green mites need a dry season lasting 2–6 months with rainfall below 60 mm/month to become economic pests (Bellotti et al. 1987, 2012). This condition is met across the locations where M. mcgregori was reported as an invasive pest in Asia. However, the extent to which M. mcgregori may impact cassava during the wet season, or in locations without a dry season, merits empirical attention.
For locations where cassava green mites are already established as invasive pests, classical biological control by phytoseiid predators should be considered. Based on their climatic homology to potential target areas, our predictions suggest Colombia’s inter-Andean valleys rank among the best sites to import them from. Explorations conducted in Colombia during the mid-1980s identified 46 phytoseiid species associated with cassava mites (Bellotti et al. 1987). The list includes Typhlodromalus aripo, a predator introduced into Africa to target M. tanajoa, resulting in a highly successful case of classical biological control (Yaninek and Hanna 2003). Efforts to test the potential of T. aripo or an alternative phytoseiid predator against M. mcgregori in Asia are therefore warranted.
References
Baldwin RA (2009) Use of maximum entropy modeling in wildlife research. Entropy 11:854–866
Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT, Soberón J, Villalobos F (2011) The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Modell 222:1810–1819
Bellotti AC, van Schoonhoven A (1978) Mite and insect pests of cassava. Annu Rev Entomol 23:39–67
Bellotti AC, Mesa N, Serrano M, Guerrero J, Herrera C (1987) Taxonomic inventory and survey activity for natural enemies of cassava green mites in the Americas. Int J Trop Insect Sci 8:845–849
Bellotti AC, Smith L, Lapointe SL (1999) Recent advances in cassava pest management. Annu Rev Entomol 44:343–370
Bellotti AC, Herrera Campo BV, Hyman G (2012) Cassava production and pest management: present and potential threats in a changing environment. Trop Plant Biol 5:39–72
Ceballos H, Fregene M, Pérez JC, Morante N, Calle F (2007) Cassava genetic improvement. In: Kang MS, Priyadarshan PM (eds) Breeding major food staples. Blackwell, Ames, pp 365–391
Chen Q, Lu F, Huang G, Li K, Ye J, Zhang Z (2010) General survey and safety assessment of cassava pests. Chin J Trop Crops 31:819–827
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A (2006) Novel methods improve prediction of species distributions from occurrence data. Ecography 29:129–151
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of maxent for ecologists. Divers Distrib 17:43–57
FAOSTAT (2014) Cassava. http://faostat.fao.org/site/567/default.aspx#ancor. Accessed 28 Nov 2014
Gutierrez A, Yaninek JS, Wermelinger B, Herren H, Ellis C (1988) Analysis of biological control of cassava pests in Africa. III. Cassava green mite Mononychellus tanajoa. J Appl Ecol 25:941–950
Herrera Campo BV, Hyman G, Bellotti AC (2011) Threats to cassava production: known and potential geographic distribution of four key biotic constraints. Food Sec 3:329–345
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Jiménez-Valverde A, Peterson AT, Soberón J, Overton J, Aragón P, Lobo JM (2011) Use of niche models in invasive species risk assessments. Biol Invasions 13:2785–2797
Kearney M, Porter W (2009) Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett 12:334–350
Lebot V (2009) Tropical root and tuber crops: cassava, sweet potato, yams and aroids. CABI, Wallingford
Lu H, Ma Q, Chen Q, Lu F, Xu X (2012) Potential geographic distribution of the cassava green mite Mononychellus tanajoa in Hainan, China. Afr J Agric Res 7:1206–1213
Lu F, Chen Q, Chen Z, Lu H, Xu X, Jing F (2014a) Effects of heat stress on development, reproduction and activities of protective enzymes in Mononychellus mcgregori. Exp Appl Acarol 63:267–284
Lu H, Lu F, Xu XL, Chen Q (2014b) Potential geographic distribution areas of Mononychellus mcgregori in Guangxi province. Appl Mech Mater 522:1051–1054
Merow C, Smith MJ, Silander JA (2013) A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36:1058–1069
Parsa S, Kondo T, Winotai A (2012) The cassava mealybug (Phenacoccus manihoti) in asia: first records, potential distribution, and an identification key. PLoS ONE 7:e47675
Peterson AT, Papes M, Eaton M (2007) Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography 30:550–560
Peterson AT, Moses LM, Bausch DG (2014) Mapping transmission risk of Lassa Fever in West Africa: the importance of quality control, sampling bias, and error weighting. PLoS ONE 9(8):e100711. doi:10.1371/journal.pone.0100711
Phillips SJ, Dudík M, Schapire RE (2004) A maximum entropy approach to species distribution modeling. Proceedings of the 21st international conference on machine learning. ACM Press, New York, pp 655–662
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell 190:231–259
Radosavljevic A, Anderson RP (2014) Making better Maxent models of species distributions: complexity, overfitting and evaluation. J Biogeogr 41:629–643
Reddy S, Davalos L (2003) Geographical sampling bias and its implications for conservation priorities in Africa. J Biogeogr 30:1719–1727
Vásquez-Ordóñez AA, Parsa S (2014) A geographic distribution database of Mononychellus mites (Acari, Tetranychidae) on cassava (Manihot Esculenta). ZooKeys 407:1–8
Venette RC, Kriticos DJ, Magarey RD, Koch FH, Baker RH, Worner SP, Raboteaux NNG, McKenney DW, Dobesberger EJ, Yemshanov D (2010) Pest risk maps for invasive alien species: a roadmap for improvement. Bioscience 60:349–362
Yaninek JS (1988) Continental dispersal of the cassava green mite, an exotic pest in Africa, and implications for biological control. Exp Appl Acarol 4:211–224
Yaninek JS, Hanna R (2003) Cassava green mite in africa: a unique example of successful classical biological control of a mite pest on a continental scale. In: Neuenschwander P (ed) Biological control in IPM systems in Africa. CABI, Wallingford, pp 61–75
Yaninek JS, Herren H (1988) Introduction and spread of the cassava green mite, Mononychellus tanajoa (Bondar) (Acari: Tetranychidae), an exotic pest in Africa and the search for appropriate control methods: a review. Bull Entomol Res 78:1–13
Yaninek JS, Herren H, Gutierrez AP (1989) Dynamics of Mononychellus tanajoa (Acari: Tetranychidae) in Africa: seasonal factors affecting phenology and abundance. Environ Entomol 18:625–632
Acknowledgments
We thank Rodrigo Zuñiga for his curatorial work at CIAT’s Arthropod Reference Collection (CIATARC). We also thank the International Institute of Tropical Agriculture (IITA) for generously sharing their M. tanajoa distribution database with us. Emmanuel Zapata and Julian Ramirez (CIAT) kindly helped with GIS methods. This research was supported by the Research Program on Roots, Tubers, and Bananas (RTB) of the Consultative Group on International Agriculture Research (CGIAR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parsa, S., Hazzi, N.A., Chen, Q. et al. Potential geographic distribution of two invasive cassava green mites. Exp Appl Acarol 65, 195–204 (2015). https://doi.org/10.1007/s10493-014-9868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9868-x