Abstract
Aims
Recently, cancer-derived exosomes were shown to have pro-metastasis function in cancer, but the mechanism remains unclear. Angiogenesis is essential for tumor progression and is a great promising therapeutic target for advanced cervical cancer. Here, we investigated the role of cervical cancer cell-secreted exosomal miR-221-3p in tumor angiogenesis.
Methods and results
miR-221-3p was found to be closely correlated with microvascular density in cervical squamous cell carcinoma (CSCC) by evaluating the microvascular density with immunohistochemistry and miR-221-3p expression with in situ hybridization in clinical specimens. Using the groups of CSCC cell lines (SiHa and C33A) with miR-221-3p overexpression and silencing, the CSCC exosomes were characterized by electron microscopy, western blotting, and fluorescence microscopy. The enrichment of miR-221-3p in CSCC exosomes and its transfer into human umbilical vein endothelial cells (HUVECs) were confirmed by qRT-PCR. CSCC exosomal miR-221-3p promoted angiogenesis in vitro in Matrigel tube formation assay, spheroid sprouting assay, migration assay, and wound healing assay. Then, exosome intratumoral injection indicated that CSCC exosomal miR-221-3p promoted tumor growth in vivo. Thrombospondin-2 (THBS2) was bioinformatically predicted to be a direct target of miR-221-3p, and this was verified by using the in vitro and in vivo experiments described above. Additionally, overexpression of THBS2 in HUVECs rescued the angiogenic function of miR-221-3p.
Conclusions
Our results suggest that CSCC exosomes transport miR-221-3p from cancer cells to vessel endothelial cells and promote angiogenesis by downregulating THBS2. Therefore, CSCC-derived exosomal miR-221-3p could be a possible novel diagnostic biomarker and therapeutic target for CSCC progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Cervical cancer remains one of the leading causes of cancer-related deaths among females in developing countries [1]. While early-stage and locally advanced cancers can often be cured with current standard treatments, metastatic or recurrent cervical cancer has limited treatment options and a high rate of mortality [2]. Angiogenesis is essential for tumor progression and has shown great promise as a therapeutic target for the treatment of advanced cervical cancer [3]. Recently, the addition of bevacizumab, an antibody directed against vascular endothelial growth factor (VEGF), to chemotherapy was shown to improve median overall survival in patients with recurrent, persistent, or metastatic cervical cancer [4]. However, anti-angiogenesis therapies mostly target the VEGF axis [5], and treatment with angiogenesis-targeted combination regimens is marred by variable responses, nonnegligible toxicity, and possible drug resistance [6]. Therefore, the identification of novel anti-angiogenetic factors or predictive biomarkers will be critical for future drug development and anti-cancer therapy.
Within the tumor microenvironment (TME), intercellular communication between malignant and stromal cells of the host is necessary [7]. In recent years, exosomes, a group of extracellular 30–100 nm membrane vesicles secreted by multiple cell types, have emerged as a novel pattern of intercellular communication [8]. Recently, accumulating evidence has revealed that cancer-derived exosomes play an important role in tumor initiation, progression, metastasis, drug resistance, and angiogenesis due to the vast array of cancer-associated contents, including microRNAs, mRNAs, transcription factors, proteins, and lipids [9]. microRNAs (miRNAs) are a class of endogenous 22- to 25-nt noncoding single-stranded RNA molecules that are stable in exosomes because RNase degradation is prevented [10]. miRNAs have been identified as major players in posttranscriptional gene regulation in diverse biological processes [11].
Our previous study discovered that miR-221-3p induced epithelial-to-mesenchymal transition (EMT) in cancer cells and promoted tumor progression in cervical squamous cell carcinoma (CSCC) [12]. In addition to EMT, local angiogenesis is critical for cancer progression. We further explored whether CSCC-derived miR-221-3p could affect angiogenesis via an exosome carrier.
Materials and methods
Cell culture
Human CSCC cell lines SiHa and C33a, human umbilical vein endothelial cells (HUVECs), and human embryonic kidney 293T cells were purchased from the American Type Culture Collection (ATCC). SiHa, C33a, and 293T cells were cultured in DMEM (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS). HUVECs were cultured in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated FBS.
Transient expression and inhibition of miR-221-3p were performed by transfecting with miRNA oligonucleotides (miR-221-3p mimics, inhibitors, and their negative controls, RIBO Bio.) using Lipofectamine 2000 (Invitrogen). In addition, overexpression of THBS2 in vitro was performed by transfection with pCMV-THBS2(Sino Biological Inc.).
The stable overexpression and inhibition of miR-221-3p in SiHa and C33a cell lines were established by stable transduction using lentivirus according to the manufacturer’s protocol. Lenti-mCherry containing an miR-221-3p overexpression sequence and its negative control vector (miRNA-NC) or containing an miR-221-3p inhibiting segment (si-miR-221-3p) and its negative control vector (si-miRNA-NC) were purchased from GeneChem Inc. The transfected cells were designated both in SiHa and C33a cell lines as following: miRNA-NC, miR-221-3p, si-miRNA-NC, and si-miR-221-3p.
Clinical specimens
The clinical specimens were collected at Nanfang Hospital from 2013 to 2015. Fifteen samples of normal cervical tissue were collected from patients who underwent hysterectomy and were diagnosed with uterine leiomyoma but were not diagnosed with HPV infection or a cervical lesion. Forty-five CSCC samples were collected from patients who underwent abdominal radical hysterectomy (ARH) without prior radiotherapy and chemotherapy. The clinical and pathological characteristics of the cervical cancer specimens are shown in Table 1 and Supplementary Table 1 (Table S1). All samples were submitted for pathological examination after the operation. The study was approved by the Institutional Research Ethics Committee of Southern Medical University. Informed consent was obtained from each patient before collecting samples.
In situ hybridization
In situ hybridization was performed as described previously [13]. Briefly, after incubation with 3% H2O2, digestion with pepsin for 2 min at 37 °C, and fixation with 1% paraformaldehyde (PFA) in diethyl pyrocarbonate (DEPC) for 5 min, the slides were prehybridized in hybridization buffer at 42 °C with miR-221-3p or U6 synthetic oligonucleotide probes (Exiqon). The slides were then incubated with streptavidin–biotin complex (SABC) and horseradish peroxidase (HRP) polymer. Subsequently, slides were stained with 3,3-diaminobenzidine and counterstained with hematoxylin (Sigma). For semiquantitative evaluation of the level of miR-221-3p in tissue, an immunoreactivity “histo” score (H score) was used [14]. First, the staining intensity (0, 1 +, 2+, or 3+) was determined for each cell in a fixed field. Then, the percentage of cells at each staining intensity level was calculated, and an H score was assigned using the following formula: [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3 +)].
Immunohistochemistry
Tissue sections were used for Immunohistochemistry (IHC) as described previously [15]. Mouse anti-human cluster of differentiation (CD31) antibodies (Zsgb-Bio), rat anti-mouse CD31 antibodies (Abcam, ab56299), and rabbit anti-Thrombospondin2 antibodies (Abcam, ab84469) were used as primary antibodies. Horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (Zsgb-Bio) and HRP-conjugated goat anti-rat antibody (Zsgb-Bio) were used as secondary antibodies. In cervical cancer samples, peritumoral vessels were counted and matched with the field of the slide by in situ hybridization. In a mouse tumor model, intratumoral and peritumoral blood vessels were counted in every sample. In addition, the H score system was used to evaluate the relative expression of THBS2.
Exosome isolation
Exosome isolation was performed as previously described [16]. In brief, cancer cells were cultured with exosome-free FBS media and when cells were grown to 70% confluency [17], washed 3 times with PBS, and incubated for 24 h in serum-free media and the supernatant was collected. The supernatant was centrifuged at 3000 × g for 15 min to remove cells and cell debris and it was then mixed with ExoQuick exosome precipitation solution (SBI) and incubated overnight, according to the manufacturer’s protocol. Then, the mixture was centrifuged at 1500 × g for 30 min at 4 °C. The pelleted exosomes were dissolved in phosphate-buffered saline (PBS) and were subsequently split and transferred to RNase-free tubes to be stored or undergo electron microscopy, protein assays, RNA extraction, and to be used for in vitro or in vivo treatment.
Electron microscopy
EM imaging of exosomes was performed as previously reported [18]. Briefly, exosomes were fixed in 2% PFA and absorbed by a Formvar–carbon-coated 400 mesh copper grid (Electron Microscopy Sciences) for 20 min. The grid was postfixed with 1% glutaraldehyde for 5 min. The samples were stained first with uranyl-oxalate solution at pH 7, for 5 min and then with a 9:1 ratio of 2% methyl cellulose at pH 4 and 4% uranyl acetate for 10 min. After the grids were air-dried, micrographs were captured with a CM20 Twin Phillips electron microscope (Phillips) at 80 kV.
Exosome uptake assay
For exosome uptake experiments, exosomes were labeled with a PKH67 Green Fluorescent Cell Linker Kit (Sigma) following the manufacturer’s protocol. 10 µg of exosomes was resuspended in 100 µl PBS and were added to 1 × 105 HUVECs. HUVECs were harvested at different time points (0 h, 6 h, 12 h, 24 h, and 36 h) for qRT-PCR and immunofluorescence analysis. Rhodamine phalloidin (Thermo Fisher) was used to stain actin stress fibers in HUVECs.
qRT-PCR analysis
Total RNA was extracted from cells and exosome samples with TRIzol reagent (Invitrogen). U6 was used as an endogenous control for miRNA, and GAPDH was chosen as the internal control for mRNA. The primer sequences are shown in Table 2. Quantitative mRNA and miRNA expression was measured with ABI Prism 7500 Software v2.0.6 and calculated based on the comparative threshold cycle (CT) method. The expression level of miRNA or mRNA was normalized to that of U6 or GAPDH and converted to fold changes (2− ΔΔCt) and expression as the n-fold difference relative to the control.
Western blotting
Protein samples were prepared using a BCA Protein Assay Kit (Beyotime). A total of 50 µg of protein were separated by 10% SDS–PAGE and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% bovine serum albumin (BSA) for 1 h before being incubated overnight at 4 °C with the primary antibodies, including an Exosomal Marker Antibody Sampler Kit (CST, #74220), anti-THBS2 (Abcam, ab84469), and anti-GAPDH (Abcam, ab9485) antibodies. The membranes were washed three times with PBST and then incubated with HRP-conjugated secondary antibodies (Abcam, ab6721, and ab6789) for 1 h. After the final wash with PBST, the proteins were detected using enhanced chemiluminescence (ECL) reagents (Pierce).
Matrigel tube formation assay
A Matrigel tube formation assay was performed as previously described [19]. In brief, HUVECs were treated with exosomes (10 µg of exosomes resuspended in 100 µl PBS were added to 1 × 105 HUVECs) or were transfected with miRNA oligonucleotides or plasmid for 24 h; HUVECs were then harvested. HUVECs were diluted with serum-free RPMI 1640 and 15,000 cells in 100 µl were added per well to a 96-well culture plate precoated with basement membrane matrix (BD). Then, the plate was incubated at 37 °C for 6 to 8 h. The tube formation was visualized under an inverted microscope. Enclosed networks of tube structures from three randomly chosen fields were recorded under a Leica DC300F microscope (Leica).
Spheroid sprouting assay
A three-dimensional (3D) spheroid sprouting assay was performed as described previously [20]. In brief, HUVECs were treated with exosomes (10 µg of exosomes resuspended in 100 µl PBS were added to 1 × 105 HUVECs) or were transfected with miRNA oligonucleotides or plasmid for 24 h; HUVECs were then harvested. A total of 1 × 105 HUVECs were added to each well of a 96 U-well suspension plate (Corning) in 150 µl of endothelial cell (EC) growth medium-2 (EGM-2) (Lonza), with 20% Methocel (v/v). Cells formed spheroids overnight at 37 °C. Afterward, a solution of 3 mg/ml of rattail collagen type I (BD) was prepared in EGM-2 medium, and the pH was neutralized by 1 M NaOH. Spheroids were suspended with an equivalent solution of collagen type I, deposited over the first layer, and incubated at 37 °C for 1.5 h. After the collagen gels were set, 100 µl of RPMI 1640 medium containing 5% FBS was added to each well, and the spheroids formed sprouts after 48 h. Sprouts were imaged with a Leica TCS SP8-X confocal microscope (Leica) at 100 × magnification.
Transwell migration assay
The upper chambers were rehydrated with RPMI 1640 (serum-free) for 2 h at 37 °C with no Matrigel coating. In the lower chamber, RPMI 1640 medium containing 10% FBS was added as a chemoattractant. HUVECs were treated with exosomes (10 µg of exosomes resuspended in 100 µl PBS were added to 1 × 105 HUVECs) or were transfected with miRNA oligonucleotides or plasmid for 24 h; HUVECs were then harvested and diluted with serum-free RPMI 1640 (1 × 105 cells in 200 µl) and added to the upper compartment of the chamber. After incubation for 24 h, invading cells on the undersides of the membranes were stained with hematoxylin and five random visual fields (200 × ) were counted under a light microscope. Each experiment was repeated three times.
Wound healing assay
HUVECs were treated with exosomes (10 µg of exosomes resuspended in 100 µl PBS were added to 1 × 105 HUVECs) or transfected with miRNA oligonucleotides or plasmid for 24 h; HUVECs were then were harvested and seeded in a 6-well plate at a density of 1 × 105 cells/well. A scratch wound was generated using a sterile 200 µl pipette tip, and floating cells were removed by washing with PBS. Images of the scratches were taken using an inverted microscope at 100 × magnification at 0 h and 24 h after scratching. The percentage of the healed wound area was measured as a ratio of the occupied area to the total area using Image Olympus IX71 (Olympus).
miRNA target prediction
The analysis of miR-221-3p predicted targets was determined by miRwalk (http://zmf.umm.uni-heidelberg.de/) and Pictar (http://pictar.bio.nyu.edu/). Gene Ontology (http://geneontology.org/) was used to identify negative regulation of angiogenesis genes. Then, Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/) was used to combine the data and determine the most probable target. miRanda (http://www.microrna.org) was used to identify the seed sequences and regions of potential base-pairing of the target gene.
Mouse xenograft model
Six-week-old female athymic nude mice were purchased from the Experimental Animal Center at Southern Medical University (Guangzhou, PR China). Animal handling and experimental procedures were approved by the Institutional Animal Research Ethics Committee of Southern Medical University. Tumor cells (SiHa or C33a, 5 × 106 cells/mouse) were implanted subcutaneously into the flank of nude mice. Tumor volume (mm3) was measured every 3 days and calculated by the formula: volume = (width)2 × length/2. When tumors reached a minimal volume of 50 mm3 (approximately 12 days after tumor inoculation), the animals were randomly divided into five groups for further experiments. 10 µg of exosomes resuspended in 20 µl PBS was injected into the center of the xenograft tumors every 3 days [16, 21]. The same volume of PBS was used as a control. After five injections, primary tumors had reached a volume of approximately 300 mm3, and mice were euthanized and tumors were excised for IHC analysis.
Luciferase activity assay
A luciferase activity assay was performed as described previously [22]. In brief, the 3′ UTR segment of the THBS2 gene was amplified by PCR and inserted into the vector. A mutant construct that disrupted the miR-221-3p binding sites of the THBS2 3′ UTR region was also generated using a Quick Change Site-Directed Mutagenesis Kit (Agilent). Cotransfection of THBS2 3′ UTR or mut-THBS2 3′ UTR plasmid with miR-221-3p mimic into the cells was performed using Lipofectamine 2000 (Invitrogen). After 48 h of transfection, luciferase activity was analyzed by a Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
The SPSS (version 20.0) software package was used for statistical analysis. Data are presented as the mean ± standard error of the mean (SEM), and the graphs and diagrams were generated by GraphPad Prism. Statistical analysis between two groups was performed using Student’s t-test, and analysis between multiple groups was conducted by one-way analysis of variance (ANOVA) with the Bonferroni correction. Correlation analyses were performed using the Spearman rank test. Differences were considered statistically significant at P value < 0.05.
Results
miR-221-3p expression positively correlates with microvascular density in CSCC
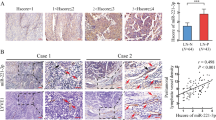
To identify the correlation between miR-221-3p expression and microvascular density(MVD) in CSCC, miR-221-3p was detected by in situ hybridization (ISH), and the blood vessels were stained with the vascular endothelial cell marker CD31 in paraffin-embedded human CSCC serial sections. Normal cervical tissue was used as a negative control. An H score system was established to semiquantitatively assess the expression of miR-221-3p in tissue (Fig. S1). Compared to the low expression of miR-221-3p in normal cervical samples, significantly higher expression of miR-221-3p was detected at the primary tumor sites of CSCC samples (Fig. 1a, c, **P < 0.0001). Correspondingly, the expression of miR-221-3p was positively correlated with an increase in MVD in serial sections of CSCC specimens (Fig. 1B, D, r2 = 0.6544; P < 0.0001). Taken together, these results suggested that miR-221-3p may be an angiogenesis-promoting miRNA in CSCC.
miR-221-3p expression positively correlates with microvascular density in CSCC. a The expression of miR-221-3p was detected in paraffin-embedded normal cervical tissues and CSCC samples by in situ hybridization (ISH). Representative micrographs are shown at × 200 magnification. b In CSCC samples, miR-221-3p was detected by ISH, and blood vessels were stained with the vascular endothelial cell marker CD31 in serial sections. Representative cases with low (Case 1) and high levels (Case 2) of miR-221-3p that were correspondingly stained CD31 are shown (magnification 200 × ). c Scatter diagrams of the H score of miR-221-3p in normal cervical tissues and CSCC samples (30.27 ± 4.036 vs 148.6 ± 9.808, **P < 0.0001). d Correlation analysis of the H score of miR-221-3p and the microvascular density in CSCC tissues (n = 45, r2 = 0.6544, P < 0.0001)
Exosomes transport miR-221-3p from CSCC cells to HUVECs in vitro
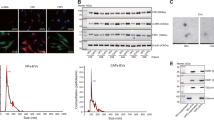
Based on the findings in clinical samples, we also confirmed the relative higher level of miR-221-3p in CSCC cell lines (SiHa and C33a) than in HUVECs (Fig. S2A). We explored how cancer-derived miR-221-3p affected angiogenesis in HUVECs. Previous studies have revealed that exosomes transport functional small RNAs from cancer cells to other stromal cells [25]. Cancer cell-derived exosomes were isolated from the supernatant of two CSCC cell lines, SiHa and C33a, and 30–100 nm cup-shaped exosomes were characterized by the expression of CD9, CD54, and Annexin and by the lack of GM130 expression with western blot analysis (Fig. 2a, b). The uptake of CSCC exosomes into HUVECs was confirmed by the presence of PKH67-labeled exosomes in HUVECs after exosome addition (Fig. 2c).
Exosomes transport miR-221-3p from CSCC to HUVECs in vitro. a Exosomes were isolated from the supernatant of SiHa and C33a cells and the typical cup-shaped morphology and a size range of 30–100 nm were confirmed by transmission electron microscopy (scale bar = 100 nm). b Exosomes (SiHa-Exo and C33a-Exo) were analyzed by western blotting using anti-CD9, anti-CD54, anti-Annexin, and anti-GM130 antibodies. Cellular lysates (SiHa and C33a) were used as positive loading controls. c HUVECs were stained with rhodamine phalloidin (red) and a nuclear marker (DAPI, blue) and viewed under confocal microscopy after treatment with PKH67 labeling exosomes (SiHa-Exo and C33a-Exo) (magnification 1800 × ). d qRT-PCR analysis of the relative expression of miR-221-3p in SiHa cells and their exosomes. The data represent the means ± SEM of triplicates (*P < 0.05). e HUVECs were treated with SiHa cell-derived exosomes for different lengths of time (0 h, 6 h, 12 h, 24 h, and 36 h) and then miR-221-3p was detected by qRT-PCR. The data represent the means ± SEM of triplicates (*P < 0.05; **P < 0.001)
We further investigated whether exosomes transferred miR-221-3p into vessel endothelial cells. First, to prove that miR-221-3p was enriched in CSCC exosomes, stable cell lines that were overexpressing or inhibiting miR-221-3p were established by lentiviral vectors in the SiHa and C33a cell lines (Fig. S2B). We found that the expression of miR-221-3p in CSCC-secreted exosomes was higher than that of their parental cells (Figs. 2d and S3A; *P < 0.05). To confirm the role of exosomes in transporting miR-221-3p from tumor cells to vessel endothelial cells, HUVECs were incubated with exosomes for different lengths of time (0 h, 6 h, 12 h, 24 h, and 36 h) and were harvested for miR-221-3p detection. We found that exosome addition obviously increased the level of miR-221-3p in a time-dependent manner (Figs. 2e, S3B, *P < 0.05, **P < 0.001). These results suggested that CSCC-secreted exosomes were enriched with miR-221-3p from cancer cells and transported miR-221-3p into HUVECs in vitro.
CSCC exosomal miR-221-3p promotes HUVEC angiogenesis in vitro
To investigate the function of CSCC exosomal miR-221-3p in vessel endothelial cells, the angiogenic ability of HUVECs was examined by a Matrigel tube formation assay, spheroid sprouting assay and migration assay. HUVECs were pretreated with miR-221-3p overexpressing exosomes (miR-221-3p-Exo), miR-221-3p-silenced exosomes (si-miR-221-3p-Exo), control group exosomes (miRNA-NC-Exo and si-miRNA-NC-Exo) at a concentrate of 10 µg/1 × 105 cells for 24 h. Treatment with miR-221-3p-overexpressing exosomes induced HUVEC tube-like structure formation in the Matrigel tube formation and spheroid sprouting assays (Figs. 3a, b, S3C, D, **P < 0.001) and cell migration in the transwell migration assay and wound healing assay (Figs. 3c, d, S3E, F, *P < 0.05, **P < 0.001). miR-221-3p-silenced exosomes (si-miR-221-3p) had opposite effects on HUVECs. The similar proliferation level of HUVECs in all groups excluded a proliferation induced effect that promoted angiogenesis (Fig. S3G, H, P > 0.05). To exclude the effect of soluble factors than exosomes on angiogenesis, HUVECs were treated with exosome-free conditioned medium from SiHa cells for 24 h and were then harvested for Matrigel tube formation assay and transwell migration assay. The results revealed that exosome-free conditioned media of CSCC cells had no effect on angiogenesis (Fig. S6). These results reveal that CSCC exosomal miR-221-3p promoted HUVEC angiogenesis in vitro.
CSCC exosomal miR-221-3p promotes HUVEC angiogenesis in vitro. HUVECs were treated with exosomes isolated from SiHa stable cell lines for 24 h before the following assays. The control group was treated with an equal volume of PBS. a An in vitro Matrigel tube formation assay was performed to evaluate the angiogenic ability of HUVECs, and representative micrographs images are shown at 200 × magnification. The number of branches per high-power field was analyzed, and the data represent the means ± SEM of triplicates (*P < 0.05; **P < 0.001). b Representative micrographs of the 3D spheroid sprouting assay (magnification 100 × ). Means of the sproutings per high-power field from three independent experiments were analyzed (*P < 0.05; **P < 0.001). c Representative micrographs of the transwell assay (magnification 100 × ). Invasive cells were calculated per high-power field from three independent experiments (*P < 0.05; **P < 0.001). d Representative micrographs of the wound healing assay. The average migration distance was calculated by the difference of gap widths of the same area. The data represent the means ± SEM of triplicates (*P < 0.05; **P < 0.001)
CSCC exosomal miR-221-3p promotes tumor growth in mouse models
We proved that CSCC exosomal miR-221-3p promoted angiogenesis in HUVECs in vitro. However, the tumor microenvironment (TME) is complicated. We further investigated whether CSCC exosomal miR-221-3p was also pro-angiogenic in the TME. CSCC mouse xenograft tumor models (SiHa and C33a cell lines) were used to investigate the function of exosomal miR-221-3p in vivo. 10 µg of exosomes (miRNA-NC-Exo, miR-221-3p-Exo, si-miRNA-NC-Exo, and si-miR-221-3p-Exo) were injected into the center of tumors every 3 days, starting from the time that the tumor reached the minimal volume of 50 mm3 (approximately 12 days after tumor inoculation) [16, 21]. After five injections, primary tumors reached a volume of approximately 300 mm3, and mice were euthanized and tumors were harvested by surgical removal on day 27. We found that the administration of miR-221-3p-Exo significantly promoted tumor growth (SiHa, Fig. 4a–c, *P < 0.05, **P < 0.001; C33a, Fig. S4A-C, *P < 0.05, **P < 0.001). The tumor volume in the SiHa miR-221-3p-Exo group increased from 62.67 ± 11.23 mm3 to 376.33 ± 21.56 mm3 during exosome treatment, whereas the tumor volume in the SiHa miRNA-NC group increased from 54.00 ± 8.54 mm3 to 313.33 ± 20.81 mm3 (Fig. 4a, **P < 0.001). Tumor volume increased from 54.33 ± 7. 09 mm3 to 318.33 ± 18.92 mm3 in the C33a miR-221-3p-Exo group and from 51.67 ± 7.64 mm3 to 251.67 ± 10.41 mm3 in the C33a miRNA-NC group (Fig. S4A, **P < 0.001). In contrast, when miR-221-3p was silenced in exosomes (si-miR-221-3p-Exo), the growth of the tumor was repressed (Figs. 4a, S4A, *P < 0.05). The blood vessels were evaluated in tumors by IHC using anti-CD31 antibody. We found that miR-221-3p-Exo significantly promoted angiogenesis at both peritumoral (SiHa, Fig. 4d, 7.6 ± 0.54 vs 5.6 ± 0.54, **P < 0.001; C33a, Fig. S4D, 8.4 ± 0.89 vs 5.6 ± 1.14, **P < 0.001) and intratumoral sites (SiHa, Fig. 4d; 7.8 ± 0.83 vs 4.8 ± 0.83, **P < 0.001; C33a, Fig. S4D, 7.8 ± 0.83 vs 5.6 ± 0.55, **P < 0.001) compared to that of the miRNA-NC-Exo group. In addition, there was no significant difference between the control groups (miRNA-NC-Exo, si-miRNA-NC and PBS). Together, these results indicated that CSCC exosomal miR-221-3p significantly increased blood vessel formation and promoted tumor growth in vivo.
CSCC exosomal miR-221-3p promotes tumor growth in mouse models. a Growth curves of tumors (SiHa) were generated by measuring tumor volumes every three days (*P < 0.05; **P < 0.001). Arrows mark that intratumoral exosome injection occurred at the indicated times. An equal volume of PBS was injected as a blank control. b Images of tumors excised from mice (n = 3). c Means of the weight of tumors. The data represent the means ± SEM of triplicates (*P < 0.05; **P < 0.001). d The blood vessels in tumors were detected by immunohistochemistry using an anti-CD31 antibody. The peritumoral (black arrows, magnification 200 × ) and intratumoral (red arrows, magnification 400 × ) CD31 + vessels were measured. The data represent the means ± SEM of triplicates (**P < 0.001)
THBS2 is a direct target of miR-221-3p
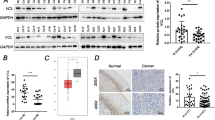
As miRNAs mainly inhibit target genes at the posttranscriptional level, the negative regulation of angiogenic genes regulated by miR-221-3p was our candidates of interest. Using bioinformatic tools (miRWalk and PicTar databases), 61 candidate genes were identified as the most probable downstream target genes of miR-221-3p (Fig. 5a). Gene Ontology analyses indicated that there were 401 anti- angiogenic genes. By combining the data presented above using Venny 2.1.0, THBS2 was identified as a putative miR-221-3p target gene involved with the process of angiogenesis (Fig. 5a), and the presence of seed regions with potential base-pairing with miR-221-3p in the 3′ UTRs of THBS2 mRNA were verified by miRanda analysis (http://www.microrna.org) (Fig. 5b).
THBS2 is a direct target of miR-221-3p. a Venn diagram of predicted miR-221-3p targets by three programs (miRwalk, PiTar, and Gene Ontology). b The seed regions of miR-221-3p, the seed-recognizing sites in the THBS2 3′ UTR, and the nucleotides mutated in THBS2 mutant 3′ UTR are shown. c Luciferase activity of wild-type 3′ UTR-THBS2-luc and mutant 3′ UTR-THBS2-luc constructs in HUVECs and 293T cells after transfection with miR-221-3p oligonucleotides. The data represent the means ± SEM of triplicates (*P < 0.05). d HUVECs were transfected with mimics-NC, inhibitor-NC, miR-221-3p mimics, and miR-221-3p inhibitor. The expression of miR-221-3p was detected by qRT-PCR after transfection. The data represent the means ± SEM of triplicates (*P < 0.05). e qRT-PCR analysis of THBS2 mRNA in HUVECs after transfection. The data represent the means ± SEM of triplicates (*P < 0.05). f Western blotting analysis of THBS2 protein in HUVECs after transfection. GAPDH was used as a loading control. g To further confirm the regulatory effect of exosomal miR-221-3p on THBS2 in vivo, the expression of THBS2 in a mouse xenograft model was also detected by IHC (magnification 200 × ). h The expression of THBS2 was analyzed by the H score system. The data represent the means ± SEM of triplicates (*P < 0.05)
To confirm this prediction, a 1187-bp fragment of the 3′ UTR region of THBS2 mRNA that was a putative binding site for miR-221-3p was subcloned and inserted into a luciferase reporter plasmid. miR-221-3p binding sites in the 3′ UTR region of THBS2 were mutated to obtain the 3′ UTR-Mut THBS2-luc plasmid (Fig. 5b). Transient transfection of wild-type THBS2-luc reporter with miR-221-3p mimic into 293T cells and HUVECs led to a significant decrease in luciferase activity compared with that of control groups (Fig. 5c; *P < 0.05). However, miR-221-3p did not decrease the luciferase activity of the mutant construct-3′ UTR-Mut THBS2-luc (Fig. 5c).
To further confirm the regulatory effect of miR-221-3p on THBS2, miR-221-3p mimics, inhibitors, and negative controls were transfected into HUVECs. The expression of miR-221-3p in HUVECs after transfection was confirmed by qRT-PCR (Fig. 5D, *P < 0.05). We found that miR-221-3p mimics decreased the expression level of THBS2 at both the mRNA (Fig. 5e, *P < 0.05) and protein levels (Fig. 5f, Fig. S5A), but the miR-221-3p inhibitor increased the expression levels (Fig. 5e, f, Fig. S5A). Similarly, in vivo, miR-221-3p manipulation of THBS2 was also observed in mouse tumors (Fig. 5g, h, *P < 0.05; Fig. S4E, *P < 0.05). These results verified that miR-221-3p downregulated the expression of THBS2 by directly binding to the 3′ UTR region of THBS2 mRNA.
Overexpressing THBS2 rescues the angiogenic effect of miR-221-3p
To further investigate whether overexpressing THBS2 could rescue the biological effects of miR-221-3p, HUVECs were transfected with miR-221-3p mimics, mimics-NC, pCMV-THBS2 plasmid, pCMV-NC plasmid, and miR-221-3p mimics plus pCMV-THBS2 plasmid. The expression of THBS2 mRNA and protein was significantly upregulated upon transfection with the pCMV-THBS2 plasmid (Fig. 6a, b, *P < 0.05). In the Matrigel tube formation assay, 3D spheroid sprouting assay, wound healing assay, and transwell assay, miR-221-3p significantly increased angiogenesis in vitro (Fig. 6c–f, *P < 0.05). However, overexpression of THBS2 in HUVECs significantly decreased the angiogenic effects of miR-221-3p (Fig. 6c–f, *P < 0.05). These findings revealed that overexpressing THBS2 could rescue the angiogenic function of miR-221-3p in HUVECs. Additional evidence supported that miR-221-3p promoted angiogenesis by downregulating THBS2.
Overexpressing THBS2 rescues the angiogenic effect of miR-221-3p. HUVECs were transfected with mimics-NC, miR-221-3p mimic, pCMV-NC plasmids, pCMV-THBS2 plasmids, and miR-22-3p mimics plus pCMV-THBS2 plasmids. An equal volume of PBS was used as a blank control. a Western blot analysis of THBS2 protein in HUVECs after transfection. GAPDH was used as a loading control. b The expression of THBS2 mRNA in HUVECs after transfection was detected by qRT-PCR. The data represent the means ± SEM of triplicates (*P < 0.05; **P < 0.001). c Representative micrographs of the in vitro Matrigel tube formation assay (magnification 200 × ). The number of branches per high-power field was analyzed and the data represent the means ± SEM of triplicates (*P < 0.05). d Representative micrographs of the 3D spheroid sprouting assay (magnification 100 × ). Means of the sproutings per high-power field were analyzed (*P < 0.05). e Representative micrographs of the transwell assay (magnification 100 × ). Invasive cells per high-power field from three independent experiments were calculated (*P < 0.05). f Representative micrographs of the wound healing assay. The average migration distance was calculated by the difference of gap widths of the same area. The data represent the means ± SEM of triplicates (*P < 0.05)
Discussion
Angiogenesis is critical to cervical cancer development and progression [23]. When tumor size exceeds 1 to 2 mm in diameter, angiogenetic factors activate the angiogenic network and result in the sprouting of blood vessels from the surrounding tissues and into the tumor [24]. Anti-angiogenic therapy has been proven to be an effective treatment strategy for advanced or recurrent cervical cancer patients [25], and anti-VEGF strategy effectively improves progression-free survival. However, the single target and potential resistance may limit the efficiency of anti-angiogenesis [3]. Recently, because their expression profile potentially reflected deregulated expression patterns in multiple human cancer types and high stability in serum [26], we investigated cancer-derived exosomal miRNAs as biomarkers of angiogenesis in CSCC. In our study, we initially found that miR-221-3p was upregulated in CSCC patients and that miR-221-3p expression positively correlated with the microvascular density of tumors. Second, cancer-resourced exosomes transported miR-221-3p from CSCC cells to vessel endothelial cells, and exosomal miR-221-3p significantly induced angiogenesis to promote tumor growth. Finally, miR-221-3p manipulated THBS2 in vessel endothelial cells to promote angiogenesis.
We first found that, in cervical cancer, CSCC-derived miR-221-3p directly affected vessel endothelial cells to promote angiogenesis by downregulating THBS2 in vessel endothelial cells. In previous reports, endogenous miR-221-3p of endothelial cells was found to have a pro-angiogenic function in metastatic renal cell carcinoma [27], hepatocellular carcinoma [28], and even in embryogenesis [29], with the involvement of c-kit molecules and PI3K-AKT pathways [30]. Our studies demonstrated that exogenous CSCC-derived miR-221-3p entered vessel endothelial cells and directly promoted angiogenesis, similar to the endogenous miR-221-3p.
The short life span of and obstacles to cell entrys of exogenous miRNA in serum restricts persistent function, and it is difficult to detect reliably. The exosomes were found to be mediating the transport of cellular communications both locally and distally [31, 32]. Cancer cells secrete millions of exosomes containing a wide variety of miRNAs, and exosome carriers protect them from degradation and phagocytosis and help them to enter into other cancer cells and normal cells, then allowing them to reprogram their surroundings to generate tumor-promoting microenvironment [26]. Our study has shown that miR-221-3p is enriched in CSCC exosomes. CSCC-derived exosomes carried exogenous miR-221-3p into vessel endothelial cells. After encapsulation in exosomes, exogenous CSCC-derived miR-221-3p was successfully transferred into HUVECs and induced angiogenesis in vivo and in vitro. The long-lived exosomal miRNA-221-3p in serum is a potential biomarker and therapeutic target for diagnosis and therapy.
In previous study, the pro-angiogenic properties of endogenous miR-221-3p involved c-kit and PI3K-AKT in vessel endothelial cells [30]. We first identified that the miR-221-3p-THBS2 axis in HUVECs promoted angiogenesis and tumor growth in a cervical cancer model. THBS2 has been found to be a potent endogenous inhibitor of angiogenesis [33]. As it was involved with activation of the PI3K-AKT pathway, THBS2 suppressed VEGF and nitric oxide (NO) [34], harnessed endothelial cell migration, and induced endothelial cell apoptosis [35]. THBS2 knockout mice had a complex phenotype characterized chiefly by abnormalities in blood vessels [36]. The newly discovered axis implicated miR-221-3p in multiple regulatory functions in angiogenesis.
In addition, our previous study suggested that the miR-221-3p-THBS2 axis stimulated EMT in cancer cells and promoted tumor progression [12]. This axis increased stromal cell trophoblast growth and migration [37]. In addition to THBS2 in HUVECs, our group also found that CSCC exosomal miR-221-3p enhanced lymphangiogenesis and lymphatic metastasis, by manipulating VASH-1 in lymph endothelial cells [16]. In summary, multiple mechanisms involving CSCC exosomal miR-221-3p enhanced the pro-tumor microenvironment that promoted tumor growth and metastasis. Therefore, CSCC exosomal miR-221-3p could be a possible novel diagnostic biomarker and therapeutic target for CSCC progression.
Many other studies have also shown that other exosomal miRNAs derived from cancer cells have angiogenic potential in several types of cancer. For example, Umezu et al. reported that multiple myeloma-derived exosomes in hypoxic bone marrow contain high levels of miR-135b and that exosomal miR-135b from hypoxia-resistant multiple myeloma cells enhanced angiogenesis [38]. miR-23a from cancer cell-derived exosomes increased angiogenesis and vascular permeability in lung cancer [39] and mediated angiogenesis and promoted metastasis in nasopharyngeal carcinoma [40].The cancer-derived exosomal miRNA may be potential biomarker and therapeutic targets. Moreover, some studies reported that the growth factors(EGF, HGF) and cell confluency could affect the cancer exosomal miRNA secretion, such as nonsmall cell lung cancer [41], renal cancer [42], Hepatocellular carcinoma [43], colorectal cancer cells, cervical cancer, and hepatocellular carcinoma [44], even in the renal tubular cells [45], which need thoughtful attention to cancer-exosomal miRNAs in different conditions.
Conclusion
In summary, we found that CSCC exosomes transferred miR-221-3p from cancer cells to vessel endothelial cells and promoted angiogenesis by downregulating THBS2. CSCC-derived exosomal miR-221-3p could be a possible novel diagnostic biomarker and therapeutic target for CSCC progression.
Change history
19 December 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10456-022-09864-3
Abbreviations
- VEGF:
-
Vascular endothelial growth factor
- EM:
-
Electron microscopy
- CSCC:
-
Cervical squamous cell carcinoma
- THBS2:
-
Thrombospondin-2
- NC:
-
Negative control
- HUVEC:
-
Human umbilical vein endothelial cell
- qRT-PCR:
-
Quantitative real-time reverse transcriptase-polymerase chain reaction
References
Shrestha AD, Neupane D, Vedsted P, Kallestrup P (2018) Cervical cancer prevalence, incidence and mortality in low and middle income countries: a systematic review. Asian Pac J Cancer Prevent 19(2):319–324
Li H, Wu X: Advances in diagnosis and treatment of metastatic cervical cancer. 2016, 27(4):e43
Jayson GC, Kerbel R, Ellis LM, Harris AL (2016) Antiangiogenic therapy in oncology: current status and future directions. Lancet 388(10043):518–529
Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM et al (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370(8):734–743
Alldredge JK, Tewari KS (2016) Clinical trials of antiangiogenesis therapy in recurrent/persistent and metastatic cervical cancer. Oncologist 21(5):576–585
Symonds RP, Gourley C, Davidson S, Carty K, McCartney E, Rai D, Banerjee S, Jackson D, Lord R, McCormack M et al (2015) Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CIRCCa): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol 16(15):1515–1524
Svensson KJ, Belting M (2013) Role of extracellular membrane vesicles in intercellular communication of the tumour microenvironment. Biochem Soc Trans 41(1):273–276
van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G (2013) Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev 65(3):331–335
Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W (2015) Exosomes in cancer: small particle, big player. J Hematol Oncol 8:83
Falcone G, Felsani A, D’Agnano I (2015) Signaling by exosomal microRNAs in cancer. J Exp Clin Cancer Res 34:32
Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M (2012) microRNAs in cancer management. Lancet Oncol 13(6):e249–e258
Wei WF, Zhou CF, Wu XG, He LN, Wu LF, Chen XJ, Yan RM, Zhong M, Yu YH, Liang L et al (2017) MicroRNA-221-3p, a TWIST2 target, promotes cervical cancer metastasis by directly targeting THBS2. Cell Death Dis 8(12):3220
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR et al (2014) Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25(4):501–515
Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C, Franklin WA (2003) Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 21(20):3798–3807
Wei WF, Han LF, Liu D, Wu LF, Chen XJ, Yi HY, Wu XG, Zhong M, Yu YH, Liang L et al (2017) Orthotopic xenograft mouse model of cervical cancer for studying the role of MicroRNA-21 in promoting lymph node metastasis. Int J Gynecol Cancer 27(8):1587–1595
Zhou CF, Ma J, Huang L, Yi HY, Zhang YM, Wu XG, Yan RM, Liang L, Zhong M, Yu YH et al (2018) Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene 38:1256–1268
Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chap. 3:Unit 3.22
Chan YK, Zhang H, Liu P, Tsao SW, Lung ML, Mak NK, Ngok-Shun Wong R, Ying-Kit Yue P (2015) Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. Int J Cancer 137(8):1830–1841
Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van Beijnum JR, Bender RHF et al (2018) Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 21:425–532
Maracle CX, Kucharzewska P, Helder B, van der Horst C, Correa de Sampaio P, Noort AR, van Zoest K, Griffioen AW, Olsson H, Tas SW (2017) Targeting non-canonical nuclear factor-kappaB signalling attenuates neovascularization in a novel 3D model of rheumatoid arthritis synovial angiogenesis. Rheumatology 56(2):294–302
Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, Li X, Chen J, Liu K, Li C et al (2016) Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res 76(7):1770–1780
Huang TH, Chu TY (2014) Repression of miR-126 and upregulation of adrenomedullin in the stromal endothelium by cancer-stromal cross talks confers angiogenesis of cervical cancer. Oncogene 33(28):3636–3647
Yang P, Chen N, Yang D, Crane J, Huang B, Dong R, Yi X, Guo J, Cai J, Wang Z (2017) Cervical cancer cell-derived angiopoietins promote tumor progression. Tumour Biol 39(7):1010428317711658
Hoff PM, Machado KK (2012) Role of angiogenesis in the pathogenesis of cancer. Cancer Treatm Rev 38(7):825–833
Tomao F, Papa A, Rossi L, Zaccarelli E, Caruso D, Zoratto F, Benedetti Panici P, Tomao S (2014) Angiogenesis and antiangiogenic agents in cervical cancer. OncoTargets Therapy 7:2237–2248
Kalluri R (2016) The biology and function of exosomes in cancer. J Clin Invest 126(4):1208–1215
Garcia-Donas J, Beuselinck B, Inglada-Perez L, Grana O, Schoffski P, Wozniak A, Bechter O, Apellaniz-Ruiz M, Leandro-Garcia LJ, Esteban E et al (2016) Deep sequencing reveals microRNAs predictive of antiangiogenic drug response. JCI Insight 1(10):e86051
Gramantieri L, Fornari F, Callegari E, Sabbioni S, Lanza G, Croce CM, Bolondi L, Negrini M (2008) MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med 12(6a):2189–2204
Nicoli S, Knyphausen CP, Zhu LJ, Lakshmanan A, Lawson ND (2012) miR-221 is required for endothelial tip cell behaviors during vascular development. Dev cell 22(2):418–429
Urbich C, Kuehbacher A, Dimmeler S (2008) Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res 79(4):581–588
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM (2015) Exosome mediated communication within the tumor microenvironment. J Control Release 219:278–294
Meehan K, Vella LJ (2016) The contribution of tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin Lab Sci 53(2):121–131
Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M (1999) Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA 96(26):14888–14893
Simantov R, Febbraio M, Silverstein RL (2005) The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol 24(1):27–34
Koch M, Hussein F, Woeste A, Grundker C, Frontzek K, Emons G, Hawighorst T (2011) CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res Treatm 128(2):337–346
Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P (1999) Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc Natl Acad Sci USA 96(8):4449–4454
Yang Y, Li H, Ma Y, Zhu X, Zhang S, Li J (2018) MiR-221-3p is down-regulated in preeclampsia and affects trophoblast growth, invasion and migration partly via targeting thrombospondin 2. Biomed Pharmacother 109:127–134
Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH (2014) Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 124(25):3748–3757
Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL (2017) Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 36(34):4929–4942
Bao L, You B, Shi S, Shan Y, Zhang Q, Yue H, Zhang J, Zhang W, Shi Y, Liu Y et al: Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. 2018, 37(21):2873–2889
Garofalo M, Romano G, Di Leva G, Nuovo G, Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G et al (2011) EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med 18(1):74–82
Teixeira AL, Dias F, Ferreira M, Gomes M, Santos JI, Lobo F, Mauricio J, Machado JC, Medeiros R (2014) Combined influence of EGF + 61G> A and TGFB + 869T> C functional polymorphisms in renal cell carcinoma progression and overall survival: the link to plasma circulating MiR-7 and MiR-221/222 expression. PloS ONE 10(4):e0103258
de Conti A, Ortega JF, Tryndyak V, Dreval K, Moreno FS, Rusyn I, Beland FA, Pogribny IP (2017) MicroRNA deregulation in nonalcoholic steatohepatitis-associated liver carcinogenesis. Oncotarget 8(51):88517–88528
Yoon S, Kovalenko A, Bogdanov K, Wallach D (2017) MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity 47(1):51–65.e57
Zhou X, Zhang W, Yao Q, Zhang H, Dong G, Zhang M, Liu Y, Chen JK, Dong Z (2017) Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am J Phys Renal Physiol 312(6):F963–Ff970
Funding
This work was supported by the National Natural Science Foundation of China [Grant Nos.: 81672589, 81372781, 81304078], the Shenzhen Science and Technology Programme [Grant No.: JCYJ20160429161218745], the National Key Research and Development Program of China [2016YFC1302901], and the Natural Science foundation of Guangdong province [Grant Nos.: 2017A030313872, 2018A030313804] The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
The study was approved by the Institutional Research Ethics Committee of Southern Medical University. Informed consent was obtained from each patient before collecting samples.
Additional information
Communicated by V. Loia.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s10456-022-09864-3"
Electronic supplementary material
Below is the link to the electronic supplementary material.
10456_2019_9665_MOESM1_ESM.tif
Supplementary Figure 1. H score system for miR-221-3p expression. The H score system was established to semiquantitatively assess the expression of miR-221-3p in paraffin embedded samples. Representative images of different score groups are shown (magnification 200 × ). (TIF 7869 KB)
10456_2019_9665_MOESM2_ESM.tif
Supplementary Figure 2. Stable cell lines were established by lentivirus. (a) The expression of miR-221-3p in CSCC cell lines (MS751, ME180, Caski, C33a and SiHa) and vessel endothelial cells (HUVECs) was detected by qRT-PCR. (b) The stable overexpression or silenced miR-221-3p SiHa and C33a cell lines were established by stable transduction with lentivirus (mCherry labeled): miRNA-NC lentivirus, miR-221-3p lentivirus, si-miRNA-NC lentivirus and miR-221-3p inhibitor lentivirus (si-miR-221-3p). The stable cell lines were imaged by light microscope and using the mCherry fluorescence channels (magnification 200 × ). (TIF 5452 KB)
10456_2019_9665_MOESM3_ESM.tif
Supplementary Figure 3. C33a-secreted exosomes transfer miR-221-3p into HUVEC and promote angiogenesis in vitro. (a) qRT-PCR analysis of the relative expression of miR-221-3p in C33a cells and their exosomes. The data represent the means ± SEM of triplicates (*P < 0.05). (b) HUVECs were treated with C33a cell-derived exosomes for different lengths of time (0 h, 6 h, 12 h, 24 h and 36 h) and then miR-221-3p was detected by qRT-PCR. The data represent the means ± SEM of triplicates (*P < 0.05). (c) HUVECs were treated with exosomes isolated from C33a stable cell lines for 24 h before the following assays. The control group (PBS) was treated with an equal volume of PBS. Representative micrographs of Matrigel tube formation assay are shown at 200 × magnification. The number of branches per high-power field was analyzed (*P < 0.05; **P < 0.001). (d) Representative micrographs of the 3D spheroid sprouting assay (magnification 100 × ). Means of the sproutings per high-power field from three independent experiments were analyzed (*P < 0.05; **P < 0.001). (e) Representative micrographs of the transwell assay (magnification 100 × ). Invasive cells were calculated per high-power field from three independent experiments (*P < 0.05). (f) Representative micrographs of the wound healing assay. The average migration distance was calculated by the difference of gap widths of the same area. The data represent the means ± SEM of triplicates (*P < 0.05; **P < 0.001). (g-h) The proliferation rate of HUVECs treated with SiHa and C33a cell-derived exosomes were detected by Cell Counting Kit-8 assay. (TIF 17336 KB)
10456_2019_9665_MOESM4_ESM.tif
Supplementary Figure 4. C33a-derived exosomal miR-221-3p promotes tumor growth in mouse models. (a) Growth curves of tumors (C33a) were generated by measuring tumor volumes every three days (*P < 0.05; **P < 0.001). Arrows mark that intratumoral exosome injection occurred at the indicated times. An equal volume of PBS was injected as a blank control (PBS). (b) Images of tumors excised from mice (n=3). (c) Means of the weight of tumors. The data represent the means ± SEM of triplicates (*P < 0.05; **P < 0.001). (d) The blood vessels in tumors were detected by IHC using an anti-CD31 antibody. The peritumoral (black arrows, magnification 200 × ) and intratumoral (red arrows, magnification 400 × ) CD31+ vessels were measured. The data represent the means ± SEM of triplicates (*P < 0.05). (e) To further confirm the regulatory effect of exosomal miR-221-3p on THBS2 in vivo, the expression of THBS2 in mouse xnograft model was also detected by IHC (magnification 200 × ) and was analyzed by H score system (*P < 0.05). (TIF 12287 KB)
10456_2019_9665_MOESM5_ESM.tif
Supplementary Figure 5. miR-221-3p represses the expression of THBS2 in HUVECs. (a) HUVECs were transfected with miR-221-3p oligonucleotides and viewed under confocal microscopy. Representative micrographs of HUVECs stained with THBS2 (green) and a nuclear marker (DAPI, blue) (magnification 1200 × ). (TIF 3201 KB)
10456_2019_9665_MOESM6_ESM.tif
Supplementary Figure 6. Exosome-free conditioned media of CSCC cells has no effect on the angiogenic ability of HUVECs. Exosome-free conditioned media was isolated from supernatants of different groups of SiHa cell lines by ultracentrifugation. HUVECs were treated with different groups of exosome-free conditioned media for 24 h and then harvested for a Matrigel tube formation assay and transwell migration assay. The same volume of RPMI 1640 culture media was used as a blank control (Ctrl). (a) Representative micrographs of the Matrigel tube formation assay are shown at 200 × magnification. The number of branches per high-power field was analyzed (P > 0.05). (b) Representative micrographs of the transwell migration assay (magnification 100 × ). Invasive cells were calculated per high-power field from three independent experiments (P > 0.05). (TIF 6760 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, XG., Zhou, CF., Zhang, YM. et al. RETRACTED ARTICLE: Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 22, 397–410 (2019). https://doi.org/10.1007/s10456-019-09665-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-019-09665-1