Abstract
We investigated a novel community-based HIV testing and counseling (HTC) strategy by recruiting men from bars in northern Tanzania in order to identify new HIV infections. All bars in the town of Boma Ng’ombe were identified and male patrons were systematically invited to participate in a health study. HIV testing was offered to all enrolled participants. Outputs included HIV test yield, cost per diagnosis, and comparison of our observed test yield to that among male patients contemporaneously tested at five local facility-based HTC. We enrolled 366 participants and identified 17 new infections – providing a test yield of 5.3% (95% Confidence interval [CI] 3.3–8.4). The test yield among men contemporaneously tested at five local HTC centers was 2.1% (95% CI 1.6–2.8). The cost-per-diagnosis was $634. Our results suggest that recruiting male bar patrons for HIV testing is efficient for identifying new HIV infections. The scalability of this intervention warrants further evaluation.
Resumen
Investigamos una novedosa estrategia comunitaria de asesoramiento y pruebas de VIH (HTC) reclutando hombres de los bares del norte de Tanzania para identificar nuevas infecciones de VIH. Se identificaron todos los bares de la ciudad de Boma Ng'ombe y se invitó sistemáticamente a los clientes varones a participar en un estudio de salud. Se ofrecieron pruebas de VIH a todos los participantes inscritos. Los resultados incluyeron los resultados de las pruebas de VIH, el costo por diagnóstico y la comparación de nuestros resultados observados con los de los pacientes varones que simultáneamente se sometieron a pruebas en cinco centros locales de HTC. Se inscribieron 366 participantes y se identificaron 17 nuevas infecciones, proporcionando un resultado en las pruebas del 5.3% (intervalo de confianza [IC] del 95%: 3.3-8.4). Los resultados de las pruebas realizadas simultáneamente en cinco centros locales de HTC fue del 2.1% (IC del 95%: 1.6-2.8). El costo por diagnóstico fue de $634. Nuestros resultados sugieren que el reclutamiento de clientes masculinos para las pruebas de VIH fue eficiente para identificar nuevas infecciones de VIH. La escalabilidad de esta intervención merece una evaluación adicional.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the expansion of HIV testing services, testing uptake remains suboptimal in sub-Saharan Africa (SSA). In particular, men in SSA consistently access HIV testing services at lower rates in comparison to women [1]. Consequently, men who are living with HIV are less likely to initiate antiretroviral therapy, are more likely to suffer from HIV-related morbidity and mortality in comparison to women, and remain an ongoing source for forward transmission to their sexual partners [2]. In order to achieve the 90–90-90 targets and the promise of an HIV-free generation, there is an urgent need to identify strategies to increase the uptake of HIV testing among men in SSA.
Several factors that lead to the poor uptake of HIV testing among men in SSA have been described. The most commonly cited factors are gender norms and practices, specifically around views of masculinity [3]. Masculine ideals that emphasize strength and independence may tacitly discourage health seeking behaviors and are commonly perceived as incompatible with HIV testing. Other research suggest that men’s low testing uptake is not entirely about individual choices but rather a reflection of the differential positioning of men and women within HIV programs [4]. The most evident example is the routine testing of all pregnant women while failing to engage their male partners. Furthermore, several studies suggest that the gendered structuring of HIV testing has led to the perspective among some men that HIV clinics are “female spaces” which may negatively influence their test seeking behaviors [4,5,6,7].
Recently, a number of innovative strategies have been described to engage men in HIV testing. These interventions recognize that facility-based HIV testing and counseling (HTC) have achieved limited testing coverage among men and thus emphasize delivering testing services within the community setting [8, 9]. Community-based HTC, defined as HTC done outside of health facilities, are generally more acceptable to men, attract more first-time male testers, diagnose men at earlier stages of their HIV disease, and more efficiently reach men with undiagnosed HIV [10]. Generally, community-based HTC strategies fall into one of three main categories: mobile, home-based, and venue-based testing. The provision of oral self-testing within each of these categories has emerged as an innovative strategy to offer discreet and convenient testing [11, 12]. In all epidemic settings, community-based HTC is increasingly recognized as a strategy to overcome individual and facility-level barriers that prevent men and other hard to reach populations from accessing HIV testing services [13].
In SSA, alcohol-serving establishments offer unique opportunities within the community to expose men to HIV testing. These venues are patronized by large numbers of men and their importance as sites to implement HIV prevention is enhanced by the consumption of alcohol, a well-established risk factor for HIV acquisition [14, 15]. In the present study, we recruited men attending bars in northern Tanzania and offered them HIV testing in a non-clinical setting. We describe the HIV testing acceptance, HIV testing yield, and cost-per-diagnosis of our testing strategy. Furthermore, we obtained testing data from local facility-based HTC centers and compare our observed testing yield to that of these facilities.
Methods
Setting
The study was conducted in the town of Boma Ng’ombe, elsewhere referred to as Boma, which is located in the Hai District of the Kilimanjaro Region of Tanzania. Boma has a population of approximately 17,000 persons and lies on a major highway connecting the Kilimanjaro Region to the Arusha Region. HIV prevalence among adults aged 15 years or older in the Kilimanjaro Region is estimated at 2.6% with a prevalence of 2.0% among men and 3.1% among women [16].
Bar Enrollment and Sampling
A bar was defined as an establishment that sells alcohol and provides seating for the consumption of alcohol. All bars in Boma were eligible for participation. A study team member with local guides systematically surveyed the town to map all venues meeting the study definition of a bar. If a venue met this definition, the objective and purpose of our study were explained to the bar owner, and we requested permission to recruit male bar patrons. Bar owners were asked to provide the opening hours of their bar as well as the times in which the bar had the highest customer traffic. This information was used to determine the days and times in which recruitment activities would lead to the highest participant yield. The global positioning system (GPS) coordinates for each bar was recorded using Garmin eTrex handheld devices (Garmin, Olathe, Kansas). This GPS location was used to locate bars during the participant recruitment period.
The days and times to visit bars for participant recruitment were randomized and adapted from previously described venue sampling methods [17]. Each month, 16 to 20 bars were randomly selected for recruiting activities using randomly generated numbers. Once bars were selected, each bar was assigned a random day, including weekend days, to be visited during that month. Per self-report from bar owners, we found that customer traffic in most bars was highest after 4PM on most days. Out of safety consideration for study recruiters, participant recruitment at bars stopped after 8PM. Thus, participant recruitment from bars occurred from 4PM through 8PM. A bar could be selected more than once during the duration of the study if randomly selected during multiple months.
Participant Recruitment
All males aged 18 years or older entering the bar were approached for recruitment by a male research assistant. Individuals with signs of intoxication such as slurred speech, disinhibition, or incoordination were not approached. Individuals seated in the bar at the start of the visit were not recruited to avoid disturbing the business operations. The research assistant provided each eligible male with a study recruitment card (Fig. 1). This card invited patrons to participate in a study focused on the health of male bar patrons. To participate in the study, bar patrons were invited to report to the study office, located in Boma, on a day different than the recruitment day. Our study office was opened for enrollment on Mondays, Wednesday, Thursdays, and Saturdays from 9AM to 5PM. Individuals presenting to the office without a recruitment card were ineligible to participate. Each recruitment card was uniquely numbered to track the number of cards distributed and the number of participants returning for enrollment in the study. Recruitment cards were available in English or Kiswahili. All participants were informed at the time of recruitment that they would be reimbursed 5,000 Tanzanian shillings (TSh), approximately 2.17 US Dollars in 2019 currency, for participation in the study. Eligible male participants presenting to the study office were offered enrollment into the study. During the consenting process, all participants were offered HIV testing but were also made aware that HIV testing was not required to participate in the study and that refusal of testing would not affect study compensation.
Survey
After obtaining informed consent, participants underwent a survey administered in Kiswahili by Tanzanian research assistants using Samsung Galaxy Tab A tablets (Samsung, Seoul, South Korea). The survey was designed using Open Data Kit version 1.12.2 (available online at https://opendatakit.org/). Basic sociodemographic information, including age, marital status, and highest level of education attained, was recorded. Alcohol use was measured using the Alcohol Use and Disorder Identification Test (AUDIT). The AUDIT has been validated in Tanzania [18, 19]. Participants were asked about sexual activity in the last 12 months. If sexually active, participants were asked about the number of sexual partners within the last 12 months, condom use with these partners, and concurrent sexual partners. Participants were considered to have concurrent sexual partners if they reported an ongoing sexual relationship with at least 2 partners. Lastly, participants were asked if they had previously tested for HIV and the timing of their last HIV test.
HIV Testing
HIV testing was performed by a male research assistant trained in voluntary counseling and testing. All testing was accompanied by pre- and post-test counseling and was performed on fingerstick samples using the SD-Bioline HIV-1/HIV-2 3.0 test (Standard Diagnostics Inc, Kyonggi-do, South Korea) for screening, followed by the Unigold Rapid HIV test (Trinity Biotech, Bray, Ireland) for confirmation. In the event of a positive test, the participant was referred to a facility-based treatment center of their choice. Participants received follow-up phone calls from a research assistant to encourage care engagement.
Cost
Cost (in 2019 US dollar) was calculated using the following cost categories: personnel; equipment; infrastructure; travel; and participant incentives. Personnel cost was the salary paid to two research assistants hired for seven months. Personnel cost included salaries paid during research training and enrollment of bars. Equipment cost largely consisted of the recurring cost of HIV testing kits, gloves, and phone vouchers for research assistants. The cost per test for both SD Bioline and Unigold was $1.74. Infrastructure cost included the recurring cost of the research office rental. Infrastructure cost also included the fixed one-time cost of office furnishing. Because both research assistants lived outside of Boma, travel cost included the transport cost to and from Boma. In addition, travel cost included the transport cost between the study office and bars during participant recruitment. Participant incentive cost was the sum total dollar amount given to participants for participation in the study. All cost estimates were based on receipts or documenting expenditure on a study form by research assistants if a receipt could not be obtained.
Data from HIV Testing Centers
We obtained approval from the District Medical Officer, the highest-ranking medical official within Hai District, to collect testing data from all facility-based testing centers in Boma. We categorized the service level of these facilities as dispensary, health center, or hospital [20]. Generally, dispensaries provide basic outpatient care while health centers are intended to provide preventative outpatient care but are capable of providing some basic inpatient care. Hospitals are categorized as either district, regional, zonal, or national, with district hospitals providing the most basic level of hospital-based care within the Tanzanian health system. Test centers could be either publicly or privately owned. All testing centers are required to use a standardized log book for recording and monitoring HIV testing. From these log books, we recorded the numbers of men and women who underwent HIV testing and the results of these test. We only collected testing data for men and women tested while our study was active to minimize any temporal biases relating to testing uptake and test yield. All data collected from log books were deidentified when recorded onto study forms.
Statistical Analysis
Data analysis was performed using descriptive statistics. Continuous variables were expressed using the median and the interquartile range (IQR). Categorical variables were expressed as frequencies. The outputs measured in this study included: (1) Proportion of participants enrolled (the numerator is the number of participants enrolled divided by the number of recruitment cards distributed); (2) HIV testing acceptance (the numerator is the number of participants who accepted HIV testing divided by the number of participants enrolled); (3) HIV testing yield (the numerator is the number of participants who were newly diagnosed with HIV infection divided by the number of participants who accepted HIV testing); (4) Proportion of participants enrolled with HIV-infection (the numerator is the number of participants with a positive HIV test or a prior diagnosis of HIV infection divided by the number of participants who accepted HIV testing, were previously diagnosed with HIV, or reported testing within the previous 12 months prior to enrollment); and (5) Cost-per-diagnosis (the numerator is the sum total of all cost categories divided by the number of positive HIV tests). All analyses were performed using STATA version 16.0 (StataCorp, College Station, TX).
Results
Participant Characteristics
We identified 102 bars in the town of Boma (Fig. 2). All bars owners agreed to participate in the study. From 6 December 2018 through 31 May 2019, we randomly selected 74 bars and provided recruitment cards to 1,688 men from these bars (Fig. 3). Among men who were recruited, 366 (21.7%) presented for enrollment in the study. The median (IQR) age of participants was 41 (32–50) years. The median AUDIT score (IQR) was 10 (3–16) and 209 (57.1%) participants were classified as harmful drinkers based on their AUDIT score. When asked about sexual partners in the last 12 months, 218 (59.6%) participants reported 0 to 1 sexual partner and 148 (40.4%) reported 2 or more sexual partners. Among participants reporting a sexual partner in the last 12 months, 295 (90.2%) reported no condom use with their last sexual partner and 45 (14.0%) reported concurrent sexual partners. When asked about HIV testing history, 99 (27.1%) reported an HIV test in the previous 12 months, 165 (45.1%) reported that their last HIV test was more than 12 months prior, and 102 (27.9%) reported no prior HIV testing. Participant characteristics are presented in Table 1.
(Map 1) Map of Tanzania divided into regions with the Kilimanjaro and Arusha Regions gray shaded. The blue shaded area reflects the general location of Boma Ng’ombe, where this study was conducted. (Map 2) We present a close-up view, using OpenStreetMap, of the town of Boma Ng’ombe which is located in the Hai District of the Kilimanjaro Region of Tanzania
HIV Testing
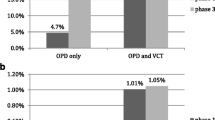
HIV testing was offered to all participants, and 321 (87.7%) accepted the testing offer (Fig. 3). Among the 102 men who reported no prior HIV test, 95 (93.1%) accepted the testing offer. We identified 17 HIV infections among all participants who accepted testing, resulting in a test yield of 5.3% (95% confidence interval [CI] 3.3–8.4). Among participants who tested more than 12 months prior or had never tested, the test yields were 6.8% (95% CI 3.6–12.2) and 6.3% (95% CI 2.8–13.5), respectively. Among the 45 participants who declined testing, 7 reported being previously diagnosed with HIV. Eight participants reported no HIV testing in the previous 12 months and declined our testing offer. Thus, the total number of participants with HIV infection in our cohort was 24, resulting in a proportion of enrolled participants with HIV infection of 6.7% (95% CI 4.5–9.8).
Test Yield from Local Testing Centers
We identified 5 testing centers in Boma. Of these, 2 were privately owned dispensaries, 1 was a public dispensary, 1 was a privately-owned health center, and 1 was a district hospital (Table 2). During the period of active recruitment and enrollment in our study, these testing centers tested 2,464 men and identified 52 HIV infections. Thus, the overall testing yield at these testing centers was 2.1 (95% CI 1.6–2.8).
Cost
The total costs, in 2019 USD, of study implementation was $10,782. Per cost category, the costs were $6,720 for personnel; $879 for equipment; $822 for infrastructure; $1,565 for travel, and $796 for participant incentives. Given the 17 newly identified HIV infections, the overall cost-per-diagnosis was $634.
Discussion
Recruiting and incentivizing male bar patrons to test for HIV in the context of a health survey was efficient for identifying new HIV infections in the Kilimanjaro Region. The percentage of HIV infection among men consenting to participate in this study was 6.7%, a percentage considerably higher than the background prevalence of 2.0% reported in demographic surveillance data for this region [16]. Furthermore, the testing yield of newly diagnosed infections of 5.3% was 2.5-fold higher than contemporaneous testing yields from local facility-based testing centers. Our results suggest that community-based HTC targeting male bar patrons holds considerable potential to identify men living with HIV who might not otherwise seek testing.
Community-based HTC designed for men should be implemented in settings frequented by large numbers of men exhibiting behaviors which place them at risk for HIV infection and transmission. Bars across many settings in SSA meet these criteria. Yet, few studies have evaluated implementing HIV prevention programs in these venues [21]. Given the support of our study by bar owners and community leaders, the high proportion of participants without recent HIV testing, and the high yield of testing, we believe that HIV testing services targeting these venues are welcomed and appropriate. One factor that may challenge the population-level impact of this testing strategy is testing uptake. While testing acceptance was high among enrolled participants, the overall uptake of testing, using the 1,668 men who accepted our recruitment cards, was low. A potential strategy to improve testing uptake is the inclusion of multi-disease screening or services alongside HIV testing. This approach may reduce the stigma associated with HIV testing while providing a comprehensive men’s health service which could include alcohol use interventions [22, 23]. Providing the option of self-testing may also improve testing uptake as recent studies suggest the integration of this strategy into community-based HTC modalities is well-accepted by men and recognizes that some men may prefer to test in private or are reluctant or unable to present to a testing site [12, 22, 24, 25].
As more persons living with HIV are diagnosed, finding those who are undiagnosed becomes increasingly difficult. While further scale-up of available testing services may help to reach those who are undiagnosed, resource limitations have forced HIV programs across SSA to emphasize testing efficiency. For this reason, test yield, which is dependent on the HIV prevalence of the target population, has emerged as a relevant metric to guide which testing services should be implemented and scaled [26]. We observed a 2.5-fold higher test yield than that reported among men contemporaneously tested at five local facility-based testing centers. Our results suggest that further optimization of test yield among male bar patrons could be achieved by targeting testing towards men with a distant or no prior history of HIV testing. Overall, our test yield results are consistent with previous studies that have described higher test yields with targeted community-based HTC in comparison to facility-based HTC but with a lower overall testing coverage [13, 26]. These findings highlight the need for complementary approaches to HIV case finding that reconcile the high absolute number of infections identified through facility-based HTC and the high yield of targeted community-based HTC.
A limitation of some community-based HTC strategies is cost, because case finding often requires intensive resource commitments. For this reason, cost-per-diagnosis is increasingly recognized as a useful metric to measure the cost-effectiveness of novel testing modalities [27]. The estimated cost-per-diagnosis for our bar-based testing strategy was $634. Studies across SSA evaluating different community-based HTC strategies have reported cost-per-diagnosis (in USD) ranging from $66 to $1095 [28,29,30,31,32,33]. However, comparisons across studies are difficult since costs varies by testing modality, scale of the intervention, HIV prevalence of the target population, and the country where the intervention is set. Future studies are needed to establish cost-per-diagnosis thresholds modelled across different epidemic or programmatic scenarios typical for countries in SSA. Despite these limitations, important insights can be extracted from our cost estimates. First, we provided an accurate assessment of the costs to implement this project by including all costs related to project implementation, including data collection procedures for research that would not be needed in routine implementation. Thus, the testing strategies evaluated in our research context may be higher than the true operational cost. Second, consistent with other studies, the largest component of cost was personnel [34, 35]. This highlights the importance of finding approaches to improve personnel efficiency. Third, further reductions in costs could likely be achieved by utilizing existing infrastructure, more selective patron recruitment guided by prior HIV testing history, and by probing the impact of lower cost incentives.
Our study has several limitations. First, although we facilitated linkage to care for all men who tested positive for HIV infection, we did not actively track linkage. Linkage to care is a key constraint to the effectiveness of community-based HTC [36]. While men may prefer to test outside of health facilities, they still must travel to these facilities to access treatment. Some evidence suggests that community-based HTC with facilitated linkage, such as HIV counselor reminders to establish care, can achieve linkage to care rates comparable to facility-based HTC [10]. Second, comparisons between our study population and those presenting to local health facilities were limited. Notably, we were not able to compare the number of first-time testers, rates of linkage to care, or the severity of HIV disease at diagnosis. Assessing such parameters in future studies is critical to understand the true impact of our testing strategy. Third, the extent of selection bias in our study is difficult to measure. Notably, it is unclear how participants who presented for enrollment differed from those who were recruited and how those who were recruited differed from the larger male bar patron population. Fourth, more robust cost effectiveness analyses are needed to assess the value of our testing strategy in comparison to other public health interventions, including facility-based HTC and other community-based HTC strategies, and to determine the optimal mix of testing modalities that warrant implementation.
In summary, recruiting and incentivizing male bar patrons for HIV testing was efficient for identifying new HIV infections in the Kilimanjaro Region. The scalability of this intervention warrants further evaluation, including if testing uptake could be improved using multi-disease screening or self-testing, if test yield could be optimized by more selective patron recruitment guided by prior HIV testing history, and if cost-per-diagnosis can be reduced by using existing health-related infrastructure and evaluating thresholds for effective incentivization. Furthermore, formal cost effectiveness assessments of such community-based HTC, compared to traditional facility-based HTC, are needed.
References
Joint United Nations Programme on HIV/AIDS. Knowledge is Power Knowledge is power: know your status, know your viral load. Geneva: Joint United Nations Programme on HIV/AIDS; 2018.
Tsai AC, Siedner MJ. The missing men: HIV treatment scale-up and life expectancy in Sub-Saharan Africa. PLoS Med. 2015;12(11):e1001906.
Sileo KM, Fielding-Miller R, Dworkin SL, Fleming PJ. What role do masculine norms play in men’s HIV testing in Sub-Saharan Africa?: A scoping review. AIDS Behav. 2018;22(8):2468–79.
Dovel K, Yeatman S, Watkins S, Poulin M. Men’s heightened risk of AIDS-related death: the legacy of gendered HIV testing and treatment strategies. AIDS. 2015;29(10):1123–5.
Leichliter JS, Paz-Bailey G, Friedman AL, Habel MA, Vezi A, Sello M, et al. “Clinics aren’t meant for men”: sexual health care access and seeking behaviours among men in Gauteng province, South Africa. Sahara J. 2011;8(2):82–8.
Pascoe L, Peacock D, Stemple L. Reaching men: addressing the blind spot in the HIV response. Int J Mens Soc Community Health. 2018;1(SP1):e57-70.
Fleming PJ, Colvin C, Peacock D, Dworkin SL. What role can gender-transformative programming for men play in increasing South African men’s HIV testing and engagement in HIV care and treatment? Cult Health Sex. 2016;18(11):1251–64.
Colvin CJ. Strategies for engaging men in HIV services. The Lancet HIV. 2019;6(3):e191-200.
Sharma M, Barnabas RV, Celum C. Community-based strategies to strengthen men’s engagement in the HIV care cascade in sub-Saharan Africa. PLoS Med. 2017;14(4):e1002262.
Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77-85.
Hlongwa M, Mashamba-Thompson T, Makhunga S, Muraraneza C, Hlongwana K. Men’s perspectives on HIV self-testing in sub-Saharan Africa: a systematic review and meta-synthesis. BMC Public Health. 2020;20(1):66.
Hatzold K, Gudukeya S, Mutseta MN, Chilongosi R, Nalubamba M, Nkhoma C, et al. HIV self‐testing: breaking the barriers to uptake of testing among men and adolescents in sub‐Saharan Africa, experiences from STAR demonstration projects in Malawi, Zambia and Zimbabwe. J Int AIDS Soc. 2019;22(Suppl Suppl 1):e25244
WHO. Consolidated guidelines on HIV testing services 2015. Genera: WHO; 2015. p. 2015.
Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in Sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8(2):141.
Fritz KE, Woelk GB, Bassett MT, McFarland WC, Routh JA, Tobaiwa O, et al. The association between alcohol use, sexual risk behavior, and HIV infection among men attending Beerhalls in Harare, Zimbabwe. AIDS Behav. 2002;6(3):221–8.
Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC). Tanzania HIV Impact Survey (THIS) 2016–2017: Final Report. Dar es Salaam, Tanzania. December 2018. [Internet]. Available from: http://phia.icap.columbia.edu
Muhib FB, Lin LS, Stueve A, Miller RL, Ford WL, Johnson WD, et al. A venue-based method for sampling hard-to-reach populations. Public Health Rep. 2001;116(Suppl 1):216–22.
Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–32.
Francis JM, Weiss HA, Helander A, Kapiga SH, Changalucha J, Grosskurth H. Comparison of self-reported alcohol use with the alcohol biomarker phosphatidylethanol among young people in northern Tanzania. Drug Alcohol Depend. 2015;156:289–96.
Kwesigabo G, Mwangu MA, Kakoko DC, Warriner I, Mkony CA, Killewo J, et al. Tanzania’s health system and workforce crisis. J Public Health Policy. 2012;33(Suppl 1):S35-44.
Carrasco MA, Esser MB, Sparks A, Kaufman MR. HIV-alcohol risk reduction interventions in Sub-Saharan Africa: a systematic review of the literature and recommendations for a way forward. AIDS Behav. 2016;20(3):484–503.
Mak J, Mayhew SH, von Maercker A, Integra Research Team IRT, Colombini M. Men’s use of sexual health and HIV services in Swaziland: a mixed methods study. Sex Health. 2016;13(3):265–74.
Chamie G, Hickey MD, Kwarisiima D, Ayieko J, Kamya MR, Havlir DV. Universal HIV testing and treatment (UTT) integrated with chronic disease screening and treatment: the SEARCH study. Curr HIV/AIDS Rep. 2020;17(4):315–23.
Corbett EL, Dauya E, Matambo R, Cheung YB, Makamure B, Bassett MT, et al. Uptake of workplace HIV counselling and testing: a cluster-randomised trial in Zimbabwe. PLoS Med. 2006;3(7):e238.
Shapiro AE, van Heerden A, Krows M, Sausi K, Sithole N, Schaafsma TT, et al. An implementation study of oral and blood-based HIV self-testing and linkage to care among men in rural and peri-urban KwaZulu-Natal, South Africa. J Int AIDS Soc. 2020;23(S2):e25514.
De Cock KM, Barker JL, Baggaley R, El Sadr WM. Where are the positives? HIV testing in sub-Saharan Africa in the era of test and treat. AIDS. 2019;33(2):349–52.
Phillips AN, Cambiano V, Nakagawa F, Bansi-Matharu L, Wilson D, Jani I, et al. Cost-per-diagnosis as a metric for monitoring cost-effectiveness of HIV testing programmes in low-income settings in southern Africa: health economic and modelling analysis. J Int AIDS Soc. 2019;22(7):e25325.
Chang W, Chamie G, Mwai D, Clark TD, Thirumurthy H, Charlebois ED, et al. Cost and efficiency of a hybrid mobile multi-disease testing approach with high HIV testing coverage in East Africa. J Acquir Immune Defic Syndr. 2016;73(3):e39-45.
Lasry A, Bachanas P, Suraratdecha C, Alwano MG, Behel S, Pals S, et al. Cost of community-based HIV testing activities to reach saturation in Botswana. AIDS Behav. 2019;23(4):875–82.
de Beer I, Chani K, Feeley FG, Rinke de Wit TF, Sweeney-Bindels E, Mulongeni P. Assessing the costs of mobile voluntary counseling and testing at the work place versus facility based voluntary counseling and testing in Namibia. Rural Remote Health. 2015;15(4):3357.
Meehan S-A, Beyers N, Burger R. Cost analysis of two community-based HIV testing service modalities led by a Non-Governmental Organization in Cape Town, South Africa. BMC Health Serv Res [Internet]. 2017 Dec 2 [cited 2020 Jan 16];17. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5712171/
Hauck K. The costs of home-based HIV testing and counselling in sub-Saharan Africa and its association with testing yield: a literature review. Afr J AIDS Res. 2019;18(4):324–31.
Johnson C, Dalal S, Baggaley R, Hogan D, Parrott G, Mathews R, et al. Systematic review of HIV testing costs in high and low income settings [Internet]. Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection 2015. World Health Organization; 2015 [cited 2020 Oct 24]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK316032/
Tabana H, Nkonki L, Hongoro C, Doherty T, Ekström AM, Naik R, et al. A cost-effectiveness analysis of a home-based HIV counselling and testing intervention versus the standard (Facility Based) HIV testing strategy in rural South Africa. PLoS ONE. 2015;10(8):e0135048.
Mangenah C, Mwenge L, Sande L, Ahmed N, d’Elbée M, Chiwawa P, et al. Economic cost analysis of door-to-door community-based distribution of HIV self-test kits in Malawi, Zambia and Zimbabwe. J Int AIDS Soc. 2019;22(Suppl 1):e25255.
Ruzagira E, Baisley K, Kamali A, Biraro S, Grosskurth H. Linkage to HIV care after home-based HIV counselling and testing in sub-Saharan Africa: a systematic review. Tropical Med Int Health. 2017;22(7):807–21.
Acknowledgements
The authors would like to thank the study participants as well as the clinical staff and administration at the facility-based testing centers in Boma Ng’ombe. In addition, we would like to thank the village leaders and ward leaders in Hai district who supported the study.
Funding
This research was supported by the US NIH Fogarty International Center grant D43TW009337. DBM and PM received support from the NIH Ruth L. Kirschstein National Research Service Award (NRSA) 5T32AI007392.
Author information
Authors and Affiliations
Contributions
DBM and NMT conceived the study; DBM and NMT designed the study protocol; DBM, BN, and NMT implemented and supervised the study; DBM performed the data analysis; DBM drafted the manuscript; DBM, PM, AM, TP, JO, BN, and NMT critically revised the manuscript for content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest related to this work.
Ethical Approval
Ethics approval was obtained from the Institutional Review Board of Duke University, the Research Ethics Committee of Kilimanjaro Christian Medical Centre, and the Ethics Coordinating Committee of the Tanzanian National Institute for Medical Research.
Informed Consent
Written consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Madut, D.B., Manavalan, P., Mtalo, A. et al. Increasing the Uptake of HIV Testing among Men in Tanzania: A Novel Intervention for Bar Patrons. AIDS Behav 25, 2014–2022 (2021). https://doi.org/10.1007/s10461-020-03131-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-020-03131-6