Abstract
Bioaerosols are particles of great importance for several fields of research, and spores produced by fungi can exist as bioaerosols when suspended in the air. Microbiological standards for environmental monitoring of outdoor air parameters can be achieved by analyzing the relationship between airborne microorganisms and the prevailing environmental conditions. The outdoor air of the Metropolitan Region of São Paulo and the rural area in a city of the state of São Paulo (Ibiúna/SP), both in Brazil, were evaluated for the presence of microorganisms using the MAS-100 ECO (Merck®, Fr.) and M Air T (Millipore®) air sample collectors. Dichloran Rose-Bengal Chloramphenicol and Tryptic Soy Agars were used for fungal and bacterial isolation, respectively. Bacterial colonies were counted, and the plates with fungal colonies were sent for phenotypic identification up to genus and species level, respectively. Data on pollutant concentrations were obtained from the Environmental Company of the State of São Paulo. The highest number of Colony-Forming Units/m3 (CFU/m3) of microorganisms was measured in the winter and summer seasons, respectively, but the greatest Spore-Forming Units (SFU) of fungi were found in the rural area, where pollutant concentrations were lower. Nitrogen dioxide (NO2) had a slightly positive influence on the concentration of SFU of fungi in both areas studied. Sulfur dioxide (SO2) pollutant concentrations had both positive and negative great relations showing influence on microbial counts in the air of the rural area. In the rural area, the low bacteria count was influenced negatively by the low concentration of carbon monoxide (CO). The microbial counts were related to each other, as well as to the concentrations of pollutants, shown by all the correlations seen, indicating microorganisms as biomarkers of pollution in outdoor areas. The influence of environmental factors on the population and outdoor air biome is also explicit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aerosols are particles of great importance for several areas of research involving atmospheric chemistry, physics, biosphere, climate, and public health (Farmer & Riches, 2020; Pöschl, 2005). These aerosols can be divided into two fractions: the fine fraction, less than 2 μm and produced mainly by the conversion into particles of gases, and the coarse fraction, with particles greater than 2 μm (Theotônio et al., 2007; Andreeva et al., 2024). Aerosols of biological origin are generally part of this last fraction and are called bioaerosols, which consist of fragments of leaves, pollen grains, and fungi (Theotônio et al., 2007; Andreeva et al., 2024).
At certain places and times of the year, bioaerosols contribute up to 50% of the total number of aerosols or particles in the air (Morris et al., 2004; Pescott et al., 2015Yao, 2018). Bioaerosols can change atmospheric thermodynamic properties such as temperature profile and variability of relative humidity, and they depend on these factors to circulate in the environment. The average length of stay in the atmosphere is variable, ranging from days to weeks. Many of these bioaerosols have defense mechanisms that allow them to withstand the environmental stresses of air transport, including exposure to UV radiation, dehydration, and pH in the cloud water, and also to survive long-range travel (Andreeva et al., 2024; Burrows et al., 2009; Farmer & Riches, 2020; Rosenfeld et al., 2008).
These particles are not suspended in the air as independent elements, and cell agglomerations or airborne transport in plant or animal fragments, soil particles, pollen, or spores may occur. The intact particles that are part of bioaerosols have several sizes: Most pollen types grains are 17–58 μm, fungal spores are 1–30 μm, bacteria are 0.25–8 μm, and viruses are less than 0.3 μm in diameter (Jones et al., 2004).
Kingdom Fungi can produce bioaerosols including fragments of hyphae, single-celled, and multicellular spores, allowing their dispersion. In general, diversity is inherent to the size of fungal particles, associated with the strong influence of their diameter. The taxonomic composition of these anemophilous fungi can determine how they will be distributed in the atmosphere. All fungi are active participants in the cycle of elements in nature; approximately 28 to 50 tons of fungal materials are emitted annually in the Earth's atmosphere, and fungal particles can constitute up to 420% of primary organic aerosol emissions (Bernardi, 2007; Elbert et al., 2006; Heald & Spracklen, 2009; Yamamoto et al., 2012).
Microbiological analysis of the air can be performed by several techniques, but sample collection using impactors can determine the exact value of the air analyzed; due to its flow control, it can determine the amount of air collected (Lacey & Venette, 2020). A successful fungal analysis depends largely on the used technique, as well as the choice of the appropriate culture media in the collection, which allows a comprehensive evaluation of quantitative and qualitative characteristics (Gutarowska & Piotrowska, 2007). The characterization of bioaerosols, of fungal origin, can be done by analyzing small air samples that can provide a reasonable estimate of the typical concentration of spores. These analyses may allow the identification of potentially harmful fungal contamination, even when surface colonies are not easily visible (Egan et al., 2014).
Parameters on microbiological air conditions could be performed by analyzing the relationship between airborne fungi and bacteria present in outdoor air and the environment where they are isolated. However, other microorganisms are used in environmental controls, for their sensitivity to pollution, especially in rural areas (Martins et al., 2008; Munzi et al., 2007; Ristic et al., 2017; Stamenković et al., 2016).
On the other hand, according to the World Health Organization (World Health Organization, 2003), air quality standards are variable according to the approach adopted to balance health risks, technical feasibility, economic considerations, and various other political and social factors, which in turn depend, among other things, on the level of development and the national capacity to manage air quality, in which the number of spores in the air is one of the factors (Resolução CONAMA No 491, 2018).
The measurement of anemophilous microorganisms is necessary to evaluate the effectiveness of microbial control strategies integrated by environmental monitoring.
Pollution influences on respiratory disorders have a great impact on public health and are related to anthropic activities producing pollutants, whether particulate matter or toxic gases such as sulfur dioxide (SO2), nitric dioxide (NO2), and carbon monoxide (CO), among others, which also cause enormous inferences to the environment (Martins et al., 2002; Parsi and Görecki, 2006; Jasinski et al., 2011).
In Brazil, environmental control analyses of outdoor air are carried out characterizing the aerosols. The organic fraction has been mainly related to biomass burning and combustion, although there is a significant presence of green areas in cities that make biogenic emissions an additional source of organic carbon (Rackes & Waring, 2013; Ana Paula Mendes Emygdio, Cristiane Degobbib, Fábio Luiz Teixeira Gonçalves, 2018). This control does not focus on microorganisms in the atmosphere.
The Metropolitan Region of São Paulo (MRSP) is characterized by a large megalopolis with a high population and number of vehicles. This condition favors the emission on a scale of pollutants (particulate matter and toxic gases), creating its own biome in relation with the atmospheric and biological characteristics of the air of the region. The large circulation of people and vehicles observed in highly populated and industrialized regions can produce a high dispersion of microorganisms in these areas (Chiquetto et al., 2021).
Several types of researches conducted with environmental samples in China and India showed a strong correlation between air pollutants and the diversity of microorganisms, using culture techniques for the bioaerosols identification, showing the influence of environmental factors on the concentration of Colony-Forming Units/m3 (CFU/m3) of fungi and bacteria, correlating the concentrations of pollutants with the amount of CFU of microorganisms (Fan et al., 2019; Roy & Gupta Bhattacharya, 2020).
Identification of airborne fungi, especially those belonging to the Ascomycota phylum, can provide important information which, when related to atmospheric conditions or the pollutants concentration, would turn these microorganisms into strong predictors of environmental conditions (Roy & Gupta Bhattacharya, 2020).
In Brazil, there is a growing concern in monitoring indicators of environmental pollution and the need to expand the knowledge about these microorganisms in the atmosphere, as well as to analyze the relationship between these microorganisms and air pollutants; beyond that, we have a lack of studies on this board realized on Latin America. Based on this information, the aim of this study was to collect MRSP air samples and analyze their relationships with air pollutant concentrations during the period.
2 Materials and methods
Outdoor air was evaluated in the MRSP and in the rural area of a city of the state of São Paulo (Ibiúna/SP) regarding the presence of fungi and bacteria, for six years, amounting to 736 collections; each collection presented a sample for fungi and another for bacteria, respectively.
Two points were analyzed: one in the city of São Paulo at the Adolfo Lutz Institute (IAL), located at Cerqueira César neighborhood. The rural area analyzed was the Votorantim neighborhood in the city of Ibiúna/SP. The two cities are 60.6 km apart (Table 1; Fig. 1). The first point is located at the downtown, and Ibiúna is considered rural.
The distribution of the collected samples is described according to the collection site and during the season of the year (Table 2).
Air was sampled using the air compactors MAS-100 ECO (Merck®, Fr.) and M Air T (Millipore®). Both have the same air flow capacity and final volume of sample collected (Moura, Caldas et al., 2015).
Three daily samples were collected at 1-h intervals during the morning (9 AM, 10 AM, and 11 AM), based on mutual schedules with highest flow of people and vehicles in the capital and the city of the interior. Each presented the final volume of 250 L (0.25 L/m3), totaling 750 L (0.75 L/m3), using the modified Dichloran Rose-Bengal Chloramphenicol (DRBCm) culture media (de Matos Castro e Silva et al., 2015) for isolation of fungi, while Tryptic Soy Agar (TSA) was supplemented with cycloheximide for count of CFU/m3 of bacteria.
The samples collected from the TSA plates were incubated at 30 °C for three days. After this period, was performed the colony counting, and after that, the plates were discarded, since there is no need for more applied identifications for its correlation with the environment (Brągoszewska & Pastuszka, 2018).
The DRBCm media inoculated with fungi were incubated for up to seven days at 30 °C. The resulting fungal colonies after the period of incubation were counted, and only one isolate of each fungal genera was identified using phenotypic characteristics such as the macro-micromorphology and the presence of pigments (hyaline and dematiaceous), among others (Hoog, Guarro, Gené, 2014).
Data on pollutant concentrations at the time of air collection were obtained from the Environmental Company of the State of São Paulo (CETESB) in daily online reports of the stations: Cerqueira César, Pinheiros, and Sorocaba.
2.1 Statistical analysis
For statistical analysis, we applied the Kolmogorov–Smirnov test, but it was seen that none of the variables had normal distribution. Then, we performed the factorial analysis of variables and applied the Kaiser–Meyer–Olkin (KMO) and Bartlett's tests to attest to the feasibility on factorial analysis, looking for the variance of the data, where the test could tell us whether factor analysis was appropriate or not. Spearman's nonparametric correlation test was performed to verify the strength of the relationships between the variables, and where p > 0.005, we applied the Mann–Whitney U test to verify whether the comparison of two unpaired groups (≤ 100 and ≥ 101 CFU/m3) was statistically significant. All tests were performed using the Biostat software.
3 Results
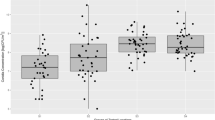
No molecular analysis was performed to adjust or categorize the methodology errors due to the scarcity of resources for these analyses. All samples collected showed the presence of bacteria and fungi, respectively (Fig. 2).
The concentrations of microorganisms were distributed in a similar way, but in the winter season, there was an increase in CFU/m3 of fungal spores, while in the summer season, the increase was in the CFU/m3 of bacteria (Fig. 3).
During sampling campaign, 1630 fungal isolates were obtained; 219 did not present reproduction structures and were classified as non-sporulating species. According to phenotypic analyses performed in 1411 isolates, 17 different genera were identified (Table 3).
The highest incidence of different fungal genera in the same air sample occurred in the rural area, with up to seven concomitant genera (Table 4).
Statistical analyses were based on factorial data represented in Table 5.
In the rural area, by the KMO–Bartlett's test, the analyzed data showed no significant relevance in the correlation of bacterial and fungal counts with the other variables, different from the relationship observed in the low positive influence of pollutant concentration on the number of bacterial counts (Table 6) dispersed on the air in the urban area (p ≤ 0.001).
Analyzing the differences in the concentrations of SO2 in the samples collected between urban and rural areas, the result was quite expressive. In the rural area, when it presented low rates, it promoted a positive influence with the increase of up to 45% in the presence of microorganisms in the samples (p ≤ 0.001), mainly fungi. The low concentration of bacterial counts was negatively influenced by the low concentration of CO (p = 0.003); on the other hand, the fungi showed a significant positive influence in relation with the low concentrations of NO2 (p = 0.014) (Table 7).
On the other hand, in the urban area, the concentrations of SO2 in the analyzed data did not impact the concentration of microorganisms. High CO concentrations did not decrease bacterial concentrations, and even NO2, with high levels in the air, continued to have a slight significant positive influence (p = 0.006) with CFU/m3 of fungi (Table 8).
For the Mann–Whitney U test, the variable of microorganisms was split into two groups (≤ 100 and ≥ 101 CFU/m3) for the analyses, and the results of their frequency are described in Table 9.
It was observed that NO2 showed positive influences in relation with the fungal counts in both studied areas and had no correlation with the concentrations of bacteria in the analyzed samples. Mann–Whitney U test (Table 10) verified statistical significance in the adequacy of data from these populations, for fungi (p = 0.004) and bacteria (p ≤ 0.001).
4 Discussion
The role of the atmosphere in the dispersion of fungi is quite nonlinear (Franić et al., 2023), in which each biological, pollution, and meteorological variable can have antagonistic or summation effects depending on the situation. What is known is that there are few investigations on microbial bioaerosols, among them, that they can be indicators of the level of biological pollution of the air and the attention that has been given to their relations as well. Since fungi and bacteria can be found as part of the microbial flora in the atmosphere, they deserve a more detailed analysis of their particles present and transported by air, as fungal spores provide a better understanding of these phenomena, and a more detailed survey of airborne particles is required (Grinn-Gofroń et al., 2011; Nowakowicz-Dębek et al., 2017).
Regarding the presence of pigments in fungal colonies, some species of dematiaceous fungi may take up to 21 days to develop (Sterflinger et al., 2012), creating a bias in the differentiation of hyaline and dematiaceous fungi in the seven-day period of growth.
Spore-forming units varied according to seasons, thus characterizing the seasonal number of fungi dispersed. Locality and seasons have already been described as influencing the aerial dispersion of the fungi most commonly isolated in the outdoor air of the city of São Paulo, and this influence was recorded during this study (Amend et al., 2010; Onofre., 2010; Borges, Monteiro, Monteiro, 2012; Filali Ben Sidel et al., 2015).
The relationships between fungal and bacterial communities are not fully understood yet. Since the environmental conditions of an area (temperature, humidity, physical and chemicals patterns) can allow the high concentration of fungi, it is more than likely that bacterial communities can proliferate in these environments, given their ease of assembly (Schmidt et al., 2014).
Previous studies have shown that regardless of the area analyzed, the greatest diversity and number of fungi occur during the winter season, which corroborates the finding of this research, where there was a significant increase in the number of fungal spores during the winter season (Dannemiller et al., 2016; Fan et al., 2019; Oliveira et al., 2009; Temperini et al., 2019). In other studies, conducted in the city of São Paulo, the highest concentrations of CO, NO2, and SO2 were recorded during the winter period, between the months of May and September, because of low rainfall rates, weak winds, and higher occurrence of temperature inversions, which corroborate the findings of this research (Aguiar, 2015; Carvalho et al., 2015; Grinn-Gofroń et al., 2011). On the flip side, these same parameters are related to the increase in the number of fungal spores dispersed and may be related to the values of the relationships found between microorganisms and pollutants (Arbex et al., 2012; Dong et al., 2016; El-Batrawy, 2010; Mitchell et al., 2007).
Pollution concentrations have a strong impact on the diversity of genera and the number of microorganisms present in the air. Similarly, in this study, in the rural region where there is less pollution, the diversity of fungal genera is greater, and, as pollutant concentrations increase, there is a progressive decline of the diversity of microorganisms dispersed in the air, safeguarding the concentrations of bacteria that did not get affected by the presence of CO (Liu et al., 2019; Oliveira et al., 2009).
Aspergillus sp. showed as the most present fungi in the samples in all seasons and in both areas analyzed, corroborating other studies of the same area (Liu et al., 2019; Oliveira et al., 2009). Some species of this genus can cause opportunistic diseases, such as aspergillosis, especially in people with immunity issues (Cuervo-Maldonado et al., 2010); other species found, such as Trichoderma sp., Penicillium sp., and Alternaria sp., are much less harmful to human health, even though they also can cause infections in immunosuppressed patients (Stathakis et al., 2015; Recio et al., 2019; ‘BI17: Opportunistic fungal infection with Alternaria in the immunosuppressed’, 2021), but these species are excellent indicators in the areas of pollution and biotechnology (Tiwari, Misra and Sangwan, 2013; Filali Ben Sidel et al., 2015; Morales-Oyervides et al., 2020).
The number of fungi and bacterial counts in the samples between urban and rural areas remained the same throughout the seasons; however, there was a big growth in the presence of fungal and bacterial spores during the winter and summer seasons, respectively. In addition, the genera diversity was lower in the urban area samples, where SO2, NO2, and CO concentrations were higher, revealing that the pollutants do not impact in the number of microorganisms dispersed, but at the variety of genera found (Liu et al., 2019; Oliveira et al., 2009).
The relation between low SO2 concentrations and the number of microorganisms present in the air of the rural area could be explained by the action of this substance in the germination of spores (fungi), when high levels of this pollutant can lead to form H2SO4, which is toxic to fungi and bacteria. This action mechanism has been used in the agricultural sector, and its usefulness is now revealed and studied in the monitoring of outdoor air (Schoenlein-crusius et al., 2001; Ana Paula Mendes Emygdio, Cristiane Degobbib, Fábio Luiz Teixeira Gonçalves, 2018).
In studies already published, NO2 concentrations negatively impacted the concentration of fungi dispersed in the air (Schoenlein-crusius et al., 2001), different from the slightly positive correlation found in the period studied. However, other studies reveal that the influence of this pollutant on the concentration of microorganisms depends on other parameters and changes temporally (Abdel Hameed et al., 2012; Gao et al., 2016).
The analysis of these pollutants, such as CO, has already revealed positive and negative influences according to the environmental conditions. As in this study, CO concentrations impacted the diversity and number of bacteria dispersed mainly in urban areas, where the concentration of this pollutant is higher (Dong et al., 2016; Liu et al., 2019).
5 Conclusions
During the collection of samples carried out in the urban region of São Paulo and in the rural region of Ibiúna for six years and in the four seasons, the highest concentration of fungi occurred in the winter season, and the concentration of bacteria did not vary during the study period. The greatest diversity of fungal genera was found in the rural area, where pollutant concentrations were lower.
Regarding the influence of pollutants NO2, CO, and SO2, dispersed in the air from the MRSP, the presence of NO2 had a positive influence in the concentration of fungi in both studied areas. The presence of low CO concentrations had a negative influence on the concentration of bacteria, and the low concentration of the pollutant SO2 impacted positively on the concentration of airborne microorganisms in the rural area.
The results indicated negative influences on the correlations between bacteria and CO concentration.
The monitoring of airborne fungi allows further studies to assist the analyses, determining bioindicators, based on their frequency and sensitivity to pollutants, which may be the most appropriate way to obtain parameters directly linked to environmental conditions for the benefit of human health.
This study has provided a possible baseline for further studies on analyzing the relationship between microorganisms and chemical components in outdoor air.
Availability of data and materials
Not applicable.
References
BI17: Opportunistic fungal infection with Alternaria in the immunosuppressed. (2021). British Journal of Dermatology. Oxford Academic, 185(S1), pp. 150–150. https://doi.org/10.1111/bjd.20315
‘Resolução CONAMA No 491 DE 19_11_2018 - Federal - LegisWeb’ (no date).
Abdel Hameed, A. A., et al. (2012). Study on some factors affecting survivability of airborne fungi. Science of the Total Environment., 414, 696–700. https://doi.org/10.1016/j.scitotenv.2011.10.042
Aguiar, L. (2015). Estudo da relação da qualidade do ar e variáveis meteorológicas na ocorrência de morbidade respiratória e circulatória na Região Metropolitana de São Paulo, p. 105. Available at: http://repositorio.utfpr.edu.br/jspui/bitstream/1/1384/1/LD_PPGEA_M_Aguiar%2C Lais Sinhorini_2015.pdf (Accessed: 7 May 2018)
Amend, A. S., et al. (2010). Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proceedings of the National Academy of Sciences, 107(31), 13748–13753. https://doi.org/10.1073/pnas.1000454107
Andreeva, I. S., et al. (2024). Culturable microorganisms of aerosols sampled during aircraft sounding of the atmosphere over the Russian Arctic Seas. Atmosphere, 15, 365. https://doi.org/10.3390/ATMOS15030365
Arbex, M. A., et al. (2012). A poluição do ar e o sistema respiratório. Jornal Brasileiro De Pneumologia, 38(5), 643–655.
Bernardi, E. (2007). Fungos anemófilos e suas relações com fatores abióticos, na praia do Laranjal, Pelotas, RS. Revista De Biologia e Ciências Da Terra, 7(2), 91–96.
Borges, K. R. A., Monteiro, S. G., & Monteiro, C. A. (2012). Diversity and prevalence of airborne fungi isolated from São Luís, Northeast Brazil. Revista Brasileira De Análises Clínicas, 44(3–4), 132–138.
Brągoszewska, E., & Pastuszka, J. S. (2018). ‘Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia, 34(2), 241–255. https://doi.org/10.1007/s10453-018-9510-1
Burrows, S. M., et al. (2009). Bacteria in the global atmosphere – Part 2: Modelling of emissions and transport between different ecosystems. Atmospheric Chemistry and Physics Discussions, 9(3), 10829–10881. https://doi.org/10.5194/acpd-9-10829-2009
Carvalho, V. S. B., et al. (2015). Air quality status and trends over the Metropolitan Area of São Paulo, Brazil as a result of emission control policies. Environmental Science & Policy, 47(November), 68–79. https://doi.org/10.1016/j.envsci.2014.11.001
Chiquetto, J. B., et al. (2021). Impact of a truck Driver’s strike on air pollution levels in São Paulo. Atmospheric Environment, 246, 118072. https://doi.org/10.1016/j.atmosenv.2020.118072
Cuervo-Maldonado, S. I., et al. (2010). Actualización en Aspergilosis con énfasis en Aspergilosis invasora. Infectio, 14, 131–144.
Dannemiller, K. C., et al. (2016). Indoor microbial communities: Influence on asthma severity in atopic and nonatopic children. Journal of Allergy and Clinical Immunology. https://doi.org/10.1016/j.jaci.2015.11.027
de Matos Castro e Silva, D., et al. (2015). A new culture medium for recovering the agents of cryptococcosis from environmental sources. Brazilian Journal of Microbiology, 46(2), 355–358. https://doi.org/10.1590/S1517-838246220130726
Dong, L., et al. (2016). Concentration and size distribution of total airborne microbes in hazy and foggy weather. Science of the Total Environment, 541, 1011–1018. https://doi.org/10.1016/j.scitotenv.2015.10.001
Egan, C., Li, D. W., & Klironomos, J. (2014). Detection of arbuscular mycorrhizal fungal spores in the air across different biomes and ecoregions. Fungal Ecology, 12, 26–31. https://doi.org/10.1016/j.funeco.2014.06.004
El-Batrawy, O. A. (2010). Relationships between personal, indoor and outdoor PM 10 in the residential environment in Damietta, Egypt. Journal of American Science., 6, 1413.
Elbert, W., et al. (2006). Contribution of fungi to primary biogenic aerosols in the atmosphere: Active discharge of spores, carbohydrates, and inorganic ions by Asco- and Basidiomycota. Atmospheric Chemistry and Physics Discussions, 6, 11317–11355. https://doi.org/10.5194/acpd-6-11317-2006
Emygdio, A. P. M., Degobbib, C., Gonçalves, F. L. T., & de Fátima, A. M. (2018). ‘One year of temporal characterization of fungal spore concentration in São Paulo metropolitan Area Brazil. Journal of Aerosol Science, 115, 121–132.
Fan, X. Y., et al. (2019). More obvious air pollution impacts on variations in bacteria than fungi and their co-occurrences with ammonia-oxidizing microorganisms in PM2.5. Environmental Pollution. Elsevier Ltd, 251, 668–680. https://doi.org/10.1016/j.envpol.2019.05.004
Farmer, D. K., & Riches, M. (2020). Measuring biosphere-atmosphere exchange of short-lived climate forcers and their precursors. Accounts of Chemical Research, 53(8), 1427–1435. https://doi.org/10.1021/ACS.ACCOUNTS.0C00203
Filali Ben Sidel, F., et al. (2015). Airborne fungal spores of Alternaria, meteorological parameters and predicting variables. International Journal of Biometeorology, 59(3), 339–346. https://doi.org/10.1007/s00484-014-0845-1
Franić, I., et al. (2023). Climate, host and geography shape insect and fungal communities of trees. Scientific Reports. https://doi.org/10.1038/s41598-023-36795-w
Gao, M., et al. (2016). Variation of correlations between factors and culturable airborne bacteria and fungi. Atmospheric Environment, 128, 10–19. https://doi.org/10.1016/J.ATMOSENV.2015.12.008
Grinn-Gofroń, A., Strzelczak, A., & Wolski, T. (2011). The relationships between air pollutants, meteorological parameters and concentration of airborne fungal spores. Environmental Pollution, 159(2), 602–608. https://doi.org/10.1016/j.envpol.2010.10.002
Gutarowska, B., & Piotrowska, M. (2007). Methods of mycological analysis in buildings. Building and Environment, 42(4), 1843–1850. https://doi.org/10.1016/j.buildenv.2006.02.015
Heald, C. L., & Spracklen, D. V. (2009). Atmospheric budget of primary biological aerosol particles from fungal spores. Geophysical Research Letters. https://doi.org/10.1029/2009GL037493
Hoog, G. S., Guarro, J., Gené, G., & Figueras, M. (2014). Atlas of Clinical Fungi (3rd ed.). CBS-KNAW Fungal Biodiversity Centre.
Jasinski, R., Pereira, L. A. A., & Braga, A. L. F. (2011). Poluição atmosférica e internações hospitalares por doenças respiratórias em crianças e adolescentes em Cubatão, São Paulo, Brasil, entre 1997 e 2004. Cadernos De Saúde Pública, 27(11), 2242–2252. https://doi.org/10.1590/S0102-311X2011001100017
Jones, A. M., et al. (2004). The effects of meteorological factors on atmospheric bioaerosol concentrations - A review. Science of the Total Environment, 326(1–3), 151–180. https://doi.org/10.1016/j.scitotenv.2003.11.021
Lacey, J., & Venette, J. (2020). Outdoor Air Sampling Techniques, Bioaerosols Handbook. CRC Press, pp. 407–471. https://doi.org/10.1201/9781003070023-16.
Liu, H., et al. (2019). ‘The distribution variance of airborne microorganisms in urban and rural environments. Environmental Pollution, 247, 898–906. https://doi.org/10.1016/j.envpol.2019.01.090
Martins, L. C., et al. (2002). Poluição atmosférica e atendimentos por pneumonia e gripe em São Paulo Brasil. Revista De Saúde Pública, 36, 88–94. https://doi.org/10.1590/S0034-89102002000100014
Martins, M. S. D. A., Isabel, M., & Lemos, A. (2008). Liquens como bioindicadores da qualidade do ar numa área de termoelétrica, Rio Grande do Sul, Brasil. Hoehnea, 35(3), 425–433.
Mitchell, C. S., et al. (2007). Current state of the science: health effects and indoor environmental quality. Environmental Health Perspectives, 115(6), 958–964. https://doi.org/10.1289/ehp.8987
Morales-Oyervides, L., et al. (2020). Biotechnological approaches for the production of natural colorants by talaromyces/penicillium: a review. Biotechnology Advances. https://doi.org/10.1016/J.BIOTECHADV.2020.107601
Morris, C. E., Georgakopoulos, D. G., & Sands, D. C. (2004). Ice nucleation active bacteria and their potential role in precipitation. Journal De Physique IV, 121, 87–103. https://doi.org/10.1051/jp4:2004121004
Moura, M. L., Caldas, C. C., et al. (2015). The impaction capacity of Millipore M air T ® and Merck MAS- 100 ® in an external environment. Access Journal of Environmental Research, 1(November), 1–6.
Munzi, S., Ravera, S., & Caneva, G. (2007). Epiphytic lichens as indicators of environmental quality in Rome. Environmental Pollution, 146(2), 350–358. https://doi.org/10.1016/j.envpol.2006.03.042
Nowakowicz-Dębek, B., et al. (2017). Evaluating bioaerosol exposure among bus drivers in the public transport sector. Journal of Occupational and Environmental Hygiene, 14(11), D169–D172. https://doi.org/10.1080/15459624.2017.1339165
Oliveira, M., et al. (2009). The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanisation level. International Journal of Biometeorology, 53(1), 61–73. https://doi.org/10.1007/s00484-008-0191-2
Onofre., L. H. F. S. B. (2010). DETERMINAÇÃO DA PRESENÇA DE FUNGOS ANEMÓFILOS E LEVEDURAS EM UNIDADE DE SAÚDE DA CIDADE DE FRANCISCO BELTRÃO- PR, pp. 22–26
Parsi, Z., & Grecki, T. (2006). Determination of ergosterol as an indicator of fungal biomass in various samples using non-discriminating flash pyrolysis. Journal of Chromatography A, 1130, 145–150. https://doi.org/10.1016/j.chroma.2006.07.045
Pescott, O. L., et al. (2015). Air pollution and its effects on lichens, bryophytes, and lichen-feeding Lepidoptera: Review and evidence from biological records. Biological Journal of the Linnean Society, 115(3), 611–635. https://doi.org/10.1111/bij.12541
Pöschl, U. (2005). Atmospheric aerosols: Composition, transformation, climate and health effects. Angewandte Chemie - International Edition, 44(46), 7520–7540. https://doi.org/10.1002/anie.200501122
Rackes, A., & Waring, M. S. (2013). Modeling impacts of dynamic ventilation strategies on indoor air quality of offices in six US cities. Building and Environment. https://doi.org/10.1016/j.buildenv.2012.10.013
Recio, R., et al. (2019). Picture of a Microorganism Trichoderma longibrachiatum: an unusual pathogen of fungal pericarditis. Clinical Microbiology and Infection. https://doi.org/10.1016/j.cmi.2019.02.006
Ristic, S., et al. (2017). Lichens as biological indicators of air quality in the urban area of Kursumlija (Southern Serbia). Kragujevac Journal of Science, 39, 165–175. https://doi.org/10.5937/kgjsci1739165r
Rosenfeld, D., et al. (2008). Flood or drought: How do aerosols affect precipitation? Science. https://doi.org/10.1126/science.1160606
Roy, S., & Gupta Bhattacharya, S. (2020). ‘Airborne fungal spore concentration in an industrial township: Distribution and relation with meteorological parameters. Aerobiologia, 36(4), 575–587. https://doi.org/10.1007/s10453-020-09653-9
Schmidt, S. K., et al. (2014). Do bacterial and fungal communities assemble differently during primary succession? Molecular Ecology. https://doi.org/10.1111/mec.12589
Schoenlein-crusius, I. H. et al. (2001). AIRBORNE FUNGI IN THE REGION OF CUBATÃO, SÃO PAULO STATE, BRAZI, pp. 61–65
Stamenković, S., et al. (2016). Air quality lichen monitoring at three selected urban areas in the Southern Serbia. Biologica NYSSANA, 7(September), 19–29. https://doi.org/10.5281/zenodo.159100
Stathakis, A., et al. (2015). Penicillium marneffei infection in a lung transplant recipient. Transplant Infectious Disease, 17(3), 429–434. https://doi.org/10.1111/tid.12377
Sterflinger, K., Tesei, D., & Zakharova, K. (2012). Fungi in hot and cold deserts with particular reference to microcolonial fungi. Fungal Ecology, 5(4), 453–462. https://doi.org/10.1016/J.FUNECO.2011.12.007
Temperini, C. V., et al. (2019). Diversity and abundance of airborne fungal spores in a rural cold dry desert environment in Argentinean Patagonia. Science of the Total Environment, 665, 513–520. https://doi.org/10.1016/j.scitotenv.2019.02.115
Theotônio, P. et al. (2007).‘O PAPEL DAS PARTÍCULAS DE AEROSSOL NO FUNCIONAMENTO DO ECOSSISTEMA AMAZÔNICO, Mudanças climáticas/ Artigos, pp. 48–50
Tiwari, P., Misra, B. N., & Sangwan, N. S. (2013). í µí»½-glucosidases from the fungus trichoderma: an efficient cellulase machinery in biotechnological applications. BioMed Research International. https://doi.org/10.1155/2013/203735
World Health Organization. (2003). Climate Change and Human Health - Risks and Responses Summary, pp. 1–37
Yamamoto, N., et al. (2012). Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. The ISME Journal, 6(10), 1801–1811. https://doi.org/10.1038/ismej.2012.30
Yao, M. (2018). Bioaerosol: A bridge and opportunity for many scientific research fields. Journal of Aerosol Science, 115, 108–112. https://doi.org/10.1016/j.jaerosci.2017.07.010
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Dulcilena de Matos Castro e Silva contributed to conceptualization, methodology, investiagtion, resources, data curation, writing—original draft preparation, writing—review and editing, and project administration; Fábio Luiz Teixeira Gonçalves contributed to conceptualization, supervision, and funding acquisition; Rosa Maria Nascimento Marcusso contributed to software and data curation; Maria Regina Alves Cardoso contributed to validation and writing—review and editing; and Valter Batista Duo Filho contributed to resources, data curation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Matos Castro e Silva, D., Duo Filho, V.B., Marcusso, R.M.N. et al. Analyses of culturable microorganisms and chemical pollutants in the air of urban and rural areas in the region of São Paulo, Brazil. Aerobiologia (2024). https://doi.org/10.1007/s10453-024-09823-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10453-024-09823-z