Abstract
Tumor growth depends on angiogenesis and inducing angiogenesis is one of the most important hallmarks in the cancer development. Treatment with small molecules that inhibit angiogenesis has been an effective strategy for anti-cancer therapy. Some anti-angiogenic factors are derived from traditional Chinese herbs. Usnic acid (UA), an active compound mainly found in lichens, has shown some biological and physiological activities. However, the role and mechanism of UA in tumor angiogenesis are still unknown. The aim of this study was to assess the effects of UA on tumor angiogenesis. In this study, we demonstrated that UA strongly inhibited in vivo angiogenesis in a chick embryo chorioallantoic membrane assay and vascular endothelial growth factor-induced mouse corneal angiogenesis model. In a mouse xenograft tumor model, UA suppressed Bcap-37 breast tumor growth and angiogenesis without affecting mice body weight. In an in vitro assay, UA not only significantly inhibited endothelial cell proliferation, migration and tube formation, but also induced morphological changes and apoptosis in endothelial cells. In addition, UA inhibited Bcap-37 tumor cell proliferation. Moreover, western blot analysis of cell signaling molecules indicated that UA blocked vascular endothelial growth factor receptor (VEGFR) 2 mediated Extracellular signal-regulated protein kinases 1 and 2(ERK1/2) and AKT/P70S6K signaling pathways in endothelial cells. These results provided the first evidence of the biological function and molecular mechanism of UA in tumor angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis is the process of generating new blood vessels and plays a critical role in the growth of solid tumors by supplying nutrients and oxygen and removing waste products from the tumor [1]. Angiogenesis also plays essential roles in tumor invasion and metastasis [2]. Thus, anti-angiogenesis is an attractive strategy for antitumor treatment.
The angiogenic signaling pathway is mediated by various pro- and anti-angiogenic factors that ultimately lead to neovascularization. It is well known that vascular endothelial growth factor (VEGF) plays a pivotal role during the angiogenesis process [3, 4]. VEGF exerts its biological effect mainly via VEGF receptor 2 (VEGFR2, also known as KDR/Flk-1)-mediated signaling pathways. Activation of VEGFR2 leads to the activation of various downstream signal transduction proteins including extracellular signal-regulated kinase (ERK) [5], AKT (also known as Protein Kinase B) [6], Focal Adhesion Kinase (FAK) and Src family kinase [7, 8]. The AKT/Protein Kinase B (PKB) signaling pathway regulates endothelial cell functions such as migration, proliferation and apoptosis [6, 9]. In addition, ERK1/2 activity has been implicated in diverse cellular activities including cell proliferation, differentiation, migration and cell death [10, 11]. For these reasons, VEGF and its receptor signaling system are attractive targets for therapeutic intervention of tumor. Indeed, some small molecule inhibitors of VEGFR2 such as sunitinib [12] and sorafenib [13], have been approved by the Food and Drug Administration for treating tumors.
Usnic acid (UA, Fig. 1a), a dibenzofuran derivative, is an active compound mainly found in lichens [14]. Previous study shows that UA exhibits several interesting properties such as anti-microbial, anti-viral, anti-inflammatory and anti-proliferative activities [15]. Moreover, other studies indicate its anti-tumor activities via different mechanisms in various cell types [16–19]. However, the role of UA in tumor angiogenesis and the related mechanism in vascular endothelial cell have not been reported.
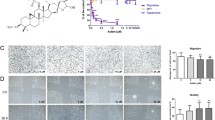
UA inhibits angiogenesis in vivo. a Chemical structure of UA with the molecular weight of 344.32. b UA inhibited the formation of new blood vessel branches in a CAM assay. Left panel, representative CAMs of control and UA-treated groups. The middle circle in the left panel is the filter paper that served as a carrier. Black indicates neovascularization in the CAM. Right panel, quantification of blood vessel branching in the CAM assay (n = 10). % is proportional to the control group. **P < 0.01. c UA inhibited VEGF-induced mouse corneal angiogenesis. Green arrows indicate the location of implanted pellets and yellow arrows indicate neovascularization. d Three parameters were quantified in the mouse cornea assay, including clock number, vessel length and area. (n = 10), *P < 0.05; **P < 0.01. (Color figure online)
In this study, we investigated the functional roles of UA in angiogenesis and breast tumor growth as well as its potential mechanism. We found that UA suppressed angiogenesis in the chick embryo chorioallantoic membrane (CAM) assay and mouse corneal micropocket model in vivo and demonstrated that UA significantly inhibited Bcap-37 breast tumor growth and angiogenesis in a xenograft tumor model. We also found that UA inhibited human umbilical vascular endothelial cells (HUVEC) proliferation, migration and tube formation in vitro. Finally, we showed that UA inhibited angiogenesis and Bcap-37 tumor growth via VEGFR2-mediated AKT and ERK1/2 signaling pathways.
Materials and methods
Cell lines, animals, and reagents
Human umbilical vascular endothelial cells (HUVECs) were obtained ScienCell Research Laboratories (San Diego, CA) and cultured in completed endothelial cell medium [20]. The human breast tumor cell line Bcap-37 was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) [21] and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % fetal bovine serum (FBS, Invitrogen, Carlsbad, CA). Both HUVECs and Bcap-37 cells were cultured at 37 °C in a humidified atmosphere containing 5 % CO2.
Fertilized chicken eggs were purchased from Shanghai Poultry Breeding Co. Ltd (Shanghai, China). C57BL/6 and nude mice were purchased from National Rodent Laboratory Animal Resources (Shanghai, China). Animals were maintained in accordance with the current regulations and standards of the United States National Institutes of Health. All experimental protocols were approved by the Animal Investigation Committee of the Institute of Biomedical Sciences, East China Normal University.
Usnic acid was 98 % pure and obtained from Sigma-Aldrich (St. Louis, MO), 10 mM solution of UA was prepared and protected from light at −20 °C and then diluted as needed concentrations in cell culture medium. Recombinant human VEGF (VEGF165) was from R&D System (Minneapolis, MN). Matrigel was purchased from BD Biosciences (San Jose, CA). Poly (2-hydroxyethyl methacrylate) (poly-HEMA) and an antibody against β-actin were purchased from Sigma-Aldrich. Antibodies for western blotting were purchased from Cell Signaling Technology.
Chick embryo chorioallantoic membrane (CAM) assay
The in vivo anti-angiogenic activity of UA was assessed by a CAM assay as described elsewhere [22]. Briefly, embryonic eggs were placed in a humidified incubator. After incubation for 5 days at 37 °C with 60 % relative humidity, a 1–2 cm2 window was opened at the blunt end of the eggs and the shell membrane was removed to expose the CAM. Then, a sterilized 5 mm diameter filter paper disk (Whatman, NJ, USA) with UA or dimethyl sulfoxide (DMSO) was placed on the CAM. The window was sealed and the egg was returned to the incubator. After further incubation for 2 days, the CAM microvessels were observed under a stereomicroscope, and the neovascularization was quantified using Image Pro Plus software.
Mouse corneal micropocket assay
A mouse corneal assay was performed according to a published method [23] with some modification. Briefly, the slow-release pellets (0.35 × 0.35 mm) containing 320 ng VEGF were prepared with a sucrose octasulfate-aluminum complex and poly -HEMA. A corneal micropocket was created in one eye of each 4–5-week-old C57BL/6 mouse with a modified needle, and then pellets were implanted into mouse corneal micropockets. Chlortetracycline hydrochloride ophthalmic ointment was applied to each operated eye to prevent infection. Then, UA-treated mice were injected with 50 mg UA/kg/day intraperitoneally every day, while the control group was treated with DMSO only. After 7 days, the vessel length and clock hours of new blood vessels were examined under a stereomicroscope and recorded. The area of neovasculature was calculated according to the formula: Area (mm2) = 0.2 × π × VL (mm) × CN (mm), where the VL is the maximal vessel length extending from the limbal vasculature toward the pellet and CN is the clock hours of neovascularization, 1 clock hour equals 30 degrees of arc.
Mouse xenograft model and immunohistochemistry
A mouse xenograft model was established as described elsewhere [24]. Briefly, 5 × 106 Bcap-37 cells were subcutaneously on the right sides of the dorsal area of 5-week-old female nude mice. After tumors grew to about 200 mm3, mice were randomly divided into two groups (n = 6) and treated intralesionally with or without UA (60 mg/kg/day). The tumor size and the body weight of each mouse were recorded every day. At the same time, solid tumor volume was determined using digital vernier caliper measurements and the formula: A × B2 × 0.52, where A is the longest diameter of the tumor and B is the shortest diameter of the tumor. After 22 days, mice were sacrificed and tumors were removed, fixed with formaldehyde and embedded in paraffin. Specific blood vessel staining was performed with an anti-CD31 antibody according to the protocol for the blood staining kit. Images were recorded using a Leica DM 4000B photomicroscope. Using Image-Pro Plus 6.0 software, we analyzed the mean integrated optical density (mean IOD) of blood vessels in tumor sections according the following formula: mean IOD = IOD/area of the tumor section.
Endothelial cell wound-healing migration assay
Based on a previously published method [25], HUVECs were seeded in 6 well plates coated with 0.1 % gelatin (Sigma-Aldrich) and cultured to confluency. After 2 μg/ml mitomycin C treatment for 2 h, the cells were scratched with a 1 ml pipette tip and washed three times with phosphate-buffered saline (PBS). Fresh medium with or without 10 ng/ml VEGF and various concentrations of UA were added into the wells. The wound area was then examined after 8–12 h of incubation under an OLYMPUS inverted microscope connected to a DXM1200 digital camera. The migration ability was expressed by the percentage of the closure of gap distance using untreated wells at 100 %. Three independent experiments were performed.
Endothelial cell transwell migration assay
The chemotactic motility of HUVECs was determined using a transwell migration assay (Corning incorporated) with an 8 μm pore size as described elsewhere [26]. Briefly, the insert of the transwell plate was coated with 0.1 % gelatin for 30 min. After washing transwells three times with PBS, fresh ECM supplemented with 4 ng/ml VEGF was placed in the lower chamber, and HUVECs (4 × 104 cells/well) were seeded in the top chamber. Then, cells were treated with UA for 4 h at 37 °C with 5 % CO2. After incubation, non-migrated cells on the top surface of the membrane were gently scraped away with a cotton swab. The membrane containing migrated cells was fixed with 4 % paraformaldehyde for 20 min and stained with hematoxylin. Images were recorded using an OLYMPUS inverted microscope, and migrated cells were quantified by manual counting. The percentage of migrated cells inhibited by UA was normalized to untreated control cell migration.
Tube formation assay
Matrigel was thawed overnight at 4 °C, and each well of a pre-chilled 96 well plate was coated with 50 μl Matrigel and then incubated at 37 °C for 30 min. HUVECs (2 × 104 cells) were seeded onto Matrigel and treated with various concentrations of UA. After 8–12 h of incubation at 37 °C with 5 % CO2, the formation of endothelial cell tubular structures was inspected under an OLYMPUS inverted microscope, and the percentage of tube formation inhibited by UA was normalized to that of untreated control cells.
Cell proliferation assay
The effect of UA on cell proliferation was measured using a CellTiter96 AQueous One solution cell proliferation assay (MTS; Promega, Madison, WI). HUVECs and Bcap-37 cells were seeded in 96 well plates (5 × 103 cells/well). After 12 h of incubation, cells were treated with various concentrations of UA for 48–72 h, and then the AQueous One solution was added, followed by measurement of absorbance with a microplate reader (SpectraMax 190; Molecular Devices).
Cell morphology assay
To further assess changes in the cellular behavior of UA-treated HUVECs, we examined changes in cell morphology. Briefly, HUVECs were trypsinized and seeded into a 6 well plate. At 40–60 % confluence, culture medium was replaced with fresh medium with or without various concentrations of UA, and cells were incubated for a further 36 h. HUVEC morphology changes were assessed under an OLYMPUS phase contrast microscope.
Apoptosis analysis
As previous study [25], cell apoptosis was analyzed by flow cytometry (FACSCalibur, BD) according to the protocol for the FITC Annexin V Apoptosis Detection Kit I (BD Biosciences). HUVECs were treated with various concentrations of UA or DMSO for 36 h. Then, the cells were harvested using trypsin, washed twice with cold PBS and resuspended in 1× binding buffer with an addition of 5 μl annexin V and 5 μl propidium iodide (PI). The mixture was incubated for 15 min at room temperature in the dark, and then, 400 μl binding buffer was added and cells were analyzed immediately by flow cytometry (BD Biosciences).
Western blot analysis
HUVECs were cultured with serum-free endothelial cell medium for 6 h and then treated with or without UA for 4 h, followed by stimulation with 100 ng/ml VEGF165. Total protein extracts were obtained by lysing cells in cold RIPA buffer (20 mM Tris, 2 mM EDTA, 1 % Triton X-100, 1 % sodium deoxycholic acid and 0.1 % Sodium dodecyl sulfate) containing a proteinase inhibitor cocktail. Proteins were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto membranes, then membranes were blocked by 5 % bovine serum albumin and probed with specific antibodies. Visualization was performed with a LI-COR Infrared Imaged Odyssey (Gene Company Limited).
Statistical analysis
Data were presented as the mean and standard error. Statistical analysis was performed using the Student’s t test. A value of P < 0.05 was considered statistically significant.
Results
UA inhibits angiogenesis in vivo
To directly examine the effects of UA on angiogenesis and vascular development in vivo, we first performed a CAM assay which is the most widely used assay to study angiogenesis [27]. As shown in Fig. 1b, in the circular area of 5 mm diameter around the filter paper disc, the formation of new blood vessels was obviously blocked by UA (1 μg/disc), suggesting that UA inhibited CAM angiogenesis.
To further investigate whether UA inhibited VEGF-induced angiogenesis in vivo, we performed a mouse corneal micropocket assay. VEGF strongly stimulated corneal neovascularization, whereas treatment with UA significantly suppressed neovascularization, compared with that in the control group (Fig. 1c). Statistical analyses of three parameters including vessel length, clock number and the area of newly formed blood vessels also showed that VEGF-induced angiogenesis in the UA-treated mouse cornea was dramatically inhibited (Fig. 1d), indicating that UA significantly suppressed VEGF-induced mouse corneal angiogenesis. During experimentation, we did not observe symptoms of eye inflammation, such as keratitis, corneal edema or advanced signs of intraocular inflammation (data not shown).
UA inhibits tumor angiogenesis and tumor growth in a mouse xenograft model
Angiogenesis is the key step in tumor growth and metastasis, which provides necessary oxygen and nutrients for the tumor [28]. To investigate the effect of UA on Bcap-37 breast tumor growth and angiogenesis, we used a mouse xenograft breast tumor model. As shown in Fig. 2b, tumor volumes in the UA-treated group (242.92 ± 159.83 mm3) were much smaller than those in control group (906.44 ± 371.31 mm3) after 22 days (Fig. 2b). Notably, in the UA-treated group, one mouse tumor was completely suppressed. The mean tumor weight of the UA-treated group (0.918 ± 0.352 g) was also much less than that of the control group (0.308 ± 0.256 g) (Fig. 2a). These data suggested that UA significantly inhibited tumor growth in the mouse xenograft breast tumor model. Results also showed that at 60 mg/kg/day, UA had no effect on the body weight of UA treated-mice compared with that of the control group (Fig. 2c), implying the potential low side-effects of UA at the therapy dosage.
UA inhibits tumor growth and angiogenesis in a mouse xenograft tumor model. Bcap-37 cells were injected into 5-week-old nude female mice (5 × 106 cells/mouse). After solid tumors grew to about 200 mm3, mice were intra-lesionally treated with or without UA (60 mg/kg/day). a After treatment for 22 days, solid tumors in UA-treated mice were significantly smaller than those in control mice (n = 6). b UA inhibited tumor growth, as measured by tumor volume. c Body weight change in UA-treated and control group mice. There was no significant difference in body weight between UA-treated and control groups. d UA inhibited tumor angiogenesis. Left panel, representative images of immunohistochemistry. Black arrows indicate blood vessels stained with an anti-CD31 antibody. Right panel, statistical results of the mean IOD for blood vessels analyzed by Image-Pro Plus software. ***P < 0.001
To further investigate whether UA inhibited tumor growth by suppressing angiogenesis, we used an anti-CD31 antibody to stain solid tumor sections. As shown in Fig. 2d, the mean IOD of tumor blood vessels in UA-treated tumors was obviously less than that in the control group. These results indicated that UA inhibited breast tumor growth through suppressing tumor angiogenesis.
UA inhibits VEGF-induced migration and capillary structure formation of HUVECs
Cell migration is a pivotal step for the formation of blood vessels by endothelial cells in angiogenesis [29]. The effects of UA on the chemotactic motility of HUVECs were measured by both wound-healing and transwell cell migration assays. We found that UA inhibited VEGF-induced HUVEC migration in a dose-dependent manner (Fig. 3a, b). The tubular formation of endothelial cells is also a key step of angiogenesis [30]. To study the effect of UA on the tubular formation of endothelial cells, we seeded HUVECs on Matrigel and treated cells with or without various concentrations of UA, and then examined the formation of capillary-like structures by HUVECs. Results showed that UA suppressed the tubular formation of endothelial cells in a dose-dependent manner (Fig. 3c). All of the above results indicated that UA inhibited angiogenesis in vitro.
UA inhibits migration and capillary-structure formation of endothelial cells. a Inhibition of HUVEC migration by UA in a wound-healing migration assay. HUVECs were treated with 2 μg/ml mitomycin C for 2 h and then 10 ng/ml VEGF. The UA group in the left panel is representative of HUVECs treated with 20 μM UA, dotted lines indicate the field of initial scraping. Data are representative of three independent experiments with similar results. b Effect of UA on endothelial cell migration in a transwell migration assay. HUVECs treated with various concentrations of UA were seeded in the upper chamber, and the bottom chamber was filled with ECM medium containing 4 ng/ml VEGF. Cells with an irregular shape in images are cells that migrated into the lower chamber. The concentration of UA in the representative image of the UA group was 20 μM. Four independent experiments were performed with similar results. c Decreased tube formation by UA-treated HUVECs. Representative photo of the UA group treated with 20 μM UA. HUVECs (2 × 104 cells) were seeded in a 96 well plate coated with Matrigel and then treated with UA for 8–12 h. % is proportional to the control group. Data are the mean ± standard deviation of three independent experiments. *P < 0.05; ** P < 0.01; ***P < 0.001
UA inhibits both HUVECs and Bcap-37 tumor cells proliferation
Endothelial cell proliferation is an essential step in the multi-step processes of angiogenesis [31] and tumor cells proliferation is required for tumor growth. To further elucidate the effects of UA on both endothelial cells and Bcap-37 breast tumor cells, we used a MTS assay to examine cell proliferation and survival. As shown in Fig. 4a and b, UA inhibited the proliferation of both HUVECs and tumor cells in a dose-dependent manner.
UA inhibits HUVEC and Bcap-37 cell proliferation and induces morphological changes in HUVECs. a UA inhibited HUVEC proliferation in a dose-dependent manner in a MTS assay. HUVECs were treated with various concentrations of UA for 60 h, followed by MTS measurement. b UA inhibited Bcap-37 cell proliferation, as measured by the MTS assay. Bcap-37 cells were also treated with UA for 60 h. c Representative morphology of HUVECs treated with or without various concentrations of UA at various time points. Cells with an irregular shape in images are cells with morphological changes. Data are representative of three independent experiments with similar results. *P < 0.05; ***P < 0.001
UA induces HUVECs morphological changes and apoptosis in HUVECs
By observation under an OLYMPUS phase contrast microscope, we found that HUVEC growth was significantly decreased at the concentration of 10–50 μM of UA, as shown in Fig. 4c. After treatment with 10 μM UA, HUVECs showed no morphological changes from 24 to 36 h. After treatment with 20 μM UA for 24 h, a reduction in cell volume and shrunken cytoplasm were observed, which became more obvious after treatment for 36 h. After 24 h of treatment, extensive vacuolization and some cellular damage was evident. Moreover, most cells detached from culture surfaces and clear cellular damage was observed after 36 h. Next, we analyzed the effect of UA on HUVEC apoptosis by flow cytometric analysis of annexin V and PI staining. Annexin V binds to cells in early apoptosis, and PI stains cells in late apoptosis and dead cells. As shown in Fig. 5a. UA increased the percentage of apoptotic HUVECs in a dose-dependent manner. This result indicated that UA induced HUVEC apoptosis.
UA induces HUVEC apoptosis. a HUVECs were treated with DMSO or various concentrations of UA for 36 h. Induction of apoptosis was determined by flow cytometric analysis of annexin V-FITC and PI staining. Cells in the lower right quadrant indicate annexin-positive early apoptotic cells. Cells in the upper right quadrant indicate annexin-positive/PI-positive late apoptotic cells. Data are representative of two independent experiments with similar results. b UA inhibited the expression of survivin, Bcl-xl and activated the cleavage of caspase 3 and PARP. Three independent experiments were performed
Bcl-xl is an anti-apoptotic member of the Bcl-2 family involved in the complex apoptptic pathways [32, 33]. Inhibitors of apoptosis proteins (IAPs) are belong to a family of anti-apoptotic proteins that regulate apoptosis and proliferation. Survivin is the smallest IAP and inhibits apoptosis [34], enhances proliferation [35] promotes angiogenesis by inhibiting the downstream portion of caspase 3 activation pathways, which induces cell apoptosis [36–41]. Therefore, we examined survivin and Bcl-xl protein levels, caspase 3 activation and poly (ADP-ribose) polymerase (PARP) cleavage using western blot analysis of HUVECs treated with various concentrations of UA. As shown in Fig. 5b, UA not only significantly decreased Bcl-xl and survivin protein expression, but also increased the expression of cleaved caspase 3 and PARP in HUVECs. These data suggested that UA induced endothelial cell apoptosis.
UA inhibits the activation of VEGFR2-mediated AKT and MAPK signaling pathways
VEGFR2 plays a major role in VEGF-dependent angiogenesis and VEGF signaling via VEGFR2 is the most important pathway that executes the angiogenesis program by inducing proliferation, survival, migration and sprouting of endothelial cells [42]. To investigate the molecular mechanism of UA-induced inhibition of VEGF-dependent angiogenesis, we examined the protein expression levels of phosphorylated (p)-VEGFR2 using western blotting. As shown in Fig. 6a, UA strongly inhibited VEGF-activated VEGFR2 phosphorylation in western blot analysis.
UA inhibits VEGFR2-mediated AKT and MAPK signaling pathways in endothelial cells. a UA suppressed VEGF-induced phosphorylation of VEGFR2 in HUVECs. b UA inhibited VEGF-induced MEK1/2 and ERK1/2 pathways in a dose-dependent manner. c UA inhibited VEGF-dependent phosphorylation of AKT and P70S6K in a dose-dependent manner. d Schematic diagram of the mechanism by which UA inhibited tumor growth and angiogenesis in HUVECs. UA inhibited the phosphorylation of VEGFR2 and its downstream AKT and ERK1/2 signaling pathways, which play important roles in endothelial cell function, and further affected angiogenesis and inhibited tumor growth. Data are representative of three independent experiments with similar results
The extracellular signal-related kinase 1/2 (ERK1/2), one of the major targets of the mitogen-activated protein kinase (MAPK) signaling pathway, has been implicated in the regulation of angiogenesis for various functions including cell proliferation, migration, and survival [11, 43]. To evaluate whether UA suppressed activation of the ERK cascade in tumor angiogenesis, we examined the phosphorylative activation of protein kinases involved in the ERK signaling pathway. Our results indicated that UA inhibited phosphorylative activation of pSer217/221- MEK1/2 and pThr202/Tyr204- pERK1/2 in a dose-dependent manner (Fig. 6b).
AKT is a serine/threonine kinase that plays a central role in a range of cellular functions including cell growth, proliferation, migration, protein synthesis, transcription procedure and survival and angiogenesis [44, 45]. P70S6K kinase (p70S6K) and S6 ribosomal protein (S6RP) are proteins downstream of AKT, and activation of p70S6K and S6RP stimulate protein synthesis and promotes cell growth and proliferation [46]. Therefore, we examined the effect of UA on the activity of AKT, P70S6K and S6RP. Results also showed that UA inhibited VEGF-dependent phosphorylation of AKT, P70S6K and S6RP in a dose-dependent manner (Fig. 6c). Together, these data showed that UA exerted its antiangiogenic function by inhibiting VEGFR2 activation and blocking VEGFR2-mediated downstream signaling cascades.
Discussion
Traditional Chinese medicinal herbs are rich sources of anti-cancer agents [47]. In this study, we found that UA inhibited angiogenesis in vivo by suppressing key angiogenic steps including proliferation, migration and tube formation of endothelial cells. Further investigations showed that UA functioned as a tumor angiogenesis inhibitor by the suppression of VEGFR2-mediated AKT and ERK1/2 signaling pathways.
Among many angiogenesis assays, the CAM assay is well established and widely used as a model to examine anti-angiogenesis [48]. Moreover, the corneal angiogenesis assay is considered as the gold standard for evaluating angiogenesis in vivo [49]. In the present study, we demonstrated that UA inhibited neovascularization in the CAM (Fig. 1b) and blocked VEGF-induced newly formed corneal blood vessels (Fig. 1c, d). These results indicated that UA is an angiogenesis inhibitor.
Angiogenesis is a hallmark of malignant neoplasia, and disruption of tumor vasculature is an active anticancer therapy in some cases [50]. In the present study, we found that UA dramatically suppressed tumor angiogenesis and tumor growth (Fig. 2). In the MTS assay, the IC50 of HUVECs was slightly higher than that of Bcap-37 tumor cells, suggesting that inhibiting tumor angiogenesis may an important aspect of the anti-tumor activity of UA at the effective dosage. Furthermore, we found that UA-treated mice did not show any body weight loss (Fig. 2c) and their survival rate was the same as that of the control group (data not shown). These results suggested that UA inhibited solid tumor growth by blocking angiogenesis with low side effects.
VEGFR2 mediates the majority of the downstream effects of VEGF in angiogenesis. Interruption of VEGFR2 signaling is thought to be necessary for tumor angiogenesis and macroscopic solid tumor growth [51]. Here, we showed that UA dramatically inhibited VEGF-induced VEGFR2 activation (Fig. 6a). In the VEGF-mediated signaling pathway, AKT is a critical regulator of cell survival, proliferation, migration and angiogenesis [45]. In addition, ERK is also an important factor for regulating endothelial cell proliferation, growth, migration, and apoptosis [52]. Activation of AKT and ERK pathways is necessary for the essential cellular processes of endothelial cells in tumor angiogenesis [52]. In the present study, we demonstrated that UA not only down-regulated the activity of MEK and ERK (Fig. 6b) but also inhibited the phosphorylation of AKT followed by suppressing the expression of AKT downstream genes including P70S6K and PS6RP (Fig. 6c). Therefore, UA may inhibit angiogenesis and tumor growth by directly inhibiting VEGFR2 activation and VEGFR2-mediated downstream signaling cascades.
Anti-angiogenic therapy targets activated endothelial cells and offers advantages over therapies directed against tumor cells. In the present study, we demonstrated that UA not only inhibited proliferation and induced apoptosis in HUVECs by decreasing Bcl-xl and survivin levels and up-regulating caspase 3 activation and cleavage of PARP, but also significantly suppressed HUVEC migration and tube formation, suggesting that UA affected angiogenesis by targeting multiple aspects of endothelial cells.
In conclusion, we found that UA potently inhibited Bcap-37 breast tumor growth and angiogenesis by suppressing VEGFR2-mediated downstream AKT and ERK1/2 signaling pathways. Therefore, our data indicate a potential role for UA in the development of new therapeutic strategies against diseases associated with dysregulation of angiogenesis.
References
Thairu N, Kiriakidis S, Dawson P, Paleolog E (2011) Angiogenesis as a therapeutic target in arthritis in 2011: learning the lessons of the colorectal cancer experience. Angiogenesis. doi:10.1007/s10456-011-9208-2
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70. doi:10.1016/S0092-8674(00)81683-9
Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J (2004) Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res 94(5):664–670. doi:10.1161/01.RES.0000118600.91698.BB
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25(4):581–611. doi:10.1210/er.2003-0027
Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC II, Vansluys J (2008) Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood 111(5):2647–2656. doi:10.1182/blood-2007-08-109710
Jiang BH, Liu LZ (2008) AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets 8(1):19–26
Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I (2001) Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J 360(Pt 1):255–264
Pyun BJ, Choi S, Lee Y, Kim TW, Min JK, Kim Y, Kim BD, Kim JH, Kim TY, Kim YM, Kwon YG (2008) Capsiate, a nonpungent capsaicin-like compound, inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity. Cancer Res 68(1):227–235. doi:10.1158/0008-5472.CAN-07-2799
Somanath PR, Razorenova OV, Chen J, Byzova TV (2006) Akt1 in endothelial cell and angiogenesis. Cell Cycle 5(5):512–518. doi:10.4161/cc.5.5.2538
Berra E, Milanini J, Richard DE, Le Gall M, Vinals F, Gothie E, Roux D, Pages G, Pouyssegur J (2000) Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol 60(8):1171–1178. doi:10.1016/S0006-2952(00)00423-8
Pages G, Milanini J, Richard DE, Berra E, Gothie E, Vinals F, Pouyssegur J (2000) Signaling angiogenesis via p42/p44 MAP kinase cascade. Ann N Y Acad Sci 902:187–200
Cabebe E, Wakelee H (2006) Sunitinib: a newly approved small-molecule inhibitor of angiogenesis. Drugs Today (Barc) 42(6):387–398. doi:10.1358/dot.2006.42.6.985633
Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, Sridhara R, Garvey P, Justice R, Pazdur R (2006) Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res 12(24):7271–7278. doi:10.1158/1078-0432.CCR-06-1249
Cocchietto M, Skert N, Nimis PL, Sava G (2002) A review on usnic acid, an interesting natural compound. Naturwissenschaften 89(4):137–146
Guo L, Shi Q, Fang JL, Mei N, Ali AA, Lewis SM, Leakey JE, Frankos VH (2008) Review of usnic acid and usnea barbata toxicity. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 26(4):317–338. doi:10.1080/10590500802533392
O’Neill MA, Mayer M, Murray KE, Rolim-Santos HM, Santos-Magalhaes NS, Thompson AM, Appleyard VC (2010) Does usnic acid affect microtubules in human cancer cells? Braz J Biol 70(3):659–664. doi:10.1590/S1519-69842010005000013
Einarsdottir E, Groeneweg J, Bjornsdottir GG, Harethardottir G, Omarsdottir S, Ingolfsdottir K, Ogmundsdottir HM (2010) Cellular mechanisms of the anticancer effects of the lichen compound usnic acid. Planta Med 76(10):969–974. doi:10.1055/s-0029-1240851
da Silva Santos NP, Nascimento SC, Wanderley MS, Pontes-Filho NT, da Silva JF, de Castro CM, Pereira EC, da Silva NH, Honda NK, Santos-Magalhaes NS (2006) Nanoencapsulation of usnic acid: an attempt to improve antitumour activity and reduce hepatotoxicity. Euro J Pharm Biopharm 64(2):154–160. doi:10.1016/j.ejpb.2006.05.018
Mayer M, O’Neill MA, Murray KE, Santos-Magalhaes NS, Carneiro-Leao AM, Thompson AM, Appleyard VC (2005) Usnic acid: a non-genotoxic compound with anti-cancer properties. Anticancer Drugs 16(8):805–809. doi:10.1097/01.cad.0000175588.09070.77
Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J (2007) Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446(7137):765–770. doi:10.1038/nature05760
Ye H, Jin L, Hu R, Yi Z, Li J, Wu Y, Xi X, Wu Z (2006) Poly(gamma,l-glutamic acid)-cisplatin conjugate effectively inhibits human breast tumor xenografted in nude mice. Biomaterials 27(35):5958–5965. doi:10.1016/j.biomaterials.2006.08.016
Cho SG, Yi Z, Pang X, Yi T, Wang Y, Luo J, Wu Z, Li D, Liu M (2009) Kisspeptin-10, a KISS1-derived decapeptide, inhibits tumor angiogenesis by suppressing Sp1-mediated VEGF expression and FAK/Rho GTPase activation. Cancer Res 69(17):7062–7070. doi:10.1158/0008-5472.CAN-09-0476
Yi ZF, Cho SG, Zhao H, Wu YY, Luo J, Li D, Yi T, Xu X, Wu Z, Liu M (2009) A novel peptide from human apolipoprotein(a) inhibits angiogenesis and tumor growth by targeting c-Src phosphorylation in VEGF-induced human umbilical endothelial cells. Int J Cancer 124(4):843–852. doi:10.1002/ijc.24027
Pang X, Yi Z, Zhang X, Sung B, Qu W, Lian X, Aggarwal BB, Liu M (2009) Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res 69(14):5893–5900. doi:10.1158/0008-5472.CAN-09-0755
Dong Y, Lu B, Zhang X, Zhang J, Lai L, Li D, Wu Y, Song Y, Luo J, Pang X, Yi Z, Liu M (2010) Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis 31(12):2097–2104. doi:10.1093/carcin/bgq167
Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, Liu M (2008) Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res 68(6):1843–1850. doi:10.1158/0008-5472.CAN-07-5944
Ribatti D, Vacca A, Roncali L, Dammacco F (1996) The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis. Int J Dev Biol 40(6):1189–1197
Tozer GM, Kanthou C, Baguley BC (2005) Disrupting tumour blood vessels. Nat Rev Cancer 5(6):423–435. doi:10.1038/nrc1628
Rousseau S, Houle F, Landry J, Huot J (1997) p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15(18):2169–2177. doi:10.1038/sj.onc.1201380
Patan S (2004) Vasculogenesis and angiogenesis. Cancer Treat Res 117:3–32
Lynch CN, Wang YC, Lund JK, Chen YW, Leal JA, Wiley SR (1999) TWEAK induces angiogenesis and proliferation of endothelial cells. J Biol Chem 274(13):8455–8459
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Annu Rev Biochem 69:217–245. doi:10.1146/annurev.biochem.69.1.217
Johnstone RW, Ruefli AA, Lowe SW (2002) Apoptosis: a link between cancer genetics and chemotherapy. Cell 108(2):153–164. doi:10.1016/S0092-8674(02)00625-6
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3(8):917–921
Yang D, Welm A, Bishop JM (2004) Cell division and cell survival in the absence of survivin. Proc Natl Acad Sci USA 101(42):15100–15105. doi:10.1073/pnas.0406665101
Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N (2001) Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer 91(11):2026–2032. doi:10.1002/1097-0142(20010601)91:11<2026:AID-CNCR1228>3.0.CO;2-E
Conway EM, Zwerts F, Van Eygen V, DeVriese A, Nagai N, Luo W, Collen D (2003) Survivin-dependent angiogenesis in ischemic brain: molecular mechanisms of hypoxia-induced up-regulation. Am J Pathol 163(3):935–946
Tu SP, Jiang XH, Lin MC, Cui JT, Yang Y, Lum CT, Zou B, Zhu YB, Jiang SH, Wong WM, Chan AO, Yuen MF, Lam SK, Kung HF, Wong BC (2003) Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res 63(22):7724–7732
Li QX, Zhao J, Liu JY, Jia LT, Huang HY, Xu YM, Zhang Y, Zhang R, Wang CJ, Yao LB, Chen SY, Yang AG (2006) Survivin stable knockdown by siRNA inhibits tumor cell growth and angiogenesis in breast and cervical cancers. Cancer Biol Ther 5(7):860–866. doi:10.4161/cbt.5.7.2893
Caldas H, Fangusaro JR, Boue DR, Holloway MP, Altura RA (2007) Dissecting the role of endothelial SURVIVIN DeltaEx3 in angiogenesis. Blood 109(4):1479–1489. doi:10.1182/blood-2006-02-003749
Botto S, Streblow DN, Defilippis V, White L, Kreklywich CN, Smith PP, Caposio P (2010) IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin. Blood. doi:10.1182/blood-2010-06-291245
Lohela M, Bry M, Tammela T, Alitalo K (2009) VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 21(2):154–165. doi:10.1016/j.ceb.2008.12.012
Risau W (1997) Mechanisms of angiogenesis. Nature 386(6626):671–674. doi:10.1038/386671a0
Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P (2008) Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol 8(4):393–412. doi:10.1016/j.coph.2008.08.004
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274. doi:10.1016/j.cell.2007.06.009
Olszewska-Pazdrak B, Hein TW, Olszewska P, Carney DH (2009) Chronic hypoxia attenuates VEGF signaling and angiogenic responses by downregulation of KDR in human endothelial cells. Am J Physiol Cell Physiol 296(5):C1162–C1170. doi:10.1152/ajpcell.00533.2008
Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3(10):768–780. doi:10.1038/nrc1189
Tufan AC, Satiroglu-Tufan NL (2005) The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr Cancer Drug Targets 5(4):249–266
Schwartz S, George J, Ben-Shoshan J, Luboshits G, Avni I, Levkovitch-Verbin H, Ziv H, Rosner M, Barak A (2008) Drug modification of angiogenesis in a rat cornea model. Invest Ophthalmol Vis Sci 49(1):250–254. doi:10.1167/iovs.06-1337
Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S (2010) Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Investig 120(4):1151–1164. doi:10.1172/JCI37223
Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ES, Curry B, Richmond GH, Wadsworth PF, Bigley AL, Hennequin LF (2002) ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 62(16):4645–4655
Murphy DA, Makonnen S, Lassoued W, Feldman MD, Carter C, Lee WM (2006) Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006). Am J Pathol 169(5):1875–1885. doi:10.2353/ajpath.2006.050711
Acknowledgments
We thank all members in the laboratory of Dr.Mingyao Liu’s lab at the Institute of Biomedical Sciences and School of Life Sciences, East China Normal University. This study was partially sponsored by the Major State Basic Research Development Program of China (2012CB910400, 2009CB918402). National Natural Science Foundation of China (30930055, 30971523, 81071807) and The Science and Technology Commission of Shanghai Municipality (11DZ2260300).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Y., Dai, F., Zhai, D. et al. Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated AKT and ERK1/2 signaling pathways. Angiogenesis 15, 421–432 (2012). https://doi.org/10.1007/s10456-012-9270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-012-9270-4