Abstract
Belowground biomass production is a vital process that contributes to carbon sequestration. The relative proportion of belowground carbon allocation, however, varies with species, age, ecoclimatic conditions and crown dominance categories of trees. Here we compare the coarse root biomass and nutrient stocks of different stem size/crown class categories of 21-year-old Grevillea robusta stand in Kerala, India and the soil carbon/nutrient stocks of the G. robusta stand with an adjoining unplanted site. Root systems of 18 trees of three stem diameter/crown classes (5–15, 15–25, and >25 cm: suppressed + intermediate, co-dominant and dominant crown classes, respectively) were excavated and their coarse root biomass estimated. Mean coarse root biomass ranged from 12.94 to 59.81 kg tree−1 with production of 18.45 Mg ha−1 (mean annual increment = 0.88 Mg ha−1 year−1). Medium sized trees (co-dominant) had the highest root:shoot ratio, followed by intermediate and suppressed crown classes and the dominant trees had the least values, implying persistence strategies of the suppressed, intermediate and co-dominant trees under conditions of resource limitation. Coarse roots accounted for 8.04 Mg ha−1 C while soil organic carbon pool (0–100 cm) was 77.56 Mg ha−1 C, as against 66.04 Mg ha−1 for contiguous treeless plots. Coarse roots accrued 24.87 kg N, 1.66 kg P and 21.06 kg K per ha. G. robusta stand exhibited higher NPK stocks in the lower soil layers, compared to treeless controls. Deep rooted G. robusta trees (>1.0 m) thus have the potential to enrich lower layers of the soil profile through plant cycling of nutrients, which is important for on-site nutrient conservation and resource sharing with associated field crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest plantations couple economic advantages with ecosystem services such as carbon sequestration (Lal 2005; Paquette and Messier 2010; Kongsager et al. 2013). In addition to aboveground C sequestration, which received considerable scientific attention in the recent past (e.g., Liao et al. 2010, Nair et al. 2009; Uri et al. 2012), trees also store substantial carbon belowground particularly in the root systems and facilitate carbon storage in the soil through litter and fine root dynamics (Kraenzel et al. 2003; Montagnini and Nair 2004). Although fine roots and litter are recognized as major pathways to enrich the soil carbon pool, coarse roots, which account for approximately 20–30 % of the total tree biomass (Young 1997; Peichl and Arain 2006), and contribute profoundly to the belowground C and nutrient pools (Nair et al. 2010), received less scientific attention (Brunner and Godbold 2007).
Relative biomass allocation between roots and aboveground plant parts, commonly expressed as root:shoot ratio (R/S), however, has been a key research theme in forestry (Klepper 1991; Nadelhoffer and Raich 1992; Gerhardt and Fredriksson 1995; Sanford and Cuevas 1996; Naidu et al. 1998; Raich et al. 2014). Cairns et al. (1997) posited that independent variables such as aboveground biomass (AGB) density, latitude, temperature, precipitation, temperature:precipitation ratios, tree type, soil texture, and age had no important explanatory value for R/S. More recently, Raich et al. (2014) proposed that rates of AGB accumulation and belowground C allocation in growing tropical forests are coupled and that both processes vary together and independently of canopy production. Yet another factor that may impact R/S ratio is crown class differentiation. In even aged populations of trees, differences in seedling performance and micro-site characteristics quickly manifest into canopy size class differences (dominant, co-dominant, intermediate and suppressed; e.g., Spurr and Barnes 1980; Nilsson and Albrektson 1994). Suppressed trees experience greater competition for site resources than dominant trees. However, the functional consequences of these in terms of R/S ratios are not entirely clear.
Despite tree roots contributing significantly to the belowground carbon and nutrient pools, studies characterizing root contribution to these pools are few. Tree roots also enrich the deep soil nutrient pools through root exudation and root turnover (Jose 2009). However, information on such enrichments, size class distribution of coarse roots of trees, and their contribution to total root biomass, carbon and nutrient storage, and turnover especially in the deeper layers of the soil profile is scarce, presumably because of the arduous nature of such studies (Jones et al. 1998; Chesney 2008). Consequently, though forest plantations of many tropical and temperate species have been established in different parts of the world (100 million ha; FAO 2006), we are still beginning to understand how these plantations will impact the soil carbon and nutrient pools. Such information, however, is crucial for projecting the consequences of plantations on CO2 emission reduction activities (e.g., Afforestation and Reforestation Clean Development Mechanisms); particularly on tropical sites that have great potential for fast growing multipurpose tree production (Nair et al. 2010).
Many authors have also reported that integration of trees into agricultural landscapes can increase the C storage potential considerably within belowground biomass (BGB), which is thought to be a viable approach for soil carbon sequestration (Nair et al. 2010; Bambrick et al. 2010; Kuyah et al. 2012). In addition, tree roots also hold substantial stocks of nutrients. However, quantitative estimates on belowground carbon and nutrient stocks of mature tropical plantation species are rare. Besides, the biomass and nutrient stocks in the tree roots, decomposition of plant residues and other organic materials in the soil is a source of C and nutrients for new growth of microbial communities and plants (Angers and Chenu 1997). This soil organic matter represents a significant carbon store and can remain in the soil for extended periods as a part of soil aggregates (Nair et al. 2010).
Biomass inventories within agroforestry systems are, however, primarily focused on measuring the aboveground component with a limited number of studies calculating root biomass (Borden et al. 2014). Although destructive sampling provides the most robust method to estimate root biomass, this method is seldom used because it is cost-, labour- and time- intensive (Kuyah et al. 2012); hence BGB estimates are often reported as a proportion of the AGB, using R/S or allometric relations (Mokany et al. 2006; Kuyah et al. 2012). Although Borden et al. (2014) suggested the use of Ground Penetrating Radar (GPR) for capturing the heterogeneity of rooting patterns non-destructively, allometric regressions developed by measuring root biomass or production and regressing these data against some easily measured variable, such as diameter at breast height (DBH), by far, form the most important method for determining root biomass production. However, such studies on tropical tree species are limited (Jangra et al. 2010; Kuyah et al. 2012). It is desirable to develop BGB equations for individual sites, owing to species-site relationships, rotation age-stand density interactions for fast growing trees.
Grevillea robusta A. Cunn. ex. R. Br. (silver oak), is an important Australian tree, which has been successfully introduced to other warm temperate, and subtropical regions of the world, including India, Africa, Ceylon, Malaysia, Madagascar etc. (Harwood 1989). The key factors that promote silver oak among farmers and planters across the world are its fast growth, ability to withstand adverse climatic and edaphic conditions (Harwood 1989) and timber quality (Shanavas and Kumar 2006). Although this tree is thought to be less competitive for site resources with the associated crops in agroforestry systems than other trees under comparable situations (Sanchez 1995), little or no information exists on its BGB production and carbon allocation. Studies on root biomass production, carbon stock and its allocation to various root size classes and how it changes with tree size and agroecology might help in exploring the potential advantages of silver oak-based polyculture systems.
In this study, we attempted to characterize the coarse root biomass and nutrient stocks of G. robusta trees grown in the humid tropical regions of peninsular India. Root biomass production and apportioning into root size classes vary with species, site conditions, tree size and stand age (Nair et al. 2010; Jangra et al. 2010). Two hypotheses were tested. First, suppressed and intermediate G. robusta trees may have relatively high R/S than the dominant and co-dominant individuals. While it has been previously reported that R/S may vary with stand/tree age (increase in R/S ratio with increasing seedling age from 5 to 32 months: Gerhardt and Fredriksson 1995), or is a function of tree species (Sanford and Cuevas 1996), canopy classes also may impact it. Suppressed and intermediate trees in an even aged stand in order to offset competition for belowground resources may allocate a relatively high proportion of biomass belowground than dominant and codominant individuals. Secondly, mature G. robusta trees, by virtue of their ability to traverse deep into the soil, may enrich the soil organic and nutrient pools, particularly in the lower layers of the soil profile. The overall objective was to evaluate the BGB carbon stock and nutrient enrichment by the experimental stand.
The specific objectives of the field study were to characterise the coarse root biomass dynamics of mature (21-year-old) G. robusta trees belonging to different crown/stem size classes. We also developed simple allometric equations to predict coarse root biomass production in G. robusta trees and estimated the NPK and C contents of coarse roots of different size classes of G. robusta trees. Finally, we compared G. robusta stands with treeless control plots to determine the long term impacts of tree planting on soil physico-chemical properties [pH, soil organic carbon (SOC), and N, P, K contents].
Materials and methods
Study area, site history, and stand characteristics
The field study was conducted in a 21 year-old G. robusta stand at Thrissur, Kerala, India (10°33′04.9″N latitude; 76°18′03.1″E longitude; and 40.29 m altitude). The experimental site was previously under rubber plantation (Hevea brasiliensis), which was clear-felled 2–3 years prior to the planting of G. robusta. The area adjoining the G. robusta plots was left unplanted and was under ruderal vegetation. The site experiences a warm humid tropical climate, with a mean annual rainfall of 2391 mm (average of 21-years from 1991 to 2012), most of which was received during the south-west monsoon (June–August). The mean maximum temperature for this period ranged from 29.1 (July) to 35.5 °C (March), while the mean minimum temperature varied from 22.2 (December) to 24.8 °C (April). The soil of the experimental site was Ultisol (Typic Plinthustult, Vellanikkara Series midland laterite-ustic moisture regimes and isohyperthermic temperature regimes) having a pH of 5.19 (authors’ data).
The experimental stand was established in June 1991 by planting approximately three month-old poly bag seedlings in a split plot design. Main plot (95 × 10 m) treatments included four population densities (D1—3333 trees ha−1: 3 × 1 m spacing; D2—2500 trees ha−1: 2 × 2 m spacing; D3—1667 trees ha−1: 3 × 2 m spacing; and D4—1111 trees ha−1: 3 × 3 m spacing), replicated thrice. Four fertilizer levels (F1—0:0:0; F2—50:25:25; F3—100:50:50 and F4—150:75:75 kg of N, P2O5 and K2O ha−1 year−1; equivalent to 0:0:0, 50:10.75:20.75, 100:21.5:41.5 and 150:32.25:62.25 kg of elemental N, P and K ha−1 year−1 respectively) formed the sub-plot treatments. The fertilizer levels were selected so as to provide a range of nutrient regimes. There were 5 m wide unplanted buffer strips separating the subplots. Each subplot of 20 × 10 m was also demarcated by 50 cm wide and tall earthen risers (“bunds”) on all sides, which were routinely reinforced prior to fertilizer application. The plots were manually weeded twice a year. After crown closure, however, weeding was less frequent and was carried out only as needed.
Fertilizers in the form of urea (46 % N), Mussorie-phos (i.e., finely powdered, 100 mesh size rock phosphate with 23–24 % P2O5, slowly available under acidic soil conditions) and Muriate of potash (58 % K2O) were applied in basins (50 cm radius and 10–12 cm deep) dug around each tree (with minimum disturbance to tree roots), as per the experimental protocol, thrice, i.e., in August 1992, and September 1993 and September 1996 (respectively at 1.25, 2.25 and 5.25 years after planting). To avoid heavy leaching losses, fertilizers were applied immediately after the heavy monsoon season. They were mixed with soil in the basins and covered with a thin layer of earth. Fertilizers were applied consecutively in the second and third year as the crown and root systems develop during this stage and growth retardation because of nutrient limitation is probable in quick growing trees. Later on (i.e., by 5.25 years of age), however, part of the nutrient requirement of the growing tree was thought to be obtained through the nutrient cycling process (Kumar 2008); hence fertilizers were applied at a longer (3-year) interval and no fertilizers were applied after 1996.
Average survival count during the first year was 83 %. Some more trees died due to natural causes in the subsequent years, besides the stand also experienced unauthorized logging at irregular intervals. With the result, the spacing and fertilizer effects, as originally envisaged, could not be determined objectively (no perceptible effects). During the reporting period (2011), the stand was enumerated by measuring DBH and total tree height using a fiberglass tape and clinometer (Suunto, PM-5, Finland) and the crown class differentiation was visually assessed based on the crown position and size, amount of crown receiving direct sunlight, and the amount of competition by surrounding trees (suppressed, intermediate, co-dominant and dominant: e.g., Spurr and Barnes 1980; Long and Smith 1984). There were 460 trees ha−1 (25 % of the initial stocking) with an average tree height of 16.91 m (±0.58) and DBH of 24.07 cm (±1.42), and having a more or less closed canopy. Given the heterogeneity of tree size in the experimental area, the stems were grouped into three diameter classes viz. 5–15, 15–25 and >25 cm each with 110, 276 and 74 trees per ha, respectively, roughly corresponding to crown classes such as suppressed and intermediate (5–15 cm), co-dominant (15–25 cm) and dominant (>25 cm) and from that population, a sample of 18 trees were selected for destructive sampling (six randomly selected trees per class). In this regard, Gill et al. (2000) fitted models of tree crown radius and found DBH as the only independent variable that was appropriate, which justifies our approach of using DBH as a surrogate for crown class.
Sampling for belowground biomass and carbon
The selected trees (18) were felled at 15 cm aboveground (for ease of cutting) using a power saw (Oleomech MTL 51, Italy) during December 2011 and the AGB components were determined (Paul 2012). Root systems of the felled trees were carefully excavated during the period from 6 to 28 December 2011 by removing the soil around the stump of the felled tree (~2 m radius and 30–40 cm depth) and then pulling it out using a powered mini earth mover (Tata-Hitachi Mini excavator, TMX 20-2). After excavation, all broken roots were carefully picked up and rearranged to reconstruct the root systems, to the extent possible. Maximum root length and spread were noted and a size-to-scale sketch made (15 × 15 cm graph paper). Roots were separated into 0–3.0, 3.1–6.0, 6.1–9.0, and 9.1–12.0 cm diameter classes, besides the larger taproots (>12 cm diameter). Fresh weight of the roots in each size class was recorded tree-wise, after thorough cleaning with a powerful water jet to remove soil particles adhering to the roots followed by rubbing with muslin cloth and drying under the sun. Triplicate samples (ca. 250 g for each size class) were collected tree-wise for moisture and chemical analyses. The samples were immediately transferred to the laboratory in double-sealed polythene bags. After recording fresh weights, they were dried to constant weights at 70 °C, and the dry weights recorded. Estimates of total root dry weights were obtained from the fresh weights of various root size categories and their corresponding moisture contents. For biomass calculation on per hectare basis, mean tree root biomass values for different tree diameter classes were multiplied by the corresponding stand density (110, 276, and 74 tree ha−1) and summed up. Root: shoot ratios were calculated by dividing the coarse root dry weight of individual trees (range: 4.54–81.43 kg tree−1) with the corresponding aboveground dry weight (range: 39.54–597.15 kg tree−1: Paul 2012) and the resultant values were pooled according to the size/crown classes.
Phytochemical analysis
Root samples belonging to various diameter classes (0.1–3.0, 3.1–6.0, 6.1–9.0, 9.1–12.0 cm and tap roots) for all three tree diameter classes were analyzed for N, P, K, and elemental carbon. For this, samples from each root size class was composited tree diameter class-wise, chopped to form smaller size fractions, dried to constants weights, ground to pass through a 2 mm sieve and stored in air-tight plastic pouches. Triplicate samples were analyzed for N (micro-Kjeldahl method), P [vanado-molybdo phosphoric yellow colour method following diacid (HNO3 and HClO4 in 9:4) digestion], and K (flame photometry). Triplicate root samples (ca. 10 mg) were also analyzed for elemental carbon using CHNS analyzer (Elementar, Vario MICRO cube, Germany). The samples were subjected to complete instantaneous oxidation (“flash combustion”) inside the Elementar micro cube. The combustion products were separated by a chromatographic column and detected by the thermal conductivity detector (T.C.D.), which gave an output signal proportional to the concentration of the individual components of the mixture.
Soil sampling
Soil samples were collected by digging profile pits at random points in the interspaces between the rows of trees and from the contiguous treeless open area. Four profile pits of one meter depth (1.5 m long and 60 cm wide) were dug in the G. robusta stand and three pits in the contiguous treeless open area. The treeless plots remained uncultivated during the life cycle of the plantation but were weeded occasionally along with the experimental plots to clear shrub growth. All profiles were demarcated into 0–20, 21–40, 41–60, 61–80 and 81–100 cm horizons and triplicate soil samples collected depth-wise. All samples were air-dried, ground, sieved (2 mm sieve), bagged and stored for analysis. Soil bulk density (BD) was determined by taking cores of undisturbed soil by using a steel cylinder (7.5 cm length and 5.5 cm diameter) following standard procedures (Jackson 1958). Soil cores were collected by inserting the cylinder horizontally on the wall of soil pits (1 × 1.5 × 0.6 m) at the centre of each depth class. Soil pH was determined using a suspension of soil and water (1:2 ratio) with a pH meter (Model Li 613, Elico, India), organic carbon estimated by permanganate oxidation method (Walkley and Black 1934) and carbon stock in the soil estimated as a product of carbon concentration, BD, soil depth and area (Anderson and Ingram 1989). Total N (micro-Kjeldahl method), available P (Bray-1 extractant followed by chloromolybdic acid blue colour method using ascorbic acid as the reducing agent), available K (flame photometry using 1 N neutral normal ammonium acetate solution, as the extractant) were determined. All nutrient concentrations were expressed on an oven dry basis. Nutrient concentrations (NPK) for various soil horizons were multiplied by corresponding soil mass (computed from soil depth and corresponding BD) to derive the nutrient stocks.
Statistical analysis
The data on root biomass, carbon content, nutrient concentrations and content were analyzed for various root size classes across tree diameter classes using ANOVA. Data on soil BD, C, N, P and K across soil depth were subjected to multivariate analysis of variance (MANOVA) and that of G. robusta stand and treeless control compared using the hierarchical analysis of variance technique. BGB equations were fitted using the statistical package SPSS (ver. 17) in which root biomass data of the 18 sampled trees were regressed on corresponding tree diameter and height, as predictor variables. Eight models were tried for predicting BGB, which included linear (single independent variable), quadratic, logarithmic, semi-logarithmic and quadratic, logarithmic and quadratic, linear (two independent variables), semi-logarithmic (two independent variables), and logarithmic (two independent variables). Models with maximum coefficient of determination (R2) and minimum Furnival index were selected. Furnival index was obtained by multiplying the square root of MSE with the inverse of geometric mean of the derivatives of the dependent variable.
Results
Coarse root biomass production
Dry matter production of coarse roots increased significantly (p < 0.01) from the smallest to the largest root diameter class (Table 1). Taproots, in general, had much higher biomass accumulation than other root classes, regardless of tree size classes (ca. 62 % of the total root biomass for medium and large sized trees). Coarse root biomass production on a stand basis was 18.45 Mg ha−1 with a mean annual increment (MAI) of 0.88 Mg ha−1 year−1. Among the tree diameter classes, medium size (15–25 cm DBH) with the largest number of trees had maximum root dry matter production on a per hectare basis.
Among the various allometric models tried, semi-logarithmic and logarithmic equations (models 7 and 8: Table 2) with DBH and tree height as independent variables gave the best fit with relatively high adjusted R2 values of 0.83 and 0.86, and low Furnival indices of 10.42 and 9.34 respectively. The residual plots (Fig. 1A, B) also imply the statistical robustness of these models. Between the two models (7 and 8; Table 2), model 8 was better (p < 0.001).
Residual plots A for semi-logarithmic with two independent variable (model 7) and B for logarithmic with two independent variable (model 8) (see Table 2 for model description and coefficients)
Root: shoot ratio
Root–shoot ratios varied considerably among the crown/diameter classes (Fig. 2). Highest ratio (0.17) was observed for mid-sized trees (15–25 cm DBH) and the lowest (0.11) for the >25 cm DBH class. Coarse roots accounted for 10.12–14.53 % of the total tree biomass for different size/crown classes (data not presented).
Root:shoot ratio among different tree size classes for 21-year-old Grevillea robusta in central Kerala, India. Shoot data source: Paul (2012)
Belowground biomass carbon stocks
No clear trend was discernible with respect to elemental carbon concentrations in the roots (Table 3). The highest value of 465.9 g kg−1 was observed for the 3.1–6.0 cm root diameter class and the least for taproots (372.9 g kg−1). Mean coarse root biomass C in the 21-year-old G. robusta was 16.63 kg tree−1 (Table 4), which increased with increasing tree size, with the largest size (>25 cm DBH) having 23.84 kg tree−1 of C. Among the various root size categories, taproots accounted for the largest proportion of total root carbon, viz., 50, 63 and 58 % in 5–15, 15–25 and >25 cm DBH classes, respectively. MAI for root carbon stock was 0.27–1.12 kg tree−1. On a unit area basis, G. robusta stored 0.62–5.65 Mg ha−1 of C with a total of 8.03 Mg ha−1 coarse root carbon stock for all three DBH classes (Table 4). Taproots accounted for 4.89 Mg ha−1 of C compared to 1.19 Mg ha−1 for the 3.1–6.0 cm root diameter class. The contribution was least for roots of 9.1–12.1 cm diameter class (0.03 Mg ha−1 of C). As regards to tree size classes, 15–25 cm diameter class sequestered the highest coarse root carbon, because of the relatively high frequency of occurrence of trees in that category.
Nutrient concentration and stocks in the coarse root biomass
Concentration of N, P and K varied considerably among the different root size classes (Table 5). But the effects of tree sizes on nutrient concentrations were not pronounced. As expected, smaller roots (0.1–3.0 cm diameter) had the highest concentration of N, P and K, which declined with increasing size (9.1–12.0 cm diameter). Total root N, P, and K stocks were 24.87, 1.66 and 21.06 kg ha−1 respectively (Table 6). Taproots accounted for the largest share of N, P, and K stocks (57.61, 61.37, 58.21 % respectively; data not presented).
Soil properties
Soil pH increased with depth except for the top layer. For example, pH of 0–20 cm layer was 5.41 and 5.37 and that of the 81–100 cm soil layer was 5.61 and 5.67 for the G. robusta and treeless control respectively (data not tabulated). However, differences between G. robusta stand and treeless control in this respect were not significant. Soil BD also increased with depth, but the differences again were not significant across the horizons. However, G. robusta showed lower soil BD compared to treeless plots (p < 0.01). G. robusta and treeless plots had values of 1.13 and 1.16 g cm−3 for the 0–20 cm layer and 1.25 and 1.23 g cm−3 for the 81–100 cm horizon respectively (data not shown).
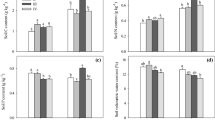
MANOVA indicated that SOC concentration declined with soil depth in both G. robusta stand as well as treeless control (p = 0.001; Fig. 3A). Hierarchical analysis of variance suggests that the differences in carbon concentration between G. robusta stand and treeless control were significant (p < 0.001). Soil carbon stocks at various depths also showed a similar trend (Fig. 3B) with the highest carbon content in the surface soil, which declined consistently (p = 0.001) with depth and the top 0–20 cm layer had about 30 % carbon. G. robusta stand showed conspicuously higher carbon build up in the soil compared to treeless open plots (p < 0.001). Of the total C sequestered within 1 m depth (77.56 Mg ha−1 for G. robusta), the top 0–40 cm accounted for about 56 % (Table 7; Fig. 3B). Belowground C stock in G. robusta stand (coarse root biomass + soil) was 85.60 Mg ha−1, which was higher than that of the treeless control plot (Table 7; 66.04 Mg ha−1; p = 0.02). The overall carbon buildup in the G. robusta stands (aboveground + coarse root biomass + soil) was 116.88 Mg ha−1, which was considerably higher than that of treeless control (p = 0.0003).
Variation in soil organic carbon levels (A) and soil carbon stocks (B) between Grevillea robusta and treeless control plots in central Kerala, India (values with the same superscripts do not differ significantly, horizon-wise differences were significant at p < 0.001 for soil of Grevillea robusta and treeless control)
As can be seen from Fig. 4, total nitrogen (p = 0.001), available phosphorus (p = 0.006) and exchangeable potassium (p = 0.069) concentrations for the G. robusta stands and treeless control declined with soil depth. Again, N, P and K concentrations (depth-wise) were higher in the G. robusta than treeless open plots. N concentration was highest in the top 0–20 cm soil layer (0.113 g kg−1) and lowest for the 80–100 cm layer (0.025 g kg−1). Consistent with the trends in nutrient concentrations, soil nutrient stocks also showed a declining trend with depth (Table 8), but the NPK stocks were always greater in G. robusta plots compared to the treeless plot (p < 0.001). For instance, NPK stocks were 692.96, 60.28 and 1084.17 kg ha−1 for G. robusta and 650.32, 44.5 and 826.9 kg ha−1 for treeless control respectively up to 1 m soil depth.
Variation in soil total nitrogen (A), soil available phosphorus (B) and soil exchangeable potassium (C) between Grevillea robusta and treeless control in central Kerala, India (Values with the same superscripts do not differ significantly, horizon-wise differences were significant at p < 0.001 for soil of Grevillea robusta and treeless control)
Differences between G. robusta stand and treeless control were statistically significant (p = 0.022) even for the lower layer (61–80 cm), implying greater N accumulation in the deeper layers of the soil profile of G. robusta plot compared to treeless plot. Available P content also showed significant variations between G. robusta and treeless plots for the entire soil depth (p = 0.0022), except 0–20 cm layer (p = 0.176). Difference in mean values for P between G. robusta and treeless plots were significant for soil depths 20–40 cm (p = 0.022), 40–80 cm (p = 0.039), 60–80 cm (p = 0.006), 80–100 cm (p = 0.0016) and 0–100 cm (p = 0.0022). The P stock in G. robusta stand at 80–100 cm layer was about 75 % more than that in treeless control. Potassium stocks were 1084.2 and 826.9 kg ha−1 respectively for G. robusta and treeless plots for 1 m soil depth (p = 0.14) and the profile variations were significant only for treeless plot (p < 0.01), signifying that G. robusta roots reduced the inherent heterogeneity in soil K distribution.
Discussion
Factors affecting coarse root biomass production in trees
Ecosystem attributes, species, stand age/density, and tree management generally determine the relative proportion of AGB and BGB. Mean coarse root dry matter production for the 21-year-old G. robusta at our humid tropical site was 39.46 kg tree−1 and the total coarse root mass 18.45 Mg ha−1 (Table 1) with a MAI of 0.88 Mg ha−1 year−1. For a 25-year-old plantation of G. robusta (550 trees ha−1) in the semi-arid tracts of northern India, Jangra et al. (2010), however, reported a much higher coarse root biomass (86.0 kg tree−1 and 47.30 Mg ha−1; Table 9) with an MAI of 1.69 Mg ha−1 year−1. Studies on coarse root biomass production of other tropical plantation species of comparable age are scarce. However, a study in the temperate region of Southern Ontario, Canada (Borden et al. 2014) indicate grossly divergent values for 25-year-old trees of five species. The mean coarse root biomass estimated from excavation method for Populus deltoides × nigra clone DN-177, Juglans nigra, Quercus rubra, Picea abies and Thuja occidentalis were 71.9 ±10.8, 75.0 ±1.4, 74.0 ±5.7, 50.1 ±21.6, 14.0 ±4.0, and 54.8 ±8.3 kg tree−1 respectively.
Higher allocation for coarse root production in the semi-arid regions compared to the humid tropical zones, is further exemplified by an average root: shoot ratio of 0.17 (Jangra et al. 2010) as against 0.14 in the present study (Fig. 2). Fast growing exotics in the arid zones may allocate relatively more root biomass to facilitate scavenging of a greater soil volume for water and minerals. This implies the adaptive strategies of trees in the water-limited environment (Markesteijn and Poorter 2009). Tomlinson et al. (2012) observed this trend in a large number of savanna species.
Our data (Fig. 2) show that crown/tree size classes exerted a profound influence on R/S. Although we expected that suppressed and intermediate trees in the lower size classes may allocate relatively more biomass belowground than other tree classes, data given in Fig. 2 clearly show that intermediate size range (co-dominant) had the highest root–shoot ratio, which was followed by the suppressed and intermediate categories (0–15 cm DBH class). Acquisition of water and nutrients may increase with high root to shoot ratio. Changes in biomass allocation pattern may thus facilitate the persistence of suppressed, intermediate and other lower size class trees under conditions of resource limitations (Naidu et al. 1998). Our estimates of BGB allocation do not include fine roots. Although seasonal production of fine roots can be high (Nadelhoffer and Raich 1992), they contribute little (2 %) to total standing biomass (Van Lear and Kapeluck 1995). Further, fine roots tend to turnover rapidly especially in humid climates and it is the coarse roots that perform long-term C sequestration. R/S can be related to factors associated with the development of stand also. Within a given ecosystem, the relative proportion of biomass allocated belowground for coarse root production for a species may be higher during the juvenile phase (2–3 years) and will decline thereafter. Above and BGB carbon allocation also may be linked and that both processes may vary together, as posited by Raich et al. (2014).
Stand density and tree management practices may also impact coarse root biomass production. Mean tree root biomass of 22-year-old G. robusta stand (1111 trees ha−1) at Thiruvazhamkunnu (within a radius of approximately 100 km from the present experimental site) in the humid tropical zone (Aneesh 2014: Table 9), managed as support trees for trailing black pepper vines, however, gave a higher coarse root content (56.96 kg tree−1 and 63.29 Mg ha−1) and mean annual production (2.58 kg ha−1 year−1 and 2.87 Mg ha−1 year−1) than the 21-year-old stand at the present experimental site. In particular, higher stand density, regular intercultural operations for the pepper vine and intensive pruning, lopping and pollarding of the support trees annually (to facilitate understory light infiltration), which resulted in an open canopy, may have facilitated greater carbon allocation belowground and deeper root penetration, compared to less intensively managed, closed canopy plantations. Enhanced allocation of carbon belowground in the pruned and lopped trees can enhance plant nutrient uptake commensurating with the enhanced growth requirements.
Among the different root fractions, taproots accounted for 53–62 % BGB, which increased with increase in tree size, implying the need for greater BGB allocation to taproots for anchorage of larger trees (Table 1). The relative proportion of BGB to that of the aboveground in the present study was in the range of 11–17 % (Fig. 2). Higher relative proportion of BGB may be possible in younger stands, which may decline as trees grow further. This is consistent with the observations of Raich et al. (2014).
Allometric equations for predicting coarse root biomass
Logarithmic equation for predicting coarse root biomass with DBH and tree height as independent variables (Table 2; Fig. 1) gave a good fit. BGB equations were previously non-existent for G. robusta at our site. Jangra et al. (2010) earlier fitted single variable-based log-linear relationship (girth at breast height as predictor) for G. robusta. The present twin variable logarithmic model clearly is an improvement over that and will be useful in the quantification of coarse root biomass and carbon stocks and monitoring the effect of stand management practices on root biomass production in G. robusta. Equations developed in the present study may also have use for other sites having similar ecoclimatic conditions. Furthermore, site specific equations have better predictability than generalized biomass equations. However, generalized biomass prediction equations can be used where the tree population under consideration is near the average for the region (Campbell et al. 1985).
Coarse root NPK concentrations/stocks
Higher nutrient concentrations were observed in the smaller sized roots (0–3.0 cm diameter) compared to the larger size fractions (Table 5), which is similar to the aboveground nutrient allocation patterns where small branches and twigs maintain higher nutrient concentrations as compared to stemwood. Studies on nutrient concentrations in roots are rare. In one such study, Kumar et al. (1998) reported a general decline in nutrient concentrations with age in fast growing tree species such as Acacia auriculiformis, Ailanthus triphysa and Casuarina equisetifolia.
Nutrient stocks in the various root size fractions also varied considerably, largely as a function of biomass yield. Consistent with this, taproots constituted about 57–62 % of the total root nutrient accumulation. Overall, G. robusta at 21-years of age accumulated 24.87 kg ha−1 N, 1.65 kg ha−1 P and 21.06 kg ha−1 K in the coarse roots. The present results thus signify the role of coarse roots in holding and releasing nutrients to the soil pool, which furthers our understanding on the belowground nutrient dynamics of mature wooded ecosystems.
Soil carbon and nutrient stocks
Higher total soil carbon stock for the G. robusta stand along the one-meter soil profile depth (77.56 Mg ha−1 as against 66.04 Mg ha−1 for a contiguous treeless plot), implies a significantly higher soil carbon build up in the tree-based production system (Table 7). Accumulation of SOC occurs primarily through the return of plant-fixed C to the soil mainly through leaves and roots (Lal and Kimble 2000; Oelbermann et al. 2006). Litterfall production, though variable (e.g., Jamaludheen and Kumar 1999), exerts a profound influence on belowground C sequestration. Kumar (2008) reported that litterfall production ranges from 1.02 to 14.5 Mg ha−1 year−1 across tropical species and ecoregions. Yet another important pathway of enriching the soil C pool is the fine root dynamics. It is well known that trees allocate a large proportion of gross primary production belowground for the production and maintenance of roots and mycorrhizae (Giardina and Ryan 2002). Indeed, more than half of the C assimilated by the plant is transported belowground via root growth and turnover, root exudates, and litter deposition (Kumar 2008). The increase in SOC content of the G. robusta stand is presumably because of litterfall and fine root dynamics, although data on these parameters from the present study site is lacking.
A general declining trend in nutrient content with increasing soil depth and a higher NPK stock in the G. robusta stand at all depths except the top layer for N was discerible (Table 8). While it is well known that soil nutrient pools are higher in the top layers of the soil profile, the higher content especially in the lower layers in G. robusta plots can be ascribed to root exudation and fine root dynamics. Deep-rooted G. robusta trees (>1.0 m) thus have the intrinsic potential to enrich the lower layers of the soil profile through plant cycling of nutrient elements, which is important from the perspective of on-site nutrient conservation and sharing.
Conclusions
Results of the present study underscore the potential of G. robusta trees for BGB production and enrichment of SOC and nutrient pools. Considerable variability, however, has been observed in biomass and carbon stocks among different tree size/crown classes implying the need for promoting tree growth and carbon storage through appropriate stand management practices (e.g., stand thinning, tree pruning and intercropping). More importantly, G. robusta enriched the lower horizons of the soil profile compared to treeless control plots, which implies the possibility of “nutrient pumping” through which the associated field crops in polyculture systems involving this tree will be benefitted. Our data support this deduction at our site. G. robusta also reduced the heterogeneity in profile distribution of nutrients, particularly K, which is susceptible to leaching losses. This nutrient buildup at deeper soil (up to 1 m depth) would enhance onsite nutrient conservation and sharing, especially in situations where nutrient leaching and soil erosion are major concerns. Integrating G. robusta trees within the landscape thus have the potential to store C. Besides, SOC and NPK may be bound in the fine root fractions, which however, have not been focused in this study. Nonetheless, the fact that such high amounts of C is sequestered by G. robusta trees is important in the context of developing strategies for mitigating rising atmospheric CO2 levels and the tree and stand management regimes hold a key role in this respect.
References
Anderson JM, Ingram JSI (1989) Tropical soil biology and fertility, a handbook of methods. CAB International, Wallingford, p 171
Aneesh S (2014) Biomass production and nutrient dynamics in a multipurpose tree based black pepper production system. Master’s Thesis, Kerala Agricultural University, 100 pp
Angers DA, Chenu C (1997) Dynamics of soil aggregation and C sequestration. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) Soil processes and the carbon cycle. CRC Press, Boca Raton, pp 199–206
Bambrick AD, Whalen JK, Bradley RL, Cogliastro A, Gordon AM, Olivier A, Thevathasan NV (2010) Spatial heterogeneity of soil organic carbon in tree-based intercropping systems in Quebec and Ontario, Canada. Agrofor Syst 79:343–353
Borden KA, Isaac ME, Thevathasan NV, Gordon AV, Thomas SC (2014) Estimating coarse root biomass with ground penetrating radar in a tree-based intercropping system. Agrofor Syst 88(4):657–669
Brunner I, Godbold DL (2007) Tree roots in a changing world. J For Res 12:78–82
Cairns MA, Brown S, Helmer EH, Baumgardner GA (1997) Root biomass allocation in the world’s upland forests. Oecologia 111:1–11
Campbell JS, Liefers J, Pielor EC (1985) Regression equations for estimating single tree biomass of trembling g aspen: assessing their applicability to more than one population. For Ecol Manag 11:283–295
Chesney P (2008) Nitrogen and fine root length dynamics in a tropical agroforestry system with periodically pruned Erythrina poeppigiana. Agrofor Syst 72(2):149–159
FAO (2006) State of the world’s forest 2005. FAO, Rome, p 166
Gerhardt K, Fredriksson D (1995) Biomass allocation by broadleaf mahogany seedlings, Swietenia macrophylla (King), in abandoned pasture and secondary dry forest in Guanacaste, Costa Rica. Biotropica 27:174–182
Giardina CP, Ryan MG (2002) Total belowground carbon allocation in a fast growing eucalyptus plantation estimated using a carbon balance approach. Ecosystems 5:487–499
Gill SJ, Biging GS, Murphy EC (2000) Modeling conifer tree crown radius and estimating canopy cover. For Ecol Manag 126(3):405–416
Harwood CE (1989) Grevillea robusta, an annotated bibliography. International Council for Research in Agroforestry, Nairobi, p 123
Jackson ML (1958) Soil chemical analysis. Asia Publishing House, New Delhi, p 498
Jamaludheen V, Kumar BM (1999) Litter of nine multipurpose trees in Kerala, India—variations in the amount, quality, decay rates and release of nutrients. For Ecol Manag 115:1–11
Jangra R, Gupta SR, Kumar R, Singh G (2010) Carbon sequestration in the Grevillea robusta plantation on a reclaimed sodic soil at Karnal in Northern India. Int J Ecol Environ Sci 36(1):75–86
Jones M, Sinclair FL, Grime VL (1998) Effect of tree species and crown pruning on root length and soil water content in semi-arid agroforestry. Plant Soil 201(2):197–207
Jose S (2009) Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor Syst 76:1–10
Klepper B (1991) Root–shoot relationships. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker, New York, pp 265–286
Kongsager R, Napier J, Mertz O (2013) The carbon sequestration potential of tree crop plantations. Mitig Adapt Strateg Glob Change 18(8):1197–1213
Kraenzel M, Castillo A, Moore T, Potvina C (2003) Carbon storage of harvest-age teak (Tectona grandis) plantations, Panama. For Ecol Manag 173(1–3):213–225
Kumar BM (2008) Litter dynamics in plantation and agroforestry systems of the tropics—a review of observations and methods. In: Batish DR, Kohli RK, Jose S, Singh HP (eds) Ecological basis of agroforestry. CRC Press, Boca Raton, pp 181–216
Kumar BM, George SJ, Jamaludheen V, Suresh TK (1998) Comparison of biomass production, tree allometry and nutrient use efficiency of multipurpose trees grown in woodlot and silvopastoral experiments in Kerala, India. For Ecol Manag 112(1–2):145–163
Kuyah S, Dietz J, Muthuri C, Jamnadas R, Mwangi P, Coe R, Neufeldt H (2012) Allometric equations for estimating biomass in agricultural landscapes: II. Belowground biomass. Agric Ecosyst Environ 158:225–234
Lal R (2005) Forest soils and carbon sequestration. For Ecol Manag 220:242–258
Lal R, Kimble JM (2000) Tropical ecosystems and the global C cycle. In: Lal R, Kimble JM, Stewart BA (eds) Global climate change and tropical ecosystems. CRC Press, Boca Raton, pp 3–32
Liao C, Luo Y, Fang C, Li B (2010) Ecosystem carbon stock influenced by plantation practice: implications for planting forests as a measure of climate change mitigation. PLoS ONE 5(5):e10867. doi:10.1371/journal.pone.0010867
Long JN, Smith FW (1984) Relation between size and density in developing stands: a description and possible mechanisms. For Ecol Manag 7:191–206
Markesteijn L, Poorter L (2009) Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J Ecol 97:311–325
Mokany K, Raison JR, Prokushkin A (2006) Critical analysis of root:shoot ratios in terrestrial biomes. Glob Change Biol 12:84–96
Montagnini F, Nair PKR (2004) Carbon sequestration: an underexploited environmental benefit of agroforestry systems. Agrofor Syst 61:281–295
Nadelhoffer KJ, Raich JW (1992) Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 73:1139–1147
Naidu SL, DeLucia EH, Thomas RB (1998) Contrasting patterns of biomass allocation in dominant and suppressed loblolly pine. Can J For Res 28(8):1116–1124
Nair PKR, Kumar BM, Nair VD (2009) Agroforestry as a strategy for carbon sequestration. J Plant Nutr Soil Sci 172(1):10–23
Nair PKR, Nair VD, Kumar BM, Showalter JM (2010) Carbon sequestration in agroforestry systems. Adv Agron 108:237–307
Nilsson U, Albrektson A (1994) Productivity of needles and allocation of growth in young Scots pine trees of different competitive status. For Ecol Manag 62:173–187
Oelbermann MTR, Voroney P, Kass DCL, Schlfnvoigt AM (2006) Soil carbon and nitrogen dynamics using stable isotopes in 19- and 10-year-old tropical agroforestry systems. Geoderma 130:356–367
Paquette A, Messier C (2010) The role of plantations in managing the world’s forests in the Anthropocene. Front Ecol Environ 8:27–34
Paul GB (2012) Biomass and carbon sequestration in silver oak (Grevillea robusta A. Cunn.) stands in the midlands of Kerala. MSc Thesis, Kerala Agricultural University, Thrissur, p 83
Peichl M, Arain MA (2006) Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric For Met 140(1–4):51–63
Raich JW, Clark DA, Schwendenmann L, Wood TE (2014) Aboveground tree growth varies with belowground carbon allocation in a tropical rainforest environment. PLoS ONE 9(6):e100275. doi:10.1371/journal.pone.0100275
Sanchez PA (1995) Science in agroforestry. Agrofor Syst 30:5–55
Sanford RL Jr, Cuevas E (1996) Root growth and rhizosphere interactions in tropical forests. In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 268–300
Shanavas A, Kumar BM (2006) Physical and mechanical properties of three agroforestry tree species from Kerala, India. J Trop Agric 44:23–30
Spurr SH, Barnes BV (1980) Forest ecology. Wiley, New York
Tomlinson KW, Sterck FJ, Bongers F, da Silva DA, Barbosa ERM, Ward D, Bakker FT, van Kaauwen M, Prins HHT, de Bie S, van Langevelde F (2012) Biomass partitioning and root morphology of savanna trees across a water gradient. J Ecol 100:1113–1121
Uri V, Varik M, Aosaar J, Kanal A, Kukumagi M, Lohmus K (2012) Biomass production and carbon sequestration in a fertile silver birch (Betula pendula Roth) forest chronosequence. For Ecol Manag 267:117–126
Van Lear DH, Kapeluck PR (1995) Above- and below- stump biomass and nutrient content of a mature loblolly pine planation. Can J For Res 25:361–367
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining organic carbon in soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–263
Young A (1997) Agroforestry for soil management, 2nd edn. ICRAF and CAB International, Wallingford, p 320
Acknowledgments
This work forms part of the master’s dissertation project of the first author. The original experiment was established using a research grant from the World Bank funded social forestry programme of Kerala Government to B. Mohan Kumar during 1991. The authors also acknowledge partial funding of the present research from the All India coordinated research project on Agroforestry of the Indian Council of Agricultural Research, New Delhi and the laboratory and other facilities provided by Kerala Agricultural University, Vellanikkara.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, S., Kumar, B.M. & Kunhamu, T.K. Coarse root biomass, carbon, and nutrient stock dynamics of different stem and crown classes of silver oak (Grevillea robusta A. Cunn. ex. R. Br.) plantation in Central Kerala, India. Agroforest Syst 89, 869–883 (2015). https://doi.org/10.1007/s10457-015-9821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-015-9821-y