Abstract
Conventional measurements of tree root biomass in tree-based intercropping (TBI) systems can be inadequate in capturing the heterogeneity of rooting patterns or can be highly destructive and non-repeatable. In this study, we estimated coarse root biomass using ground penetrating radar (GPR) of 25-year-old trees inclusive of five species (Populus deltoides × nigra clone DN-177, Juglans nigra L., Quercus rubra L., Picea abies L. Karst, and Thuja occidentalis L.) at a TBI site in Southern Ontario, Canada. Subsurface images generated by GPR were collected in grids (4.5 × 4.5 m) centred on tree stems. The predictive relationship developed between GPR signal response and root biomass was corrected for species effects prior to tree-scale estimates of belowground biomass. Accuracy of the tree-scale estimates was assessed by comparing coarse root biomass measured from complete excavations of the corresponding tree. The mean coarse root biomass estimated from GPR analysis was 54.1 ± 8.7 kg tree−1 (mean ± S.E.; n = 12), within 1 % of the mean coarse root biomass measured from excavation. Overall there was a root mean square error of 14.4 kg between measured and estimated biomass with no detectable bias despite variable conditions within the in-field and multi-species study. Root system C storage by species, calculated with species-specific root carbon concentrations, is estimated at 5.4 ± 0.7–34.8 ± 6.9 kg C tree−1 at this site. GPR is an effective tool for non-destructively predicting coarse root biomass in multi-species environments such as temperate TBI systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With more than 20 % of total tree biomass allocated belowground, tree roots comprise a substantial but understudied component of biomass in many ecosystems (Cairns et al. 1997; Mokany et al. 2006; Brunner and Godbold 2007). Ecological benefits derived from the presence of tree roots, particularly in modified agricultural systems, include soil amelioration via root exudation, turnover, and sloughing, improved water infiltration and aeration from root channels, and prevention of erosion (Schroth 1999; Jose et al. 2004; Thevathasan and Gordon 2004; Jose 2009; Nair et al. 2009). Furthermore, the incorporation of trees into agricultural landscapes can increase the C storage potential, considerably within belowground biomass, and is touted as a viable and potentially significant land-use approach to sequester atmospheric CO2 (Dixon et al. 1994; Isaac et al. 2005; Peichl et al. 2006; Bambrick et al. 2010; Kuyah et al. 2012). While potential aboveground C sequestration in temperate agroforestry systems is estimated at 1.9 × 109 Mg C year−1 (Oelbermann et al. 2004), data are limited on the belowground contribution. Improved approaches to estimate and predict the belowground biomass are required to fully capture the role of roots in C budgets for temperate TBI.
Biomass inventories within agroforestry systems are primarily focused on measuring the aboveground component with a limited number of studies also calculating root mass (e.g. Oelbermann et al. 2005; Peichl et al. 2006; Kirby and Potvin 2007; Moser et al. 2010; Kessler et al. 2012). Conventional sampling techniques of tree root systems include minirhizotrons, profile walls, and soil cores (Vogt et al. 1998; Polomski and Kuhn 2002). However, due to the spatial heterogeneity and the inherent fractal nature of root systems, these techniques generally have low accuracy particularly for coarse roots (diameter > 2 mm) (Schroth and Kolbe 1994; Taylor et al. 2013). Conversely, partial or complete root system excavations are time consuming, destructive, and non-repeatable. This has led to the wide utilization of root:shoot ratios (e.g. Jackson et al. 1996; Cairns et al. 1997; Mokany et al. 2006) or allometric equations (e.g. Kurz et al. 1996; Jenkins et al. 2003) generated from biomass studies in forest ecosystems. However, such broad generalizations of tree biomass allocation may produce inaccuracies when applied to agroforestry, or TBI sites specifically, due to variation in tree root growth in cultivated scenarios (Nair 2011; Kuyah et al. 2012). Thus, there is a need for new methodologies to study and measure root biomass in TBI systems.
Ground penetrating radar (GPR) emits electromagnetic (EM) signals into the ground and records the reflected EM waves’ amplitude, polarity, and travel time. Signal reflections occur where there is a contrast in dielectric permittivity in the subsurface (Davis and Annan 1989). Importantly, water has a high dielectric constant (Davis and Annan 1989) and when coarse roots contain greater water content than the surrounding soil matrix, the necessary contrast of dielectric properties for radar signal reflections is met (Hruska et al. 1999; Hirano et al. 2009; Guo et al. 2013b, c). As a GPR unit operates along a transect, the reflected signals can be compiled to create an interpreted image, or radargram, of the subsurface with signal reflections at a root (perpendicularly orientated to the GPR transect) depicted as a hyperbolic reflection pattern (Barton and Montagu 2004). Depth of GPR signal penetration is limited by the antenna frequency of the GPR unit and the electrical conductivity of the subsurface (Davis and Annan 1989). The water and clay content of the soil will also influence the rate of radar signal attenuation due to their dielectric properties (Butnor et al. 2001). Ideal conditions for GPR study of coarse roots are well-drained soils with low clay content and with coarse roots of sufficient water content (Guo et al. 2013a).
A key benefit of GPR estimation of root biomass is that it provides researchers the opportunity for repeated measurements of coarse roots and the capacity to examine the uniqueness of tree root system responses along gradients of biotic and abiotic constraints (Isaac and Anglaaere 2013). Previous studies on the use of GPR for biomass estimation have involved controlled experiments (Barton and Montagu 2004; Dannoura et al. 2008; Hirano et al. 2009; Cui et al. 2013; Guo et al. 2013c; Tanikawa et al. 2013) and in situ testing within tree monocultures or two-species mixtures (Butnor et al. 2003; Stover et al. 2007; Samuelson et al. 2008, 2010; Hirano et al. 2012; Raz-Yaseef et al. 2013; Day et al. 2013). Generally, physical samples of root biomass equating to corresponding areas of GPR radargrams have been compared to develop predictive relationships between a GPR signal response index and root biomass (Butnor et al. 2003; Stover et al. 2007; Guo et al. 2013a). A growing body of research has used this approach to explore root responses to variation in atmospheric CO2 (Stover et al. 2007; Day et al. 2013), forest stand management techniques (Butnor et al. 2003; Samuelson et al. 2008, 2010), and precipitation patterns (Raz-Yaseef et al. 2013). However, the utility of GPR biomass estimation has yet to be tested in a multi-species scenario or in an agroforestry system specifically. Also, prior GPR studies have not derived estimates of root biomass for individual trees, or specifically applied GPR to estimates of root carbon. Therefore, the objectives of the present study were to test the utility of GPR for coarse root biomass estimation across a variety of tree species under field conditions, and to estimate the C content of individual trees in a temperate TBI system.
Materials and methods
Study site
Ground penetrating radar data and root samples were collected below 13 individual trees at the University of Guelph Agroforestry Research Station, Guelph, Ontario, Canada (44°32′28″N, 80°12′32″W; elevation 325 m). The 30-ha site was established in 1987 as an experimental TBI system. A variety of tree species are planted in tree rows that are spaced 12.5 or 15 m apart, in between which a conventional crop rotation is practiced under no-till cultivation and annual intercropping of Zea mays L. (maize), Glycine max (L.) Merr. (soybean), Triticum aestivum L. (winter wheat), or Hordeum vulgare L. (barley) (Thevathasan and Gordon 2004).
The soil is classified as Grey-Brown Luvisol with a sandy loam texture (65 % sand, 25 % silt, and 10 % clay) (Oelbermann and Voroney 2007). The Ap horizon continues to a depth of 28–53 cm (Price and Gordon 1999) and a moraine till is located approximately 1 m below the soil surface.
The 13 studied trees were of five species that are commonly selected for agroforestry systems in the region: Populus deltoides × nigra clone DN177 (hybrid poplar; DBH = 33.5 ± 0.6 cm; n = 2), Juglans nigra L. (black walnut; DBH = 24.8 ± 1.0 cm; n = 3), Quercus rubra L. (red oak; DBH = 21.3 ± 0.5 cm; n = 3), Picea abies L. Karst. (Norway spruce; DBH = 21.5 ± 3.5 cm; n = 2), all with aboveground heights >7 m, and the multi-stem Thuja occidentalis L. (eastern white cedar; DBH = 9.1 ± 2.3 cm; n = 3)Footnote 1, with aboveground height >4 m. The trees were planted with 6 m in-row tree stem spacing, except T. occidentalis with 1 m spacing. Each of the five tree species studied were replicated with 2 or 3 randomly selected individuals. All trees in the study were approximately 25 years old and were located in flat areas of the site.
Radar survey
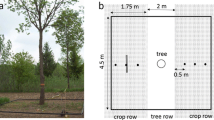
The area around the base of each target tree was cleared of leaf litter and other organic material. A 4.5 × 4.5 m grid frame was installed surrounding the base of each target tree so the tree stem was situated in the approximate centre (Fig. 1a). The grid frame was constructed of plastic pipe, which served to anchor guide-rope at 10 cm increments in both the x and y directions (Fig. 1b). To ensure straight and square transects, the GPR unit was pulled by an attached handle so that the antennae remained alongside the grid guide-rope. Data collection below 13 trees occurred between April and June, 2012. Transect increments of 10 cm were selected for GPR data collection to reduce dependency on interpolation. Each set of tree GPR data were collected on the same day and under consistent conditions with the GPR programmed to EM signal emission intervals of 0.1 ns stacked with 16 traces every 0.5 cm along each transect.
a Image of grid set-up for GPR survey of Quercus rubra in tree-based intercropping system at the University of Guelph Agroforestry Research Station, Guelph, Ontario, Canada. b Plan-view schematic of GPR data collection grid design. The base of tree stem is represented by the circle located in the centre of the grid. Red lines indicate GPR data collection transects in x and y directions with 10 cm spacing. (Color figure online)
To measure the average EM signal velocity in the subsurface, metal rods were inserted horizontally at depths of 40 cm into soil profiles adjacent to target trees. The signal travel time and distance of the GPR antenna to the rods were measured and thus average velocity of the radar signal was calculated. Velocities were measured the day of data collection for each tree and ranged between 0.08 and 0.10 m ns−1.
Tree root detection typically occurs with a GPR antenna emitting a centre frequency of 500 MHz (e.g. Barton and Montagu 2004) up to 2,000 MHz (e.g. Cui et al. 2013). For this study, we used a 1,000 MHz GPR unit (NogginPlus; Sensors and Software Inc., Mississauga, ON, Canada) for all data collection. Previous studies using this frequency have reported detection coarse roots of diameter 0.5 cm and greater (Guo et al. 2013a).
Water content of coarse roots and soil within study plots was determined by weighing samples before and after drying at 65 °C for 7 days. Coarse root gravimetric and volumetric water contents (assuming cylindrical root shapes) were 175 ± 7 and 76 ± 1 % (mean ± S.E.; n = 65), respectively, inclusive of all study trees (n = 10 or 15 per species). The soil gravimetric and volumetric water contents (using known soil sampler volume of 100 mL) to a depth of 60 cm over the entire study period were 10 ± 1 and 12 ± 5 % (mean ± S.E.; n = 20), respectively. Thus, the necessary soil to root water content gradient was satisfied for GPR detection and interpretation (Hirano et al. 2009; Guo et al. 2013c) with coarse roots containing greater than six times more water than the soil on a volume basis.
Radargram processing
Prior to image analysis of the GPR data, non-root anomalies (e.g. plane reflectors and signal “noise”) were reduced with a sequence of image processing steps (DC shift, dewow, and background removal) (Fig. 2b, c). Subsequently, dipping features, such as hyperbolic reflections of roots, were repositioned to their foci with a migration algorithm (2d FK migration with Stolt equation using known signal velocity and the angle of incidence) (Fig. 2d). Finally, an envelope algorithm known as the Hilbert transformation (operating on amplitudes of the reflected EM waves are used to interpret the data into one phase) was applied so that reflectors are more discernible (Fig. 2e). In order to enhance subsurface root reflections representatively with depth, a spreading and exponential compensation gain (SEC2) was applied to all processed GPR radargrams based on the rate of energy decay, similar to methodology in Cui et al. (2013) and Guo et al. (2013c). A colour palette of bipolar grey was selected for visualization whereby low to high amplitude response was displayed as grey to white. All GPR data processing steps were completed in EKKO_View Deluxe (Sensors and Software Inc.).
GPR data processing sequence with soil profile (0.25 × 1.0 m) and exposed roots (circles) of Juglans nigra (a) and the equivalent GPR geo-image (b) with applied background removal (c) surface reflections or banding is reduced. Hyperbola migration focuses root reflections to foci (d). The final data processing step is the Hilbert transformation whereby magnitude of reflection is brought into one phase (e). The extracted GPR index (area within an intensity range (175–255 cm2) is measured (f) to develop a GPR index–biomass relationship
Ground penetrating radar radargrams were imported into ImageJ (US National Institutes of Health, Bethesda, MD, USA) as 8-bit bmp files with pixel intensities ranging between 0 and 255. Radargrams were standardized to ensure consistent measures of distance within an image using a ratio of 400 pixels: 1 m. The final data processing step measured the number of pixels within an established threshold range (175–255) and subsequently converted to a cross-sectional area (cm2) (Fig. 2f), defined as the GPR index.
GPR index–biomass relationship
Following GPR data collection, soil profiles (0.25 m across and 1 m deep) were exposed at distances ranging from 0.5 to 2.0 m from the tree stem (n = 64) (Fig. 2a). These soil profiles were orientated along GPR data transects and included all study trees (n = 4 or 6 profiles per tree). To calculate total coarse root dry weight biomass in each excavated soil profile, the diameters of all coarse roots crossing through the exposed profiles were measured and applied to species-specific relationships of root dry weight biomass to root diameter as described in Hirano et al. (2012) and Guo et al. (2013b) (Table 1). Relationships were developed from root samples (n = 20 per species) of various diameters collected at all study trees and assumed a root length of 10 cm, which is equivalent to the GPR transect spacing.
To determine the GPR index, three pixel intensity thresholds (165–255, 175–255, and 200–255) were selected based on visually delineating root features and minimizing the inclusion of non-root anomalies. The area derived from each threshold level were compared for their correlations to coarse root biomass. The pixel intensity threshold of 175–255 (Fig. 2f) was ultimately selected to generate the area index of GPR signal response as it produced the optimal correlation with measured biomass.
Coarse root biomass estimates
The radargram processing sequence was applied to all GPR data within each tree grid. The resulting GPR index determined for each transect was used to calculate the predicted root biomass using the GPR index–biomass relationship. All transect biomass estimates were summed for total tree coarse root biomass (BGPR; kg tree−1). Since tree roots are best detected when crossing between 45° and 135° to the plane of the radargram (Butnor et al. 2001; Tanikawa et al. 2013), the biomass estimates from grid transects in both x and y directions were included to maximize root detection by capturing roots irrespective of direction of growth.
Ground penetrating radar results were compared to an estimate of coarse root biomass for the study trees using conventional allometric equations from Jenkins et al. (2003) that employed measured DBH and parameters (β) associated with species class (hardwood or softwood) and species group (hardwoods: Populus sp. = “aspen/alder/cottonwood/willow”, J. nigra = “mixed hardwood”, and Q. rubra = “hard maple/oak/hickory/beech”; softwoods: P. abies = “spruce”, and T. occidentalis = “cedar/larch”)Footnote 2 (Jenkins et al. 2003). The coarse root biomass was estimated such that:
where β0 and β1 are parameters fitted from species class data in Jenkins et al. (2003), DBH is the diameter at breast height (cm), and BGBratio is the ratio of coarse root biomass to aboveground biomass (AGB), calculated as:
where AGB is total aboveground biomass (kg), DBH is the diameter at breast height (cm), and β2 and β3 are parameters fitted from species group data in Jenkins et al. (2003).
Root excavations
Following GPR data collection, the belowground root systems of each tree were mechanically excavated using 580 Robert Tire Backhoe. A square plot equivalent to the area scanned with GPR (4.5 × 4.5 m) was removed of soil and biomass to a depth of 1 m. Coarse roots were manually extracted from the excavated soil. Due to the higher stem density of T. occidentalis, roots were collected for all T. occidentalis trees present in the excavated area. One tree replication of J. nigra was omitted for concern of inadequate collection of coarse root biomass. For each tree, all coarse roots were weighed for total wet weight. The coarse root gravimetric water content specific to each tree was applied to total wet weight to calculate the total dry weight of the coarse roots (kg tree−1).
Root carbon content
Coarse roots were randomly selected during complete excavation. Sample preparation and analysis follow a volatile C-inclusive methodology as suggested by Thomas and Martin (2012) as volatile compounds lost during oven drying constitute a non-negligible amount of C necessary for more accurate C content estimates (Lamlom and Savidge 2003; Thomas and Malczewski 2007; Martin and Thomas 2011). Roots were placed in air-tight bags and transported in a cooler to the laboratory at which point roots were washed to remove soil and stored in air-tight bags at -5 °C. Coarse roots were cut into cylindrical segments ~1 cm in length to retain representative proportions of root tissue. Root samples were dried in an 8 L freeze dryer (Labconco Co., Kansas City, MO, USA) for 7 days. The largest diameter roots were weighed for constant mass on the final day to ensure complete drying.
For each tree species (n = 3, except P. abies and Populus sp. where n = 2), coarse root samples were prepared as composite samples inclusive of four coarse root diameter classes (0.2–0.5, 0.5–1.0, 1.0–2.0, and >2.0 cm). Samples were ground in a ball grinder (MM400 Mixer Mill; Retsch, Newtown, PA, USA) and stored in snap-cap 1 mL containers at -5 °C. Total C for each sample was determined using a CHN analyzer (Thermo Flash 2000; Thermo Scientific). Samples were weighed on a microbalance for total sample mass. Elemental analysis calibrations were completed prior to each sample run using aspartic acid. Known standards (SRM 133317, Thermo Scientific) were tested during analysis to confirm instrument accuracy.
As biomass estimates are reported on a dry weight basis, the C concentrations (%) measured from freeze-dried samples were converted onto a dry weight basis:
where MF was the mass of the freeze-dried sample used for elemental analysis and MC was the mass of C in MF (Martin and Thomas 2011). The species’ mean volatile mass fraction (VMF) applied to Eq. 3 was the species-specific fraction of biomass lost during heating methods and was calculated as:
where an additional subset of freeze-dried samples were weighed (MF) and oven dried at 105 °C for 48 h and weighed again after drying (MH).
Carbon concentration analysis was repeated with oven dried roots to evaluate methodology. Finally, the total root C content for each tree species was calculated by applying the resultant C concentration values of coarse roots to GPR-derived estimates of coarse root biomass.
Statistical analysis
The GPR index–biomass relationship was developed by regressing the GPR response (area of processed radargrams above an intensity threshold; cm2) to the coarse root biomass (g) from spatially matched subsurface soil profiles to a depth of 1 m to develop predictive equations. Cook’s distance was used to identify potential outliers. One observation representing an excavated soil profile section containing an extremely large J. nigra root (>10 cm) of non-cylindrical shape was discarded on this basis. An analysis of covariance (ANCOVA) was used to test for species main effect on biomass as well as the interactive effect of species and GPR signal response (GPR index × species) on biomass in order to identify required corrected predictive equations among species.
An assessment of precision of BGPR was completed by comparing BGPR to the coarse root biomass measured from matched excavated study plots using paired t tests on means and a linear regression for all study trees. Differences among species for BGPR and C concentration were tested using one-way analysis of variance (ANOVA).
Prior to parametric tests, data were examined for equality of variance using the Bartlett test and for normality of residuals using the Shapiro-Wilks test. Statistical analyses were completed in R v.2.14.2 (R Foundation for Statistical Computing, Vienna, Austria) with the level of significance set at P < 0.05.
Results
GPR images and index–biomass relationship
Signal noise and planar reflections, specifically from surface reflections, were reduced following the image processing sequence (Fig. 2b, c). Hyperbola migration and the Hilbert transformation were successful in emphasizing root reflections in the radargrams (Fig. 2d, e). The selected image intensity threshold (pixel intensity between 175 and 255) delineated these areas of high GPR signal response (Fig. 2f). This allowed for detectable roots to be converted quantitatively to the cross sectional area (cm2) on the radargram, which became the ‘GPR index’. The area bounded in detected root signals within the subset of radargram profiles ranged from 1.56 to 615.44 cm2.
Measured coarse root biomass in the exposed soil profiles were positively correlated with the GPR index extracted from the matched radargrams (r = 0.47; n = 63). However, there was a species main effect on biomass (P = 0.0002) (Table 2) that necessitated the development of corrected relationships based on species size. When the data collected for the distinctly smaller T. occidentalis were separated from the other four species (‘pooled’), the GPR index remained significantly correlated to biomass (P < 0.0001). There was no species main effect or GPR index by species interactive effect on biomass for the remaining pooled species data (Table 2; Fig. 3). The resulting GPR index–biomass predictive equation for the corrected pooled species was y = 0.215x − 4.722 (r = 0.55; n = 51) and the GPR index–biomass predictive equation of the corrected T. occidentalis was y = 0.039x − 4.618 (r = 0.95; n = 12). These two relational equations were used for biomass estimation at the tree scale.
Transformed data used to test for species main effect and interactive effect with GPR index on measured biomass (ANCOVA). Solid symbols represent corrected pooled species data with no significant species main effect (P = 0.20), or interactive effect with GPR index (P = 0.68), on biomass (Table 2). Open circles are isolated data points collected below T. occidentalis representing the corrected data used for that species GPR index–biomass estimation equation (P < 0.0001)
Coarse root biomass
BGPR was 54.1 ± 8.7 kg tree−1 (mean ± S.E.) (n = 12), regardless of species, and the mean coarse root biomass measured from excavation was 54.8 ± 8.3 kg tree−1 (n = 12), and not significantly different (P = 0.876; Table 3). BGPR for J. nigra (omitting one excluded individual) closely matched excavated biomass. BGPR for Q. rubra was a slight overestimate of 4 % and the BGPR of P. abies was overestimated of 24 %. In contrast, the BGPR of Populus sp. was 54.6 ± 6.0 kg (n = 2) compared to an excavated mean of 71.9 ± 10.8 kg (n = 2), an underestimation of 32 %. Thuja occidentalis had a BGPR of 11.8 ± 1.5 kg tree−1 (n = 3), an underestimation of the excavated amount by 16 %. The pooled data for BGPR and excavated coarse root biomass displayed a linear relationship with no evident bias (r 2 = 0.75; P = 0.0003) and a root mean square error (RMSE) of 14.4 kg tree−1 (Fig. 4). Overall, the estimates derived from the applied allometric equations were less accurate than BGPR, underestimating the mean excavated biomass by 19 % (Table 3), and resulting in a weaker correlation (r 2 = 0.60; RMSE = 16.7 kg tree−1).
Positive correlation (solid line) between coarse root biomass estimated by GPR (kg tree−1) and coarse root biomass measured from matched excavations (kg tree−1) (r 2 = 0.75; P = 0.0003; RMSE = 14.4 kg tree−1; n = 12). BGPR for T. occidentalis is based on an area not an individual tree basis, where root area is equivalent to the other tested tree species individual root biomass area (4.5 × 4.5 m)
Root system carbon content
Among the five species, the concentration of C in the coarse roots was 45.9 ± 0.6 % (mean ± S.E.; n = 5 species) (Table 4). Carbon concentrations varied from 44.7 to 48.1 % (in J. nigra and P. abies, respectively), though no significant variation was found among species (P = 0.361). Of note, the volatile inclusive methodology captured and additional 2.1 ± 0.8 % (mean ± S.E.; n = 5 species) of C lost during oven drying methods. The C content of tree root systems using coarse root estimates from GPR data ranged between T. occidentalis with 5.4 ± 0.7 kg C tree−1 (mean ± S.E.; n = 3) to Q. rubra 34.8 ± 6.9 kg C tree−1 (n = 3) (Table 4), although no significant variations were detected among species (P = 0.361). Overall, the mean C content of tree root systems at this site was estimated at 25.7 ± 5.4 kg C tree−1 (n = 5 species), which scales to the landscape level as 2.9 Mg C ha−1.
Discussion
The GPR–biomass relationship
The use of a linear GPR index–biomass relationship was suitable in this study due to low variability of coarse root water content (Guo et al. 2013c) and a large difference between root and soil water contents (Hirano et al. 2009). A correlation of r = 0.89 between biomass from soil cores and GPR index was found in a study completed by Samueslon et al. (2008). They used a 1.5 GHz GPR unit in a Pinus taeda plantation on sandy loam soils and correlated subsurface data exclusively to a depth of 30 cm. The correlation from Day et al. (2013), also using a 1.5 GHz GPR unit, was r = 0.69 between biomass from soil cores and a GPR index inclusive of subsurface data to a depth of 60 cm in a scrub-oak ecosystem co-dominated by Quercus myrtifolia and Quercus geminata on sandy soils in Florida, USA. Although it should be expected that GPR index–biomass correlations will be reduced when there is an increase of depth of radar analysis and an increase of the variability in subsurface conditions, here we include roots to a depth of 1 m while still maintaining a reasonably strong correlation (r = 0.55) inclusive of four different tree species. The corrected relationship for T. occidentalis showed a very strong correlation (r = 0.95) in part due to the shallower root system of this relatively smaller tree species.
Ideally, the utility of GPR biomass estimation across a landscape would be greater given the applicability of one GPR index–biomass relationship to apply to all radargram data. This would reduce the number of biomass calibration points and, in scenarios where trees are proximal, overcome the limitations of determining species-specific roots detected by GPR. However, from the results of this study, two predictive equations that differentiate between the larger deep-rooted species and the smaller shallow-rooted species were appropriate in order to remove species effects. Similarly, Butnor et al. (2003) developed corrected relationships of GPR index–biomass for two contrasting scenarios of fertilizer use or no fertilizer use in a Pinus taeda stand, which altered the soil conditions for radar signals. Given reasonably consistent subsurface conditions (e.g. clay content) and soil-root moisture gradients, corrected GPR index–biomass relationships may be required for scenarios of distinct biomass gradients, a reality for temperate TBI systems.
Biomass and carbon estimates of tree root systems
Inclusion of the fraction of volatile C lost during high-heat (105 °C) drying improved accuracy of C content estimates. The highest root C concentration was for coniferous P. abies, consistent with previous reported trends where coniferous trees have a higher concentration of C than deciduous trees in temperate regions (IPCC 2006; Thomas and Martin 2012). Peichl et al. (2006) reported coarse root C concentrations, using conventional oven-drying methods, of 13-year-old P. abies at 51 %, which is ~3 % greater than those found in our study. Conversely, carbon concentrations for Populus sp. roots found by Peichl et al. (2006) (43 %) were ~3 % lower than our reported values inclusive of volatiles, but similar to the results from oven dry methods. Bert and Danjon (2006) detected variation between the interior root “wood” and the exterior root “bark” (bark + phloem) of Pinus pinaster Ait., with the root bark ~3 % greater in concentration. They also reported diameter-dependent variation notably for roots <4 cm (Bert and Danjon 2006). We did not test for variation within root tissues, but acknowledge that there may be within-root variation of C concentrations dependent on the ratio of root components, which would be inherently affected by the diameter of root samples used during elemental analysis. Additional sources of variability in C concentration of tree roots may arise from variation in sampling and C analysis protocol as well as physiological variation (Lamlom and Savidge 2003), such as tree root carbohydrate storage (Bert and Danjon 2006).
In order to show the C storage potential of trees in temperate tree-based intercropping systems, system level root C quantification was calculated using the current hardwood tree density of 111 trees ha−1. However, it should be noted that if only coniferous trees are integrated, the tree density will be much higher due to lower spacing used for coniferous trees. The estimated root C content at this site indicates an increase of belowground C storage over the last 12 years when compared to the root C content of the average reported values for 13-year-old Populus sp. and P. abies at the same site (1.8 Mg C ha−1) (Peichl et al. 2006). In temperate TBI systems, stem density, species composition, and the age of trees are highly variable. Thus reporting species root biomass and root C content at the tree scale is valuable for operational purposes that are specific to these variables (Thevathasan and Gordon 2004).
Application and limitations of GPR in tree-based intercropping
There are limitations to biomass components GPR can detect. Coarse roots smaller than 1.0 cm in diameter are less likely to cause radar signal response than larger roots (≥1 cm) (Hirano et al. 2012), coarse roots located deeper than GPR signal penetration can be undetected or misinterpreted by the GPR signal response (Hirano et al. 2009), and coarse roots located outside of the field of the radar signals, such as directly below the stem, will be undetected (Samuelson et al. 2008). As a result of these detection limitations there is an anticipated bias towards GPR underestimation of coarse root biomass, assuming proper calibration and appropriate conditions. With the exception of Populus sp., this bias was not observed in our results suggesting that the subsurface conditions and the morphological characteristics (e.g. tap roots growing horizontal from the stem) of the root systems at this study site were conducive for radar study. However, some detected biomass might be attributed to false positive GPR signal response incurred from in-field conditions.
Although GPR does require some destructive calibration sampling using soil cores or partial excavations, the amount of physical sampling required to estimate the coarse root biomass is drastically reduced compared to conventional studies in TBI systems. During the current study, 243 m2 of surface area were scanned with GPR, an area equating to over 13,500 soil cores (of 15 cm diameter). GPR techniques can provide more thorough understanding of the heterogeneity of the root systems without total excavation in agroforestry systems (Isaac et al. 2014) and unlike destructive sampling, this method of root data collection can be repeated, critical for temporal-scale studies on root system dynamics (Norby and Jackson 2000). We tested the use of pre-established species-based allometric and root:shoot equations, a more traditional approach to quantify root biomass, and found that these produced less accurate estimates of the excavated root biomass compared to the GPR estimates. Generalized equations derived from forest ecosystem data might be unsuitable for trees in agricultural landscapes where variation in management (e.g. planting density and fertilizer application) can induce differences in biomass allocation. Results from this study suggest that the overall accuracy of the allometric estimates was outperformed by GPR estimations and support the need for more site- and species-specific tree root data. Recent advancements in modelling radar signal response given variable root and soil conditions have been reported following controlled experiments (Guo et al. 2013b; Tanikawa et al. 2013). With these advancements, there is potential of enhancing accuracy of root estimation for in-field conditions and lessening the need for destructive sampling for calibrations.
Conclusions
Coarse root biomass of 12 trees, inclusive of five species, was accurately estimated with the use of GPR at a TBI site in Southern Ontario, Canada. Subsequently, C content of tree root systems was quantified using species-specific coarse root C concentrations. This was the first in-field study to test the robustness of GPR as a coarse root biomass estimation tool across multiple species. Corrected predictive relationships between GPR signal response and root biomass were required to remove a strong species effect, namely isolating data from a species with a distinctly smaller and shallower root system. We argue that this technology can be suitable for use in temperate TBI systems under well-drained, sandy loam soils. Ultimately, these results contribute to furthering methodological techniques of GPR root study for direct quantification of belowground biomass and C storage in agroforestry systems and other tree-based ecosystems.
Notes
DBH of T. occidentalis is the mean ± S.E. of the largest stem from the primary trees located in study plots.
Due to 1 m stem spacing for T. occidentalis, 1 or 2 additional trees were located at the edges of the 3 replicates of the root study area. In order to calculate root biomass in the study area from the allometric equations, these trees were assumed to contribute 50 % of their root biomass into the study area due to their location close to the boundary.
References
Bambrick AD, Whalen JK, Bradley RL, Cogliastro A, Gordon AM, Olivier A, Thevathasan NV (2010) Spatial heterogeneity of soil organic carbon in tree-based intercropping systems in Quebec and Ontario, Canada. Agrofor Syst 79:343–353
Barton CVM, Montagu KD (2004) Detection of tree roots and determination of root diameters by ground penetrating radar under optimal conditions. Tree Physiol 24:1323–1331
Bert D, Danjon F (2006) Carbon concentration variations in the roots, stem and crown of mature Pinus pinaster (Ait.). For Ecol Manag 222:279–295
Brunner I, Godbold DL (2007) Tree roots in a changing world. J For Res 12:78–82
Butnor JR, Doolittle JA, Kress L, Cohen S, Johnsen KH (2001) Use of ground-penetrating radar to study tree roots in the southeastern United States. Tree Physiol 21:1269–1278
Butnor JR, Doolittle JA, Johnsen KH, Samuelson L, Stokes T, Kress L (2003) Utility of ground-penetrating radar as a root biomass survey tool in forest systems. Soil Sci Soc Am J 67:1607–1615
Cairns MA, Brown S, Helmer EH, Baumgardner GA (1997) Root biomass allocation in the world’s upland forests. Oecologia 111:1–11
Cui X, Guo L, Chen J, Chen X, Zhu X (2013) Estimating tree-root biomass in different depths using ground-penetrating radar: evidence from a controlled experiment. IEEE Trans Geosci Remote Sens 51:3410–3423
Dannoura M, Hirano Y, Igarashi T, Ishii M, Aono K, Yamase K, Kanazawa Y (2008) Detection of Cryptomeria japonica roots with ground penetrating radar. Plant Biosyst 142:375–380
Davis JL, Annan AP (1989) Ground-penetrating radar for high-resolution mapping of soil and rock stratigraphy. Geophys Prospect 37:531–551
Day FP, Schroeder RE, Stover DB, Brown ALP, Butnor JR, Dilustro J, Hungate BA, Dijkstra P, Duval BD, Seiler TJ, Drake BG, Hinkle CR (2013) The effects of 11 yr of CO2 enrichment on roots in a Florida scrub-oak ecosystem. New Phytol 200:778–787
Dixon RK, Winjum JK, Andrasko KJ, Lee JJ, Schroeder PE (1994) Integrated land-use systems: assessment of promising agroforest and alternative land-use practices to enhance carbon conservation and sequestration. Clim Change 27:71–92
Guo L, Chen J, Cui X, Fan B, Lin H (2013a) Application of ground penetrating radar for coarse root detection and quantification: a review. Plant Soil 362:1–23
Guo L, Lin H, Fan B, Cui X, Chen J (2013b) Forward simulation of root’s ground penetrating radar signal: simulator development and validation. Plant Soil. doi:10.1007/s11104-013-1751-8
Guo L, Lin H, Fan B, Cui X, Chen J (2013c) Impact of root water content on root biomass estimation using ground penetrating radar: evidence from forward simulations and field controlled experiments. Plant Soil. doi:10.1007/s11104-013-1710-4
Hirano Y, Dannoura M, Aono K, Igarashi T, Ishii M, Yamase K, Makita N, Kanazawa Y (2009) Limiting factors in the detection of tree roots using ground-penetrating radar. Plant Soil 319:15–24
Hirano Y, Yamamoto R, Dannoura M, Aono K, Igarashi T, Ishii M, Yamase K, Makita N, Kanazawa Y (2012) Detection frequency of Pinus thunbergii roots by ground-penetrating radar is related to root biomass. Plant Soil 360:363–373
Hruska J, Čermák J, Sustek S (1999) Mapping tree root systems with ground-penetrating radar. Tree Physiol 19:125–130
IPCC (2006) Intergovernmental panel on climate change guidelines for national greenhouse gas inventories. Chapter 4: Forest lands. National greenhouse gas inventories programme, Japan
Isaac ME, Anglaaere LCN (2013) An in situ approach to detect tree root ecology: linking ground-penetrating radar imaging to isotope-derived water acquisition zones. Ecol Evol 3:1330–1339
Isaac ME, Gordon AM, Thevathasan N, Oppong SK, Quashie-Sam J (2005) Temporal changes in soil carbon and nitrogen in west African multistrata agroforestry systems: a chronosequence of pools and fluxes. Agrofor Syst 65:23–31
Isaac ME, Anglaaere LCN, Borden KA, Adu-Bredu S (2014) Intraspecific root plasticity in agroforestry across edaphic conditions. Agric Ecosyst Environ 185:16–23
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108:389–411
Jenkins JC, Chojnacky DC, Heath LS, Birdsey RA (2003) National-scale biomass estimators for united states tree species. For Sci 49:12–35
Jose S (2009) Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor Syst 76:1–10
Jose S, Gillespie AR, Pallardy SG (2004) Interspecific interactions in temperate agroforestry. Agrofor Syst 61:237–255
Kessler M, Hertel D, Jungkunst HF, Kluge J, Abrahamczyk S, Bos M, Buchori D, Gerold G, Gradstein SR, Köhler S, Leuschner C, Moser G, Pitopang R, Saleh S, Schulze SH, Sporn SG, Steffan-Dewenter I, Tjitrosoedirdjo SS, Tscharntke T (2012) Can joint carbon and biodiversity management in tropical agroforestry landscapes be optimized? PLoS ONE 7:e47192
Kirby KR, Potvin C (2007) Variation in carbon storage among tree species: implications for the management of a small-scale carbon sink project. For Ecol Manag 246:208–221
Kurz WA, Beukema SJ, Apps MJ (1996) Estimation of root biomass and dynamics for the carbon budget model for the Canadian forest sector. Can J For Res 26:1973–1979
Kuyah S, Dietz J, Muthuri C, Jamnadass R, Mwangi P, Coe R, Neufeldt H (2012) Allometric equations for estimating biomass in agricultural landscapes: II. Belowground biomass. Agric Ecosyst Environ 158:225–234
Lamlom SH, Savidge RA (2003) A reassessment of carbon content in wood: variation within and between 41 North American species. Biomass Bioenergy 25:381–388
Martin AR, Thomas SC (2011) A reassessment of carbon content in tropical trees. PLoS ONE 6:e23533
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root:shoot ratios in terrestrial biomes. Glob Change Biol 12:84–96
Moser G, Leuschner C, Hertel D, Hölscher D, Köhler M, Leitner D, Michalzik B, Prihastanti E, Tjitrosemito S, Schwendenmann L (2010) Response of cocoa trees (Theobroma cacao) to a 13-month desiccation period in Sulawesi, Indonesia. Agrofor Syst 79:171–187
Nair PKR, Kumar BM, Nair VD (2009) Agroforestry as a strategy for carbon sequestration. J Plant Nutr Soil Sci 172:10–23
Nair PKR (2011) Carbon sequestration studies in agroforestry systems: a reality-check. Agrofor Syst 86:243–253
Norby RJ, Jackson RB (2000) Root dynamics and global change: seeking an ecosystem perspective. New Phytol 147:3–12
Oelbermann M, Voroney RP (2007) Carbon and nitrogen in a temperate agroforestry system: using stable isotopes as a tool to understand soil dynamics. Ecol Eng 29:342–349
Oelbermann M, Voroney RP, Gordon AM (2004) Carbon sequestration in tropical and temperate agroforestry systems: a review with examples from Costa Rica and southern Canada. Agric Ecosyst Environ 104:359–377
Oelbermann M, Voroney RP, Kass DCL, Schlönvoigt AM (2005) Above- and below-ground carbon inputs in 19-, 10- and 4-year-old Costa Rican Alley cropping systems. Agric Ecosyst Environ 105:163–172
Peichl M, Thevathasan NV, Gordon AM, Huss J, Abohassan RA (2006) Carbon sequestration potentials in temperate tree-based intercropping systems, Southern Ontario, Canada. Agrofor Syst 66:243–257
Polomski J, Kuhn N (2002) Root research methods. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots the hidden half, 3rd edn. Marcel Dekker, New York, pp 295–321
Price GW, Gordon AM (1999) Spatial and temporal distribution of earthworms in a temperate intercropping system in southern Ontario, Canada. Agrofor Syst 44:141–149
Raz-Yaseef N, Koteen L, Baldocchi DD (2013) Coarse root distribution of a semi-arid oak savanna estimated with ground penetrating radar. J Geophys Res Biogeosci 118:135–147
Samuelson LJ, Butnor J, Maier C, Stokes TA, Johnsen K, Kane M (2008) Growth and physiology of loblolly pine in response to long-term resource management: defining growth potential in the southern United States. Can J For Res 38:721–732
Samuelson LJ, Eberhardt TL, Butnor JR, Stokes TA, Johnsen KH (2010) Maximum growth potential in loblolly pine: results from a 47-year-old spacing study in Hawaii. Can J For Res 40:1914–1929
Schroth G (1999) A review of belowground interactions in agroforestry, focussing on mechanisms and management options. Agrofor Syst 43:5–34
Schroth G, Kolbe D (1994) A method of processing soil core samples for root studies by subsampling. Biol Fertil Soils 18:60–62
Stover DB, Day FP, Butnor JR, Drake BG (2007) Effect of elevated CO2 on coarse-root biomass in Florida scrub detected by ground-penetrating radar. Ecol 88:1328–1334
Tanikawa T, Hirano Y, Dannoura M, Yamase K, Aono K, Ishii M, Igarashi T, Ikeno H, Kanazawa Y (2013) Root orientation can affect detection accuracy of ground-penetrating radar. Plant Soil. doi:10.1007/s11104-013-1798-6
Taylor BN, Beidler KV, Cooper ER, Strand AE, Pritchard SG (2013) Sampling volume in root studies: the pitfalls of under-sampling exposed using accumulation curves. Ecol Lett 16:862–869
Thevathasan NV, Gordon AM (2004) Ecology of tree intercropping systems in the North temperate region: experiences from southern Ontario, Canada. Agrofor Syst 61:257–268
Thomas SC, Malczewski G (2007) Wood carbon content of tree species in Eastern China: interspecific variability and the importance of the volatile fraction. J Environ Manag 85:659–662
Thomas SC, Martin AR (2012) Carbon content of tree tissues: a synthesis. For 3:332–352
Vogt KA, Vogt DJ, Bloomfield J (1998) Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 200:71–89
Acknowledgments
We would thank the faculty, staff, and students at the University of Guelph Agroforestry Research Station. Amy Wotherspoon from the University of Guelph graciously provided root excavation data and Stephanie Gagliardi provided important assistance during field work and laboratory analysis. We also thank Tom Meulendyk for assistance. We thank two anonymous journal reviewers for their insightful and constructive comments on earlier versions of the manuscript. Funding was provided by the Faculty of Forestry at the University of Toronto, Agriculture and Agri-Food Canada’s Agricultural Greenhouse Gases Program, and the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borden, K.A., Isaac, M.E., Thevathasan, N.V. et al. Estimating coarse root biomass with ground penetrating radar in a tree-based intercropping system. Agroforest Syst 88, 657–669 (2014). https://doi.org/10.1007/s10457-014-9722-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-014-9722-5