Abstract
Antiretroviral therapy (ART) adherence is key to achieving viral load suppression and ending the HIV epidemic but monitoring and supporting adherence using current interventions is challenging. We assessed the feasibility, acceptability and appropriateness of MedViewer (MV), a novel intervention that provides real-time adherence feedback for patients and providers using infra-red matrix-assisted laser desorption electrospray ionization (IR-MALDESI) for mass spectrometry imaging of daily ART concentrations in patients’ hair. We used mixed methods to feasibility test MV at a busy Infectious Diseases (ID) clinic, enrolling 16 providers and 36 patients. Providers underwent standardized training; patients and providers watched an 8-min informational video about MV. We collected patient and provider data at baseline and within 24 h of clinic visits and, with patients, approximately 1 month after clinic visits. MedViewer was feasible, liked by patients and providers, and perceived to help facilitate adherence conversations and motivate patients to improve adherence.
Trial Registration: NCT04232540.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide, 84% of (~ 37.7 million) people with HIV (PWH) in 2020 knew their HIV status, 73% (~ 28.2 million people) of them were accessing antiretroviral therapy (ART) and 66% (~ 18.6 million) of those accessing it were virologically suppressed [1]. Viral load suppression (VLS), which requires access to ART and optimal adherence, is a key determinant of morbidity and mortality for PWH. In addition, VLS underpins the Undetectable = Untransmittable (U = U) campaign, a key tool in counseling PWH and reducing stigma [2]. Despite decades of research devoted to optimizing ART adherence and underscoring its benefits, daily ART adherence continues to present a challenge to achieving VLS for many PWH [3]. Over a lifetime, even people with habitually perfect adherence will likely encounter treatment disruptions [4]. Importantly, variations in adherence may not be evident to prescribing providers if PWH have VLS at scheduled visits.
Many systematic reviews documenting the multifactorial nature of nonadherence to ART indicate that interventions to support adherence need to account for its multiple and individualized causes [5, 6]. It is not surprising that a broad range of approaches, including counseling, education, and those addressing health care delivery, can improve adherence, but often only modestly [7]. Optimization of ART adherence may require augmentation of currently available interventions, such as targeting multiple factors.
One domain shown consistently to influence ART adherence is the patient–provider interaction: better patient–provider relationships have a positive effect on medication adherence, including for PWH [8-13]. Providing patients with objective adherence feedback via electronic measures or biomarkers, has also been shown to enhance ART adherence, particularly when coupled with counseling [14-17]. Improving physicians' sometimes limited knowledge of patients' ART adherence can enrich their ability to provide tailored counseling about adherence [18]. Consequently, the ability to accurately, feasibly, and acceptably monitor adherence in clinical settings is increasingly recognized as an important component to augment ART adherence programs [4, 19, 20].
Reviews of studies of pharmacologic measures of adherence have shown antiretroviral concentrations in hair to be a valid biomarker of ART adherence that closely correlates with viral suppression [16, 21]. While systemic drug concentrations are incorporated into hair continuously as part of daily growth, routine methods of hair analysis require homogenization of strands or segments such that hair concentrations reflect an average of adherence behavior over a period of weeks to months, depending on hair segment length. This measure of cumulative adherence behavior, like concentrations of intracellular metabolites in blood cells, can be combined with pharmacologic measures of recent behavior (e.g., from plasma, saliva, or urine) to provide a picture of both short-term and long-term behavior [21-23], but still cannot offer a long-term daily record. Because these methods require the hair sample to be homogenized, they only provide information of cumulative or average adherence behavior over the period of growth associated with hair samples (typically 1 month or greater). Members of our research team (AK, ER, NW) have developed a novel method for profiling ARV concentrations longitudinally along hair strands using infra-red (IR) matrix-assisted laser desorption electrospray ionization (MALDESI) technology for mass spectrometry imaging (MSI) to investigate both short-term and long-term daily patterns of adherence behavior simultaneously [24, 25]. This method also eliminates the multi-step sample processing and reduces the amount of hair required by traditional methods of hair analysis, which may otherwise limit utility in clinical settings due to the time constraints and may not be acceptable to all patients [26, 27].With the goal of providing patients and providers more granular and detailed information about ART adherence in the month prior to the patient visit, we developed a novel intervention, named MedViewer (MV), utilizing this technology. We developed MV based on formative qualitative studies with patients and providers to understand their preferences for graphical display of and uses for real-time adherence feedback [28, 29]. As we described previously [29], the suggested uses of MV corresponded to constructs of the Information-Motivation-Behavioral Skills Model (IMB Model) [30, 31] of Adherence: Information provided was the important relationship between ART adherence and viral suppression and accurate knowledge of one’s personal adherence history; motivation came from comparing one’s actual to ideal adherence and reinforcing higher adherence levels; behavioral skills learned from MV included identification of patterns of missing pills, associated adherence barriers and strategies to overcome them. Specifically, the MV intervention was designed to use real-time longitudinal IR-MALDESI MSI-based adherence feedback for patients and their providers at a scheduled clinic visit combined with provider training and patient education to stimulate an adherence conversation between patient and provider. We then conducted a pilot study of MV to test the feasibility, acceptability and appropriateness of its implementation in a clinical setting.
Methods
Study Design
The study was a single-arm pilot trial to test several aspects of the feasibility, acceptability, and appropriateness of implementing the MV intervention during routine ID clinic visits at a large tertiary care center in the southeastern United States [32]. The study was designed to evaluate the use of MV as an investigational clinical adherence-monitoring tool. All study procedures were approved by the appropriate Institutional Review Board at the University of North Carolina (UNC) at Chapel Hill and the Division of AIDS (DAIDS) at the National Institutes of Health (NIH).
MedViewer Intervention
The intervention materials were developed based on an extensive literature review, formative in-depth interviews (IDI) studies with 20 patients receiving HIV care and 19 HIV care providers, input from two community advisory boards, and integration with the IMB Model [28-31]. The final MV intervention consisted of four components: (1) Standardized (in-person or virtual) 30–60 min training session for medical providers to learn about MV and how to incorporate it into routine adherence discussions with patients; (2) Informational (approximately 8-min) video for patients watched during informed consent about MV and its procedures; (3) MV assay, which included baseline sample collection of 5 hair strands, an imaging scientist running the assay in the lab and generating MV results reports (patient and provider versions) visually classifying 30 days of ART concentrations (see example reports Fig. 1), and report delivery to providers at the clinic visit; (4) One-page communication aid and reference sheet for providers to support their discussions of the MV reports with patients. Patient and provider versions of the MV reports (Fig. 1) were designed to provide information about the daily concentration level of ART in a patient’s hair as objective feedback for patient and provider. The patient version of the report was in calendar format (Fig. 1), with dichotomous daily color assignment indicating an optimal or sub-optimal medication concentration. The provider version was a bar graph (Fig. 1) displaying each median daily drug concentration as a vertical bar with whiskers representing variability among the measurements between each hair strand, along with information about the acceptable drug level threshold, and sensitivity, specificity, and reliability of the lab assay.
Participant Eligibility, Recruitment, and Screening
To be eligible, providers (attending physicians, ID fellows, nurse practitioners, physician assistants, or a designated HIV pharmacist) had to provide HIV care to patients at the study clinic, be willing to undergo the training, and provide informed consent. We invited all clinic providers through an IRB-approved secure email. Provider participants were considered lost to follow-up if, after enrollment, they did not complete any intervention activity or did not complete study activities and did not explicitly inform research staff that they would like to withdraw from the study AND were unreachable by phone, email, or in-person despite multiple attempts before study close.
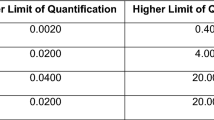
Eligible patient participants had to: be a patient living with HIV at the study clinic for at least 90 consecutive days; be ≥ 18 years of age; have at least one HIV viral load assessment per year over the 2 years before screening (to enable us to categorize them by viral load group); have attended at least one HIV appointment at study clinic within 365 days of enrollment; have been prescribed dolutegravir or emtricitabine ≥ 90 days before enrollment; have an appointment with a participating provider during the study, be literate in English; have at least 1.0 cm of natural caput hair; and provide informed consent. Patients were excluded if they met any of these criteria: had previously participated in the formative study; were deemed too ill to participate; had a history of clinically significant alteration of the gastrointestinal system or drug absorption capability; or had chemical hair treatment, such as using dye, bleach, or relaxers, within 4 weeks before hair, sampling. Screening was stratified by VL group (over the last 2 years, the first group—fully suppressed—had all VLs below the limit of quantification and second group—not fully suppressed—had at least one VL above the limit of quantification at the study site clinical laboratory, which was less than 40 copies/ml) with a maximum of 25 within each group.
Each week, a dedicated research screener provided project staff with a list of potentially eligible scheduled patients who were then contacted to verify their eligibility and invite them to participate.
Data Collection Procedures
At baseline patient participants completed an approximately 10-min self-administered questionnaire on a tablet. Within 24-h post-visit, patients completed a 12-item questionnaire that assessed their experience with MV. Prior to the COVID-19 pandemic, all study procedures for each patient were completed on the same day (January 20th, 2020, to March 16th, 2020). A hiatus in patient enrollment occurred from March 16th, 2020, to February 2nd, 2021, while study procedures were revised and approved to accommodate COVID-19 pandemic conditions. From February 2nd, 2021, to October 8th, 2021, baseline data were collected remotely up to 4 days before the provider visit and the provider visit could be conducted in-person or by telemedicine.
Provider participants completed a baseline questionnaire approximately 24 h after the provider training that assessed their demographic features and their evaluation of the training. Providers also completed a post-visit questionnaire within 48 h after each appointment with a participating patient to assess their experience with MV at that visit, including whether they showed and discussed it with the patient. Upon study completion, providers completed a questionnaire and an IDI to assess their experiences with MV after they had seen at least 2 participating patients, or after study close.
Administrative logs were maintained to track timing of each intervention procedure including hair plucking, transportation time, delivery of hair sample, start times of MV assay, and electronically time-stamped report delivery times.
Variables and Measures
Feasibility
Primary Feasibility Outcome: proportion of patient participants receiving the MV report during their provider visit as planned (defined as “the report is delivered to designated research staff member within 2 h of initiation of hair processing and discussed [by patient] with provider or pharmacist within 4 weeks of hair collection”) was assessed by combining data from study tracking logs with responses to two multiple-choice questions in the patients’ post-visit questionnaires that asked “Who showed you a copy of your MV report at your visit today?” (Response options: No one; My usual HIV medical provider; The clinic pharmacist; A clinic nurse; Someone else, please specify) and “Did this person discuss your MV report with you at your visit today (yes or no)?”.
Other Feasibility Outcomes
We assessed the total time to run the MV assay for each participant, and the proportion of assays completed within a 2-h window, using study tracking logs of MV assay start times and time-stamped electronic delivery times. We assessed time providers spent counseling patients about the MV report with one item on the provider post-visit questionnaire with 6 categorical response options (0, ≤ 1, 2–5, 5–10, 10–15, > 15 min). In instances in which patients did not receive and/or discuss the MV report with a provider, we assessed reasons using 2 multiple choice items from the provider post-visit questionnaire that included an open-ended “other” category.
We calculated the average per-patient cost for collecting, running, reporting, and discussing the MV assay. The cost estimate included costs of personnel and clinic space while showing the educational video, travel for remote visits (round-trip minutes driven to collect hair sample and number of miles X the federal mileage reimbursement rate), chemist time to run lab assay, staff time to print, deliver and review the report, and provider time to counsel (number of minutes spent self-reported by providers on a post-visit questionnaire) as well as personnel and provider costs incurred during the provider training and fixed costs for video production and equipment. All personnel times and training duration were documented using study logs in which activities were documented via start and stop time stamps as part of study administrative logs. The total cost of MV program delivery included fixed costs, such as cost of the machine, cost of creating the video and plus all summed variable costs (based upon aforementioned administrative cost data, as well as the cost of sample collection kits, lab analytes, and equipment maintenance, costs for utilizing clinical exam rooms). The total costs were then divided by the number of patients to whom the MV intervention was delivered for the estimated cost of delivery per person.
Acceptability
Primary Acceptability Outcome: Proportion of eligible contacted patients who agreed to participate in the MV Intervention pilot study was assessed using available participant screening questionnaires of patients who were contacted and found to be eligible and invited to participate in the study along with enrolled patient logs.
Other Acceptability Outcomes
We recorded reasons patients gave for declining participation when provided. Among patient and provider participants, respectively, we assessed their overall perceived usefulness of MV by asking “If MV were available for routine use, how likely would you be to use it/recommend it to some of your patients in the future?” (Response options: definitely would not, likely would not, likely would, definitely would). We also assessed the acceptability of specific components of the intervention. First, we asked providers to rate the overall quality of the training (on a scale of 1-poor to 5-excellent). Second, we assessed patients’ satisfaction with intervention components (waiting time, hair plucking, the format of the report, and the MV discussion with the provider) on a 5-point response scale ranging from “very dissatisfied” to “very satisfied.” Third, after viewing the video, we asked patients how helpful they found the video for 5 aspects of preparing for the MV assay (on a 5-point scale ranging from “extremely” to “not at all” helpful). We also used IDIs with both patients and providers to assess their views of individual MV components. Providers were asked open-ended questions about their views of the provider training. Patients and providers were asked open-ended questions about their views of both versions of MV reports, the educational materials, and several MV procedures, as well as what they thought about usefulness of MV (e.g., what aspects they liked and disliked and why and how they used them).
Appropriateness
Appropriateness of MV was assessed as providers’ perceived usefulness of MV to promote ART adherence, via the post-visit and endline questionnaires (on 5-point scales ranging from “not at all” to “extremely” useful) and qualitatively during patient and provider IDIs. We also assessed, in IDIs, perceived impact of MV on patient–provider communication and relationships. Patients were also asked how difficult or easy it was for them to understand the information in the MV Report (on a scale from “very difficult” to “very easy”) and to rate their adherence levels 1 month after receiving MV.
Statistical Analyses
We calculated descriptive statistics using SAS 9.4, SAS Institute, Cary, NC (mean and SD for continuous normally distributed variables, median and range for continuous non-normally distributed variables, frequencies, and percentages for categorical variables) of the sample characteristics of our participants as well as for each of the outcome variables. For some acceptability and the appropriateness outcomes, we conducted descriptive statistics stratified by VL group.
Qualitative Data Analyses
All interviews were digitally recorded and professionally transcribed verbatim. Study staff conducted transcription quality checks and reviewed full transcripts for content with the audio-recording in a first pass for accuracy and to develop familiarity with the data, noting emergent themes via memos. Second, a list of structural codes related to the interview questions was developed with code definitions documented in codebooks (separately for patient and provider interviews). Coding was conducted using Dedoose [33]. Trained qualitative research assistants piloted the codebook with 5 patient and 3 provider interview transcripts: each transcript was coded by 2 coders to reconcile code application, codes and rules for their application were modified as needed to achieve consensus, and new codes were added as needed. To ensure inter-coder consistency, 100% of the data were double-coded. Independent coders reviewed areas of discrepancy until complete agreement was achieved. We then summarized participant responses corresponding to each code and described variation in responses between individuals and subgroups and identified principal sub-themes within each topic using matrices. To better understand the salience of patients’ and providers’ perceptions of MV, we integrated the quantitative and qualitative results through a convergent mixed-methods approach during the interpretation phase [34, 35].
Results
Study Participation and Sample Characteristics (Table 1)
As a result of pausing study activities for several months due to the COVID-19 pandemic, we were able to recruit only 36 (74%) of the planned total of 50 participants. Specifically, while we recruited all of the 25 fully suppressed group participants during the amount of time available to conduct the study, we recruited only 11 (44%) of the 25 planned from the group of participants who were not fully suppressed, as they made up a lower proportion (< 15%) of the total scheduled clinic population. On average, 22 patients per week (68% in the fully suppressed group, and 32% in the not fully suppressed group) were eligible to be contacted by research staff based on their medical records. Research staff attempted to contact all potentially eligible patients each week, enrolling patients at a weekly rate of 0.84 patients (1.25/week pre-COVID and 0.75/week during COVID) over the 44 weeks (10 months) of active patient recruitment for a total of 36 unique participants.
Of the 75 patients who met the eligibility criteria that could be determined by chart review and who were contacted and completed a prescreening questionnaire, 7 (9.3%) were deemed ineligible during phone screening, 2 (2.6%) due to insufficient hair length, 3 (4.1%) due to hair treatment, 1 (1.3%) was in hospice and 1 (1.3%) died before the visit) leaving 68 fully eligible 58 (85.3%) of whom were scheduled for an initial visit. Of these, 22 (37.9%) did not present for their study visit while 36 (52.9% of those eligible) unique patients enrolled.
Of the 34 providers invited by secure email to participate, 24 (70%) responded and 20 (58%) agreed to participate in the study, all of whom completed the training. Of these 20, 1 moved from the university, which discontinued their eligibility, and they were moved off study. Of the remaining 19, 3 were lost to follow-up, although 2 of them had never seen patients for MV purposes. On average, the 16 providers saw 2.4 patients (SD 1.6 median 2 range 1–7). Three providers were lost to follow-up, including one who saw two patients on study but never completed endline data collection. One (6%) of the 16 providers said they were not available to complete an IDI. The 3 (19%) providers who had provided HIV care to patients in this clinic less than 1 year had all seen patients for at least 3 months; 3 (19%) additional providers had seen patients > 1 to 5 years, 3 (19%) > 5–10 years, and 7 (44%) > 10 years.
Descriptive characteristics of the 36 unique patients enrolled and of participating providers are shown in Table 1.
Feasibility of MedViewer
Primary Feasibility Outcome: Proportion of Participants Receiving the MedViewer Report During Their Provider Visit as Planned
Among the 37 patient participant clinic visits (representing 36 unique patients), the MV report was received and discussed 35 times (94%): 1 patient (not fully suppressed group) did not attend their scheduled clinic visit after the report was available, and for the other patient (fully suppressed group), the provider did not share the report with the patient. This participant was reenrolled at a later clinic visit where they discussed the new version of their report with the provider as planned. Of note, 3 (8%) of these discussions were deferred by the provider to a clinic pharmacist or other provider. Among the 37 visits, 30 (81%) had the assay completed within 2 h of initiation of hair processing. In total, of the 37 patient participant clinic visits, 28 (76%) had both their assay completed within 2 h of initiation of hair processing and the report discussed.
Secondary and Exploratory Feasibility Outcomes
Among the 35 patient/provider discussion pairs, 23 (66%) spent 2–5 min discussing the MV report, while 12 (34%) spent 5–10 min. One provider elected not to discuss the MV report with a patient as it appeared that a hair product may have interfered with the assay.
The mean assay duration was 1.8 h (SD 0.4). Among the 10 assays conducted before the COVID-19 modifications, 5 (50%) reports were delivered within 2 h of hair collection; the mean combined duration was 2.1 h (SD 0.2, median 2.0, range 1.8–2.6).
The average cost of the MV assay per patient was $198.17 ($172.17 for pre-COVID real-time visits and $224.16 for remote visits during COVID). Of this, $20 was related to supplies used in running the assay, $58.93 to chemist time to run the assay and $4.77 to other staff time to print and deliver the report to providers, while the mean cost of providers counseling patients using the report was $5.16 (range $3.17—$8.09). The remaining $109.31 was due to other costs (e.g., clinic space, staff sample collection and travel time, vehicle costs etc.). On average, patients indicated they would be willing to pay out-of-pocket a maximum of $16.73 (SD 29.2 median $10, range $0-$150) for the MV assay if it were to be routinely available in the future. Roughly 44% (16/36) reported they would pay a maximum of $10-$25 out-of-pocket, while 25% (9/36) reported they did not know how much they would be willing to pay.
Acceptability of MedViewer
Primary Acceptability Outcome: Proportion of Eligible Contacted Patients Who Agreed to Participate in the MedViewer Intervention Pilot Study
Of the 68 eligible patients contacted, 10 (14.7%) declined participation. Of these 10, 3 declined because they were unable to come to the clinic 2 h early, 3 had general time constraints, and 4 no longer wanted to participate in research in general.
Secondary and Exploratory Acceptability Outcomes (Tables 2, 3)
Overall Provider Perceived Usefulness of MedViewer (Table 2)
All 15 providers who reviewed MV reports with patients and completed the endline questionnaire said they would recommend (8 (53%) “definitely” and 7 (47%) “likely”) MV testing to some of their patients if available for routine use in the future. Nineteen (75%) patients in fully suppressed group and 9(82%) of those in the not fully suppressed group, respectively, said they “definitely would use” MV in the future if available. In IDIs, most providers stated their likelihood of recommending future MV testing to specific patients would depend on patients’ individual needs. Most providers stated that they would target it to patients with a history of detectable VL. Some providers said future MV testing for a wide spectrum of patients would be valuable as “just (be)cause someone is suppressed, doesn’t mean they’re fully adherent” and, for those who are, it “validates” their ongoing adherence.
Providers’ Views of Acceptability of Provider Training and Educational Materials (Table 2)
Providers’ mean rating of the overall quality of the training on a scale from 1 (poor) to 5 (excellent) was 4.95 (SD 0.2 median 5 range 4–5). Many providers spoke positively in IDIs about the training session, reporting that they gained understanding of the study process and left feeling prepared to discuss and review the MV report in the clinic. Many found it helpful to be in a group training where they could hear each other’s thoughts and questions about MV. As one provider put it, “to hear other people’s language or other people’s ideas about how to use the tools,” was helpful. Two providers suggested providing opportunities to practice delivering reports during training. Very few providers felt that the training session was more elaborate than needed. Providers described the communication aid and FAQ sheets as “helpful” references and “good reminder[s]” of the information discussed in the training, particularly for their first patient visit using MV. The educational video was also well-received by providers; several described it as creating a “shared experience” with patients that made the MV intervention feel “collaborative.”
Patients’ Views of Acceptability of Educational Video
Patients’ ratings of how helpful aspects of the video was to them are reported in Table 3. Most found the video “useful” to help them understand the intervention and prepare them for their MV visit with their providers. Participants liked that the video explained the entire MV process “from the hair sample on down to the end results” in a detailed and visual manner that was "informative, “straightforward, and “simple” for people of all literacy levels to understand. Participants also found the video was “pleasing to watch” and “culturally diverse,” reflecting the diversity of the HIV community. For one patient, while the video was helpful and informative, watching it led to feelings of anxiety and guilt related to ART nonadherence.
Acceptability of MedViewer Procedures
Among the 37 visits (n = 36 patients), all patients reported being comfortable and willing to provide hair samples for future MV testing, 26 (72%) reported being very satisfied with the hair plucking procedures, and 31 (86%) reported being very satisfied with the amount of time waiting for results. In IDIs, patients described the hair sampling experience as very easy, referring to it as “seamless,” “cool,” “exciting,” “painless,” and “without complications.” Patients felt excited to try something new and learn more about what was “in their system.” Patients were unconcerned about their hair being transported to the lab.
Acceptability of MedViewer Reports Delivery, Format, and Content
While some providers said they initially had a concern that incorporating the MV report into a routine visit might disrupt clinic flow, most reported that it integrated well into their clinic routine as illustrated by this quote:
I guess [I thought] if all of my patients had another sheet of paper that I had to go through, it could be disruptive, but someone handed me a folder at the beginning of my clinic session... and it was not disruptive.
Some providers said they anticipated disruptions if MV were implemented routinely for all patients due to additional questions. Some suggested that receiving the reports earlier to allow more time to review them before the patient visit could facilitate integration into clinic flow. As one provider put it, “… ideally, always it’s nice to, like, have a little bit more lead time to be able to review [the report] before, like, going in with a patient.”
Patient participants consistently stated that they would like to review the report with the clinic staff with whom they had the best rapport, which, for most, was their provider. As one patient put it, “I liked it because we have a really good rapport. Um, if it was a different provider, I might feel weird. … —it’s nice to have the one that you see [routinely] give you the information.” Some said they would be comfortable receiving their report from research staff, the phlebotomist, or HIV care pharmacist but they wanted that person to have competent understanding of the medication, be able to answer questions, and respect privacy concerns.
Most providers found the calendar version of the MV report to be helpful, simple and interpretable, using words such as “straightforward,” and “practical’ to describe it. Providers found the calendar report was easier to understand than the bar graph report and some said, as such, it helped them better “visualize the patients’ adherence during conversations.” Its dichotomous nature seemed to have made the calendar more digestible for patients.
Most patients in both groups found the calendar report easy to understand (22 (88%) in the fully suppressed group, 11 (100%) in the not fully suppressed group). Those who indicated having some difficulty comprehending the report, found the color scheme (Fig. 1) “unclear” and “unintuitive.” When asked to describe contents of the MV report, some participants incorrectly confused the colors representing no missed dose with that representing a possible missed a dose.
Among providers, 87% reported being “very satisfied” with the format and content of MV results (Table 2). Most providers thought the bar graph version of the reports helped them understand the test results. As one provider put it, “I think the bar graph is really—was helpful. It’s-it’s, uh, from a provider perspective, very visual… So, it—I think it represented the information appropriately… I wouldn’t change the structure of the report.” Providers who reported unsatisfactory experiences with the bar graph shared that they sometimes had a difficult time interpreting the data themselves. Understanding the threshold for indicating a missed dose was a particular point of confusion for a few providers. One provider explained, “I had some questions about sort of what the threshold was… I think the scale of the bar graph was a little bit confusing.”
While the provider training session explained that the bar graph version was mainly for provider use, approximately half of the providers chose to share the bar graph with their patients. Those who did considered it easy for most patients to interpret, allowing for a more detailed, “nuanced interpretation of adherence” that better emphasized trends. Providers said reviewing the bar graphs with patients served to jumpstart more nuanced adherence discussions. One provider elaborated,
I think it showed very general trends for patients, and I think that was a good starting point. I think one of the obvious things you can see is kind of the undulation of, like, the general trend, which is helpful. I thought that was especially helpful in having conversations with people who had consistent virologic suppression, um, and especially interesting for people who had detectable viral load but below 40—to kind of show them how variations in drug levels could be seen through this methodology.
Some providers viewed the bar graph report as only useful for certain patients and generally “more information that’s not really clinically significant” and “not necessarily any more helpful than the calendar view” for most patients.
I think it's maybe a little bit more confusing and nuanced to explain, like, why on certain days it was so much higher— than on other days ... so I think … maybe some training on how to sort of explain that to the patients could be helpful.
Appropriateness (Usefulness of MedViewer to Promote Adherence and Patient–Provider Communication) by Viral Load Group (Table 4)
Perceived Usefulness of MedViewer to Promote ART Adherence
In IDIs, the fully suppressed group participants found MV useful to externally motivate them to continue medication compliance. Patients that expressed high confidence in their adherence liked having a “visual representation” confirming that they were taking their medication well; they described it as something that “felt good”. As one participant described, “It would—it would just continue to empower me to do what I’m doin’ because I’m seein’ phenomenal results”. Some fully suppressed group participants reported little to no impact on their medication taking after the MV intervention because their existing strategies already supported optimal adherence. Several suggested that MV may be most beneficial for individuals newly diagnosed with HIV or struggling with medication adherence.
Most not fully suppressed participants found MV had a positive impact by increasing their motivation to adhere to ART medication or reinforcing pre-existing adherence strategies. Some suggested that MV would most benefit individuals who struggle with memory/cognitive challenges with adherence, while others believed “all patients would benefit from it.” Participants in the group not fully suppressed felt that the MV assay raised awareness of the importance of medication adherence, facilitated conversations with providers. MV was seen as a complement to routine CD4 and viral load counts. It was somewhat concerning that one not fully suppressed individual, however, viewed their adequate ART levels in the MV report, despite intentional periods of “medication vacations,” as evidence that they could use MV to monitor their medication vacations. Although the test is not validated for nor would it be recommended for this purpose, the participant stated: “It would definitely—it wouldn’t make me wanna take it every day… It would just let me know that I’m safe with how I’m takin’ it and to just continue to follow my own little guideline for my body.’”
Perceived Impact of MedViewer Use on Patient–Provider Communication and Relationship
In general, participants from the fully suppressed group described MV as having minimal impact on their relationship with their provider but felt it did support their adherence conversations with the provider by leading them to discuss specific strategies. Some not fully suppressed group patients said they felt afraid that their provider may think of them as “reckless” if future MV tests showed continued poor adherence. Some not fully suppressed patients saw MV as a way to show providers that they are “doing their best.” Patients across both groups felt that MV served as a useful communication tool that “holds you at a standard of bein[g] kinda honest about what’s goin’ on—honest about how you’re takin’ your meds.” Another put it this way, “… you know because I—you don’t wanna disappoint people. You don’t want them to ever think you’re not doin’ your part to take care of yourself. I want—it’s confirmin’ to her that I’m tryin’ everything.” One patient who reported recent suboptimal medication adherence felt that more transparent than usual discussions stimulated by reviewing MV enhanced their patient–provider relationship.
When providers were asked to rate the likely effect of implementing regular MV testing on their relationship with patients, 8 (53%) reported no change, 2 (13%) reported a somewhat positive effect, and 5 (33%) reported a very positive effect. No providers expected a negative effect. Several providers felt the MV report allowed them to be “less accusatory” when counseling patients, particularly when a discrepancy occurred between a patient's VL and their self-reported adherence.
Discussion
We developed the MV intervention, a novel hair-based clinical ART monitoring and feedback tool, as a new way to engage patients and providers to work together to address patients’ adherence. By providing them with a visual representation of the daily amount of medication in the patient’s body to review together, MV offers patient–provider dyads a tool to stimulate and support discussions about patients' medication-taking. This approach provides the advantage of assessing longer periods of adherence behavior than do other novel point-of-care adherence testing approaches currently under development (21,40) Before directly testing its efficacy to improve ART adherence, an important first step was to assess how feasible it would be to use in clinic, including how patients and providers felt about using it. In this mixed-methods study, we found that the MV intervention was generally feasible, acceptable, and appropriate for use in a busy tertiary care ID clinic as a complement to routine provider-delivered ART adherence counseling. Providers’ receipt of MV reports before routine clinic visits proved to be practical and was perceived as beneficial to review at the visit.
Each of the many aspects of the intervention that we assessed were found to be relatively feasible. During most MV visits, patients received the MV report as planned, which in our protocol was defined as “delivered within 2 h of initiating sample processing and discussed during the visit.” Because patients came to their appointment 2 hours early for the sample collection OR had it collected at locations that were remote from the clinic within 3 days before their clinic visit, MV did not impose undue burden on providers or interfere with clinic flow. That said, the low rate of patient enrollment partly reflects the fact that not all patients were willing or able to come to their appointment 2 hours early, which makes the MV less feasible when this time restriction is required. Use of remote hair collection, which was initially done in response to the COVID-19 pandemic, proved to make the use of MV more convenient and feasible for patients. The amount of time it took providers to discuss MV with patients was quite low. Furthermore, although in our formative studies some providers expressed concern that using MV might negatively affect their relationships with patients, when they actually used it in this study, providers reported no negative, and in many cases positive, effects [29]. MV was also found to be relatively affordable: only $79.00 was directly related to labor and supplies needed to run the assay itself. Both providers and patients found MV to be comprehensible, useful, and enjoyable, rating specific intervention components (e.g., video, educational materials, hair sampling process, etc.,) very favorably.
While our findings indicated that MV was feasible, acceptable, and appropriate, assessment of efficacy and optimal applications will require further study. The question remains regarding those for whom MV will most enhance adherence. All participating providers said they would recommend MV to their patients in the future, if it were available, but most thought they would order it mainly for their patients with detectable viremia. A few providers, however, believed that MV would benefit all patients since intermittent VLS represents adherence for only small windows of time. Most patients said they would “definitely” use MV in the future if available because it helped motivate them to adhere, regardless of their VLS status. For many patients, the MV report represented an expression of praise or applause. These findings are consistent with our previously published adapted IMB Model on which MV was based [30-32] as it elucidates how objective adherence feedback information from the MV report can enhance routine adherence approaches by furnishing positive reinforcement. A few patients with consistent VLS, however, saw no impact of MV on their motivation as they believed they were already optimally adherent. While most patients were inclined to think all patients could benefit from MV, some believed that the MV assay would be particularly useful to new or struggling patients. Our findings are consistent with other studies suggesting that real-time adherence feedback in clinical settings, including those using biomedical data, are a potentially useful approach but without agreement on which patients would benefit most [14-17, 36-39]. In diabetes care, adherence researchers demonstrated that targeting interventions to less adherent patients led to better clinical outcomes overall [40]. The same may be true for HIV care and future studies of MV and other ART adherence interventions that offer biomedical adherence feedback as part of clinical care will need to evaluate different tactics to targeting the intervention to determine for whom these interventions are most effective and most cost-effective [41].
In HIV care, point-of-care viral load (VL) monitoring has been shown to be cost-effective in improving viral suppression [36-38]. Real-time ART adherence monitoring has the additional potential to identify and address each individual’s adherence challenges early in treatment, to help tailor interventions to specific challenges that arise during ongoing treatment, and to motivate continued consistently high adherence [39]. So far, only few randomized trials have tested an intervention in the US to improve adherence to ART treatment specifically by giving medical providers a detailed objective adherence report before their visit with a patient [18]. Using Medication Event Monitoring System (MEMS) data for feedback to providers, the trial found no effect on adherence. However, their analyses of the audiotaped patient–provider dialogues indicated that the feedback alone was insufficient; providers also needed training on how to communicate with patients about their adherence, such as that we provided in the MV intervention. Other studies have also shown that HIV providers benefit from training in adherence counseling techniques [42]. Moreover, the lack of effect in the trial by Wilson et al., may also be attributable to the limitations of MEMS data which reflect interactions with the pill bottle rather than actual pill ingestion and do not correlate well with pharmacologic adherence measures. A similar trial in China, in which providers received MEMS feedback for patients with adherence < 95% and reviewed the feedback with these patients, counseling them on medication-taking strategies, showed improved adherence at 12 months [16]. Similarly, a multi-site randomized trial in the Netherlands of objective MEMS feedback delivered by nurses trained to use the feedback to counsel patients on adherence strategies showed modest improvement in VLS [17]. These studies suggest that objective feedback used by providers to counsel patients can be effective. Like our study, a recent study of PrEP adherence among men who have sex with men, found that a digital pill feedback system to be feasible and acceptable [43]. MV goes beyond MEMS data because it provides patients with information about what is happening daily in their bodies regarding their medication [44], something that patients mentioned as being particularly reinforcing and motivating for them.
While our findings suggest MV warrants testing of its effectiveness to promote adherence in a randomized trial, we did identify areas for improving MV before conducting a larger trial. The slow rate of recruitment in our study indicates that many patients are either ineligible, difficult to contact in advance, or uninterested in participating, suggesting it will not be a good option for all patients. Before COVID-19, 3 participants declined participation because they were unable to come the requisite 2 h before their scheduled clinic appointment. Thus, offering an option to have one’s hair collected a few days before the scheduled clinic appointment, as was done and found feasible during COVID-19, would enhance acceptability and feasibility by adding flexibility to accommodate patients’ differing circumstances. Similarly, as 22 of the 58 patients scheduled for a MV visit did not show up, most of whom had histories of detectable viral loads and thus perhaps were among those most likely to benefit from MV, we can modify the intervention to work better for patients with poor access to clinic [41, 45]. The rise of telemedicine suggests the option of going to such patients to collect the sample of hair, as we did during COVID-19, and providing the clinic visit by videoconference. This tactic could improve access to adherence support for hard-to-reach patients as was shown in a recent pilot study among African American women living with HIV and depression [46]. Additional considerations might include hair sample self-collection and mailing. Also, while most patients found that MV motivated them to improve (or continue their good) adherence, one patient with a history of having a detectable VL incorrectly concluded that despite having taken “medication vacations,” their ART levels remained sufficient; in this case MV unintentionally provided informational support for continuing medication vacations. This case emphasizes further the importance of training providers to have more nuanced conversations with patients about the complex interplay among medication-taking, dose-timing, drug concentrations, and thresholds, as some providers had requested. In addition, providers disagreed about exactly how much information to provide patients. About half of the providers shared the bar graph with their patients (although it was primarily intended for provider use) and found it helped prompt more nuanced conversations about the effects of pill-taking behavior on drug levels. Finally, while all providers and most patients found that MV had generally positive or no effects on the patient–provider relationship, a few nonadherent patients worried that their provider would judge them negatively. Future MV components should incorporate techniques for supporting patients and allaying these fears.
Interpretation of the findings of this study must consider its limitations. First, self-report measures in survey research and IDI responses may be subject to social desirability biases. Respondents may have underreported socially undesirable attitudes toward the intervention, although questionnaires were self-administered on computers/tablets whenever possible, and the confidentiality of responses was made clear to participants to mitigate bias. Second, because MV relies on patients having sufficient amounts of untreated hair, it may not be an option for all patients. Seven percent of potentially eligible patients were ineligible due to either having hair that was too short or having recently treated their hair with chemical products. Third, we did not collect information regarding why some providers spent more time than others discussing MV with their patients. This may be an important area to explore in future studies. Fourth, although the age distribution of our patient sample reflects the clinic’s population, the older median age means our findings may not generalize to younger patients. Also, the procedural changes made in response to the COVID-19 pandemic to limit in-person contact to ensure the safety of patients, research staff, and providers limited impeded our exploration of the duration of conducting all assay procedures on the same day but did provide an opportunity to test new methods of implementing MV via telehealth. In addition, while the cost of the intervention was found to be not prohibitively expensive for a US context, and some of the costs might be lower in low and middle income countries, the costs of technology, such as mass spectrometry imaging and its associated maintenance, still might be prohibitive for use in routine care in low and middle income countries.
Despite these limitations, the current pilot study presents the first effort to investigate how a novel longitudinal measure of hair concentrations as a reflection of adherence can stimulate and support adherence discussions in a clinical setting among PWH. Previous studies indicate that advanced technologies to measure ART using hair can be objective and reliable metrics of adherence [21]. Our findings indicate that MV is an appropriate, useful, and promising new tool to noninvasively measure and monitor ART daily longitudinal adherence.
Conclusion
The novel hair-based clinical ART monitoring tool, MV, with its visual representation of the daily amount of medication in the patient’s body, offers an exciting new approach to engaging patients and providers to collaborate to optimize patient adherence. This feasibility study lays the groundwork for a larger trial in the future to identify which patients to focus on for MV to achieve the most cost-effective outcomes and evaluate the impact of this monitoring on subsequent adherence. Our findings add a novel slant to a growing body of evidence of adherence monitoring tools’ impact to improve and sustain VLS, strengthen provider-patient communication, and improve engagement in long-term HIV care.
References
Global Statistics | HIV.gov [Internet]. [cited 2022 Mar 31]. https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics.
The Lancet HIV. U=U taking off in 2017. Lancet HIV. 2017;4:e475.
–90–90: good progress, but the world is off-track for hitting the 2020 targets [Internet]. UNAIDS. 2020 [cited 2022 Apr 4]. https://www.unaids.org/en/90-90-90.
Bangsberg DR, Haberer JE. Lifetime HIV antiretroviral therapy adherence intervention: timing is everything: comment on “Managed problem solving for antiretroviral therapy adherence.” JAMA Intern Med. 2013;173:306–7.
Carvalho PP, Barroso SM, Coelho HC, Penaforte FRO. Factors associated with antiretroviral therapy adherence in adults: an integrative review of literature. Cien Saude Colet. 2019;24:2543–55.
World Health Organization. Adherence to long-term therapies, evidence for action. Geneva: World Health Organization; 2003.
Rooks-Peck CR, Wichser ME, Adegbite AH, DeLuca JB, Barham T, Ross LW, et al. Analysis of systematic reviews of medication adherence interventions for persons with HIV, 1996–2017. AIDS Patient Care STDS. 2019;33:528–37.
Flickinger TE, Saha S, Moore RD, Beach MC. Higher quality communication and relationships are associated with improved patient engagement in HIV care. J Acquir Immune Defic Syndr. 2013;63:362–6.
Zolnierek KBH, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–34.
Francis V, Korsch BM, Morris MJ. Gaps in doctor–patient communication. Patients’ response to medical advice. N Engl J Med. 1969;280:535–40.
Hall J, Dl Roter, Junghans B. Doctors talking with patients—patients talking with doctors: improving communication in medical visits. Clin Exp Optometry. 1995;78:79–80.
Roter DL, Stewart M, Putnam SM, Lipkin M, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA. 1997;277:350–6.
Hall JA, Roter DL, Katz NR. Meta-analysis of correlates of provider behavior in medical encounters. Med Care. 1988;26:657–75.
Sumari-de Boer IM, Ngowi KM, Sonda TB, Pima FM, Masika Bpharm LV, Sprangers MAG, et al. Effect of digital adherence tools on adherence to antiretroviral treatment among adults living with HIV in Kilimanjaro, Tanzania: a randomized controlled trial. J Acquir Immune Defic Syndr. 2021;87:1136–44.
Gross R, Bellamy SL, Chapman J, Han X, O’Duor J, Palmer SC, et al. Managed problem solving for antiretroviral therapy adherence: a randomized trial. JAMA Intern Med. 2013;173:300–6.
Sabin LL, DeSilva MB, Hamer DH, Xu K, Zhang J, Li T, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS Behav. 2010;14:580–9.
de Bruin M, Oberjé EJM, Viechtbauer W, Nobel H-E, Hiligsmann M, van Nieuwkoop C, et al. Effectiveness and cost-effectiveness of a nurse-delivered intervention to improve adherence to treatment for HIV: a pragmatic, multicentre, open-label, randomised clinical trial. Lancet Infect Dis. 2017;17:595–604.
Wilson IB, Laws MB, Safren SA, Lee Y, Lu M, Coady W, et al. Provider-focused intervention increases adherence-related dialogue but does not improve antiretroviral therapy adherence in persons with HIV. J Acquir Immune Defic Syndr. 2010;53:338–47.
Bardon AR, Simoni JM, Layman LM, Stekler JD, Drain PK. Perspectives on the utility and interest in a point-of-care urine tenofovir test for adherence to HIV pre-exposure prophylaxis and antiretroviral therapy: an exploratory qualitative assessment among U.S. clients and providers. AIDS Res Ther. 2020;17:50.
Bardon AR, Dorward J, Sookrajh Y, Sayed F, Quame-Amaglo J, Pillay C, et al. Simplifying TREAtment and Monitoring for HIV (STREAM HIV): protocol for a randomised controlled trial of point-of-care urine tenofovir and viral load testing to improve HIV outcomes. BMJ Open. 2021;11: e050116.
Spinelli MA, Haberer JE, Chai PR, Castillo-Mancilla J, Anderson PL, Gandhi M. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV/AIDS Rep. 2020;17:301–14.
Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, Kibengo FM, et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr. 2015;68:13–20.
Adams JL, Sykes C, Menezes P, Prince HMA, Patterson KB, Fransen K, et al. Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: a new measure of antiretroviral adherence? J Acquir Immune Defic Syndr. 2013;62:260–6.
Rosen EP, Thompson CG, Bokhart MT, Prince HMA, Sykes C, Muddiman DC, et al. Analysis of antiretrovirals in single hair strands for evaluation of drug adherence with infrared-matrix-assisted laser desorption electrospray ionization mass spectrometry imaging. Anal Chem. 2016;88:1336–44.
Gilliland WM, Prince HMA, Poliseno A, Kashuba ADM, Rosen EP. Infrared matrix-assisted laser desorption electrospray ionization mass spectrometry imaging of human hair to characterize longitudinal profiles of the antiretroviral maraviroc for adherence monitoring. Anal Chem. 2019;91:10816–22.
Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3401–9.
Sykes C, Blake K, White N, Schauer AP, Guzman BB, Cottrell ML, et al. Development and validation of an LC-MS/MS assay for the quantification of dolutegravir extracted from human hair. Anal Bioanal Chem. 2018;410:7773–81.
Pack AP, Golin CE, Hill LM, Carda-Auten J, Wallace DD, Cherkur S, et al. Patient and clinician perspectives on optimizing graphical displays of longitudinal medication adherence data. Patient Educ Couns. 2019;102:1090–7.
Hill LM, Golin CE, Pack A, Carda-Auten J, Wallace DD, Cherkur S, et al. Using real-time adherence feedback to enhance communication about adherence to antiretroviral therapy: patient and clinician perspectives. J Assoc Nurses AIDS Care. 2020;31:25–34.
Fisher WA, Fisher JD, Harman J. The information-motivation-behavioral skills model: a general social psychological approach to understanding and promoting health behavior. In: Suls J, Wallston KA, editors. Social psychological foundations of health and illness. Malden: Blackwell; 2003. p. 82–106.
Rivet AK. A situated-Information Motivation Behavioral Skills Model of Care Initiation and Maintenance (sIMB-CIM): an IMB model based approach to understanding and intervening in engagement in care for chronic medical conditions. J Health Psychol. 2011;16:1071–81.
Poliseno A, Ferguson E, Perry R, Munson A, Davis A, Hill L, Keys J, White N, Farel C, Gay C, Golin C, Rosen E, Kashuba A. Establishing novel antiretroviral imaging for hair to elucidate nonadherence: protocol for a single-arm cross-sectional study. JMIR Res Protoc. 2023;12:e41188. https://doi.org/10.2196/41188.
Dedoose [Internet]. [cited 2022 Apr 1]. https://www.dedoose.com/resources/articledetail/great_new_things_dedoose_you_need_to_know_now.
Creswell JW, Plano VL. Designing and conducting mixed methods research. 3rd ed. Thousand Oaks: SAGE; 2017.
O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ. 2010;341: c4587.
Drain PK, Dorward J, Violette LR, Quame-Amaglo J, Thomas KK, Samsunder N, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV. 2020;7:e229–37.
Girdwood SJ, Crompton T, Sharma M, Dorward J, Garrett N, Drain PK, et al. Cost-effectiveness of adoption strategies for point of care HIV viral load monitoring in South Africa. EClinicalMedicine. 2020;28: 100607.
Sharma M, Mudimu E, Simeon K, Bershteyn A, Dorward J, Violette LR, et al. Cost-effectiveness of point-of-care testing with task-shifting for HIV care in South Africa: a modelling study. Lancet HIV. 2021;8:e216–24.
Gandhi M, Wang G, King R, Rodrigues WC, Vincent M, Glidden DV, et al. Development and validation of the first point-of-care assay to objectively monitor adherence to HIV treatment and prevention in real-time in routine settings. AIDS. 2020;34:255–60.
Lauffenburger JC, Lewey J, Jan S, Makanji S, Ferro CA, Krumme AA, et al. Effectiveness of targeted insulin-adherence interventions for glycemic control using predictive analytics among patients with type 2 diabetes: a randomized clinical trial. JAMA Netw Open. 2019;2: e190657.
Whiteley LB, Olsen EM, Haubrick KK, Odoom E, Tarantino N, Brown LK. A review of interventions to enhance HIV medication adherence. Curr HIV/AIDS Rep. 2021;18:443–57.
Tugenberg T, Ware NC, Wyatt MA. Paradoxical effects of clinician emphasis on adherence to combination antiretroviral therapy for HIV/AIDS. AIDS Patient Care STDS. 2006;20:269–74.
Chai PR, Mohamed Y, Bustamante MJ, Goodman GR, Najarro J, Castillo-Mancilla J, Baez A, Bronzi O, Sullivan MC, Pereira LM, Baumgartner SL, Carnes TC, Mayer KH, Rosen RK, Boyer EW, O’Cleirigh C. DigiPrEP: a pilot trial to evaluate the feasibility, acceptability, and accuracy of a digital pill system to measure PrEP adherence in men who have sex with men who use substances. J Acquir Immune Defic Syndr. 2022;89(2):e5–15. https://doi.org/10.1097/QAI.0000000000002854.PMID:34753871;PMCID:PMC8740604.
Mwangi JN, Gilliland WM, White N, Sykes C, Poliseno A, Knudtson KA, et al. Mass spectroscopy imaging of hair strands captures short-term and long-term changes in emtricitabine adherence. Antimicrob Agents Chemother. 2022;66: e0217621.
Park WB, Choe PG, Kim SH, Jo JH, Bang JH, Kim HB, et al. One-year adherence to clinic visits after highly active antiretroviral therapy: a predictor of clinical progress in HIV patients. J Intern Med. 2007;261:268–75.
Junkins A, Psaros C, Ott C, Azuero A, Lambert CC, Cropsey K, Savage R, Haberer JE, Safren SA, Kempf MC. Feasibility, acceptability, and preliminary impact of telemedicine-administered cognitive behavioral therapy for adherence and depression among African American women living with HIV in the rural South. J Health Psychol. 2021;26(14):2730–42. https://doi.org/10.1177/1359105320926526.
Acknowledgements
We would like to acknowledge and thank Ms. Cheryl Hendrickson for her diligent assistance with regulatory documentation and internal review board submissions and Dr. Michael Hudgens for advice regarding plans for statistical analyses. We would also like to acknowledge Edward Slanker for his production of the MedViewer video. The authors honor the memory of Heather Asher Prince MPA, PA-C and her contributions to this work and to the care of our patients.
Funding
This study was funded by NIAID (Grant Number R01 AI122319). This grant was partially supported by University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410). Additional trainee support is provided by the National Institute of Mental Health (K01 MH121186).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests or conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Golin, C.E., Rosen, E.P., Ferguson, E.G. et al. Feasibility, Acceptability and Appropriateness of MedViewer: A Novel Hair-Based Antiretroviral Real-Time Clinical Monitoring Tool Providing Adherence Feedback to Patients and Their Providers. AIDS Behav 27, 3886–3904 (2023). https://doi.org/10.1007/s10461-023-04104-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-023-04104-1