Abstract
Heavy metal pollution is a serious environmental problem. Most of the current techniques used to mitigate the toxic effects of heavy metals have limitations. This creates an urgent need to explore safer and more efficient methods to address these toxic effects. This study investigates the potential of nano-water hyacinth protein (nano-WHP) as an adsorbent and soil amendment to mitigate cadmium pollution. Nano-WHP is derived from water hyacinth protein and immobilized on nano-chitosan. The Cd adsorption capacity and removal efficiency of nano-WHP were determined. Nano-WHP was applied as a soil amendment to examine its impact on soil enzyme activity and the growth of common bean plants under Cd stress. Nano-WHP could remove 96% of Cd with an adsorption capacity of 150 mg Cd g⁻1. When used as a soil amendment under Cd stress, nano-WHP positively influenced soil enzyme activity, enhancing soil health and promoting the growth of common bean plants. The growth of nano-WHP-treated plants increased by approximately 35% and 50% in the first and second stages, respectively, compared to the control group under cadmium stress. Furthermore, nano-WHP significantly reduced oxidative stress markers such as lipid peroxidation, DNA oxidation, protein oxidation, and H₂O₂ levels, with reductions of about 90.63%, 85.13%, 79.35%, and 81.85%, respectively, compared to untreated plants. This reduction in oxidative stress markers is attributed to the lower availability of Cd and the heightened activity of the antioxidant machinery in nano-WHP-treated plants. These results establish a foundation for the formulation of sustainable and economically feasible methodologies to mitigate Cd contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Abiotic stress significantly impacts plants in their environment, adversely affecting plant growth and global crop productivity. Heavy metal stress, along with salt and drought, is particularly dangerous, inducing various negative effects at the cellular, physiological, and molecular levels. The toxicity of crops to heavy metals not only hampers crop production but also poses threats to human health and the ecosystem [32]. Among heavy metals, cadmium (Cd) is considered the most toxic, exhibiting toxicity in both high and low concentrations [39].
Cadmium's high solubility in water facilitates its entry into plant cells through the water stream. Additionally, it competes with essential elements like potassium (K), calcium (Ca), and iron (Fe) on transmembrane transporters, significantly reducing the absorption rate of these essential elements in the presence of higher Cd concentrations [72]. Oxidative stress represents one of the most toxic effects of cadmium on plant cells, as it generates reactive oxygen species (ROS) that subsequently deteriorate macro-biological molecules such as lipids, proteins, and nucleic acids [73]. Activating the antioxidant machinery can shield plants from the toxic effects of ROS induced by heavy metals [26].
Various strategies have been explored to enhance plant tolerance against heavy metal stress, including the use of hyperaccumulating species, plant growth-promoting rhizobacteria, plant hormones, and biochar [27, 39]. In recent years, the use of biochar and other soil amendments has gained popularity to address Cd pollution. The main goal is to chelate cadmium and convert it into an unavailable form, mitigating its toxic effects on soil microbial communities and plants [40, 42, 54, 91, 98]. The efficiency of biochar depends on its components, especially metal-chelating proteins such as phytochelatins and metallothioneins, which chelate heavy metals and sequester them into an unavailable form [5].
Water hyacinth (Eichhornia crassipes) is a pervasive invasive aquatic herb with abundant biomass. While its mechanical removal is performed to mitigate challenges in water bodies, the biomass poses ecological hazards if not repurposed effectively [35]. Water hyacinth biomass holds potential for applications such as animal feedstock, biofuels, biochar, and compost [43, 57]. Notably, water hyacinth biochar has gained attention for its efficacy in heavy metal adsorption from aqueous solutions [17, 20, 61, 70].
Water hyacinth biochar has been introduced as a soil amendment to enhance soil health and plant growth, particularly under stress conditions [21, 107]. Its ability to absorb heavy metals is attributed to its high content of metal-binding proteins, such as phytochelatins and metallothioneins [75]. However, a concern associated with biochar use is the potential introduction of contaminants into the soil. Therefore, it is preferable to employ purified soil amendments [102]. Concerns arise from factors such as variability in feedstock and raw materials, where contaminants from industrial waste or polluted areas may be retained in the biochar during pyrolysis [56, 95, 100].
The use of nano-materials loaded with adsorbent materials holds significant importance in pollutant remediation, offering advantages over traditional biochar [78]. In this study, we investigate the potential of purified protein from water hyacinth to adsorb cadmium and serve as a soil amendment by immobilizing it on nano-chitosan. The immobilization of proteins on nano-chitosan enhances their stability and activity [104]. Nano-materials, especially nano-chitosan particles, serve as excellent immobilization supports [67], demonstrating effectiveness in this role [3].
Nano-chitosan particles have gained attention across scientific and industrial fields due to their unique properties, including a large surface area, biocompatibility, ease of modification, enhanced mechanical strength, improved mass transfer, cost-effectiveness, antimicrobial properties, and pH sensitivity. These properties make nano-chitosan versatile and valuable in various applications, including biocatalysis, biomedicine, and environmental remediation [1, 90, 96].
Furthermore, nano-chitosan demonstrates promising benefits in agriculture, contributing to enhance plant growth, development, and tolerance to environmental stresses such as drought, salinity, and heavy metal toxicity [23]. As a soil amendment, nano-chitosan improves soil structure and fertility, enhances water retention, reduces soil erosion, and promotes nutrient availability for plants. The multifaceted advantages of nano-chitosan position it as a valuable material with diverse applications in agriculture and environmental remediation [2].
Most of the current techniques used to mitigate the toxic effects of heavy metals on water, soil, and plants have certain limitations [37]. Biochars themselves can be sources of contamination with heavy metals and pathogenic microbes [111]. Adding external rhizobacteria can disturb the environmental equilibrium in the soil [87]. Other synthetic materials have negative effects on the environment and human health. This underscores the urgent need to explore safer and more efficient ways to mitigate the negative effects of heavy metals on water, soil, and plants [37].
From this perspective, we aimed to establish a foundation for formulating safe sustainable and economically feasible methodologies to mitigate cadmium (Cd) contamination. Our study is based on the hypothesis that water hyacinth proteins possess a notable capability to efficiently capture and bind metals. When these proteins are immobilized on nano-chitosan, they gain higher stability and a larger surface area, enhancing their effectiveness in adsorbing metals. Additionally, the prepared nano water hyacinth protein (nano-WHP) is natural and safe for use, as it is composed of natural protein immobilized on nano-chitosan, which is biocompatible and non-toxic to the environment and living organisms.
To our knowledge, this is the first study to evaluate the adsorption of cadmium using nano water hyacinth protein. We assessed the cadmium adsorption capacity of the prepared water hyacinth protein and the impact of treating soil with nano-WHP on improving soil health and enhancing Cd tolerance in common beans.
2 Material and methods
2.1 Preparation of nano-water hyacinth protein
2.1.1 Water hyacinth protein extraction
The water hyacinth protein extraction process, following the methodology of Yifru et al. [105] is detailed as follows: Water hyacinth leaves were gathered from a canal in Sofia Village, Zagazig Governorate, Egypt. The collected leaves underwent destalking and were thoroughly washed using running tap water. Subsequently, the cleaned leaves were immersed in distilled water at a ratio of 2:1 (weight to volume) for 30 min. The soaked leaves were macerated using an electric blender. To solubilize leaf proteins, the resulting slurry's pH was adjusted to pH 9.0 with 0.1 M NaOH. The slurry was then filtered through cheesecloth. For protein coagulation, 0.1 M HCl was added to the filtrate until its pH reached 2.0. The coagulated mixture underwent centrifugation at 3800g for 10 min. The resulting pellets were collected, dried at 60°C, and stored for use as water hyacinth protein (WHP).
2.1.2 Immobilization of water hyacinth protein on nano-chitosan
The water hyacinth protein (WHP) was immobilized onto Nano-chitosan particles (NS6130-09–918) with a size range of 80–100 nm, obtained from Intelligent Materials Pvt. Ltd., USA. The immobilization procedure followed the method outlined by Badawy and Naguib [10] as follows: A 10% nano-chitosan suspension (w/v) was prepared at room temperature, following the product instructions to ensure stability. The prepared nano-chitosan suspension (100 mL) was incubated with 50g of WHP at room temperature for varying incubation periods, aiming to determine the optimal duration for maximum immobilization efficiency. After incubation, the mixture underwent centrifugation at 3800 g for 10 min. The resulting pellets were air-dried at room temperature and stored as nano-WHP. The supernatant was used for protein estimation using the Lowry assay [63] to determine immobilization efficiency, following the equation outlined by Huang et al. [45].
2.2 Cadmium removal efficiency for prepared nano-WHP in aqueous solution
2.2.1 Determination of removal efficiency and adsorption capacity
The cadmium removal efficiency and adsorption capacity of the prepared nano-WHP were evaluated in a batch system, following the method described by Wang et al. [99].
2.2.2 Evaluation the stability and reusability of prepared nano-water hyacinth protein.
In order to show the stability and reusability of the prepared nano-WHP, the adsorption–desorption cycle was repeated fifty times using the same nano-WHP using a separation column (detailed methods in the supplementary data).
2.3 Application of nano-WHP in soil for combating cadmium stress in common bean seedlings
2.3.1 Greenhouse study
The experimental setup involved plastic pots with dimensions of 25 cm in diameter and 15 cm in height, each filled with 3 kg of sandy loam soil. The pots were divided into two groups, each containing 24 pots, based on the inoculation of the soil with nano-WHP. In the first group, the soil in the pots remained untreated, serving as the control. In the second group, a blend of 15 g of nano-WHP was incorporated into each pot containing 3 kg of soil, establishing a ratio of 5 g per kg of soil (5g/kg soil). This ratio was determined from the adsorption experiment, where 5 g of nano-WHP exhibited the highest cadmium removal percentage, and further increases did not show additional benefits.
Five common bean (Phaseolus vulgaris) seeds (Agrimax Green Bean, Ag00310) were sown in each pot. After emergence, seedlings were thinned to two in each group. Cadmium (as CdCl2) was introduced at a single dose of 100 mg/kg of soil two weeks after sowing, a concentration known to induce high toxicity in bean plants [11]. The experiment included four treatments:
-
Group I: Control plants (plants grown in non-treated soil without Cd treatment).
-
Group II: Nano-WHP Plants (plants grown in nano-WHP-treated soil without Cd treatment).
-
Group III: Cd-Plants (plants grown in non-treated soil with Cd treatment).
-
Group IV: Cd-Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment).
Plants were housed in a greenhouse with day/night temperatures of 26/16 ± 2 °C and a relative humidity of 50 ± 4%. They were irrigated as needed to maintain constant soil moisture. The experiment continued for 3 weeks after the Cd treatment. This period marked the second stage, occurring before the death of untreated plants cultivated under cadmium stress. If the experiment had continued beyond this point, no untreated plants would have survived under cadmium stress conditions. Soil and shoot samples were collected after one week, which represented the initial manifestation of chlorosis and wilting symptoms due to cadmium toxicity in Cd-exposed plants.
2.3.2 Changes in soil enzymes activity
The assessment of soil enzyme activities is crucial for evaluating soil health [109]. In this study, β-glucosidase, urease, acid phosphatase, and dehydrogenase activities were determined according to the methods of Sanchez-Hernandez et al., [80] and Kaur and Kaur [53] (detailed methods in the supplementary data file).
2.3.3 Changes in growth parameters
We determined the shoot fresh and dry weights to calculate the live fine fuel moisture (LFFM) of shoots according to the following equation:
The change in growth percent was calculated according to the change in dry weight compared to the control according to the following equation:
We also determined some leaf growth parameters for the lower leaf, including leaf variables including leaf relative water content (RWC), leaf moisture (LM), and leaf dry matter content (LDMC) according to the following equations, respectively:
2.3.4 Changes in antioxidant machinery and oxidative stress markers
Oxidative stress markers
In this study, oxidative stress and damage in plant cells were assessed using various indicators. Hydrogen peroxide (H2O2), a representative reactive oxygen species in plant cells, was determined through its reaction with potassium iodide, following the method outlined by Alexieva et al. [4]. Lipid peroxidation, a key marker of oxidative damage, was quantified by measuring malondialdehyde (MDA) using the thiobarbituric acid (TBA) method, as reported by Li [58]. Protein oxidation was assessed through the measurement of tyrosine using an ELISA kit (Nikken SEIL Co., Ltd., Japan), following the procedures described by Kato et al. [51]. To evaluate DNA oxidation, the levels of 8-hydroxydeoxyguanosine (8-OHdG) were measured in prepared leaf extracts using an ELISA Kit (E-EL-0028) from Elabscience Biotechnology Inc., United States, according to the product manual. The decrease in the oxidative stress markers was calculated from the following equation:
where, Group III: Plants grown in non-treated soil with Cd treatment. Group IV: Plants grown in nano-WHP-treated soil without Cd treatment.
Antioxidant activity–Non-enzymatic antioxidant activity
The assessment of free radical scavenging capacity in shoots was conducted using the DPPH radical scavenging assay, following the method proposed by Blois [14]. The determination of non-enzymatic antioxidant compounds, namely total phenols and flavonoids, involved extracting these compounds from common bean shoots based on the procedure outlined by Campbell and Ellis [16]. The quantification of phenolic content was achieved through the Folin-Ciocalteu assay. Additionally, the determination of flavonoid content employed an aluminum chloride (AlCl3) assay, following the procedure described by Pallab et al. [74].
Antioxidant enzymes activity
Superoxide dismutase (SOD) activity was evaluated using the method of Beyer and Fridovich [13], which involves assessing the reduction of nitro blue tetrazolium (NBT). Polyphenol oxidase (PPO) activity was determined through the oxidation of pyrogallol, following the procedure outlined by Kar and Mishra [49]. The activities of both soluble and cell wall-bound peroxidases were measured according to the methodology described by Saroop et al. [82].
2.4 Statistical analysis
The experiment was systematically conducted five times, employing a completely randomized design to ensure the robustness and reliability of the results. The data collected were entered into an Excel sheet to generate figures and compute the mean values along with standard deviations (SD) from the five replicates. To assess statistical differences between distinct groups, a two-way analysis of variance (ANOVA) was performed using the Statistical Package for the Social Sciences (SPSS version 17.0 for Windows).
3 Results
3.1 Preparation of nano-water hyacinth protein and its adsorption capacity removal efficiency of cadmium in liquid solution
Figure 1A depicts that the immobilization efficiency increased with time pass till optimum incubation period. Optimal duration for achieving maximum immobilization efficiency was around 48 h. No further enhancement in immobilization efficiency was observed beyond this period.
Cadmium removal efficieny increased with increasing the amount of ano-water hyacinth protein (nano-WHP) till the concentration 5 g/L, Significantly, at a concentration of 5 g/L, nano-water hyacinth protein showcased a Cd removal efficiency of approximately 96%, with negligible alterations in efficiency beyond this concentration (Fig. 1B).
Nano-WHP exhibited an impressive adsorption capacity of about 150 mg Cd+2 g−1, as illustrated in Fig. 1B. Furthermore, it demonstrated notable stability and reusability, retaining over 75% of its removal efficiency even after 50 treatment cycles, as shown in Fig. 1C
3.2 Effect of nano-WHP on soil enzymes under Cd stress
Figure 2 presents compelling evidence of a substantial decrease in key soil enzymes (β-glucosidase, phosphatase, urease, and dehydrogenase) under cadmium stress in the no-treatment with nano-WHP. Interstingly, treatment with nano-WHP led to a significant increase in soil enzyme activity, both in normal conditions and under Cd pollution (Fig. 2). The soil enzyme activity in the nano-WHP treated group was significantly higher than that of the control under either normal or Cd contamination conditions.
Effect of nano-water hyacinth protein treatment on soil enzymes activity (glucosidase (A), dehydrogenase (B), phosphatase (C), and urease (D)) after a week (first stage) and 3 weeks (second stage) after Cd treatment. Group I: Control plants (plants grown in non-treated soil without Cd treatment); Group II: Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment); Group III: Cd-Plants (plants grown in non-treated soil with Cd treatment); and Group IV: Cd-Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment). Columns followed by different letters are significantly different according to a two-way ANOVA test (P = 0.05). The bars represent the standard deviation
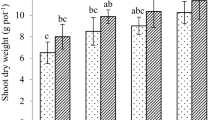
3.3 Effect of nano-WHP on plant growth under Cd stress
This study provides evidence for the efficacy of nano-Water Hyacinth Protein (nano-WHP) in alleviating the adverse effects of cadmium on common bean growth, as illustrated in Fig. 3D. Cadmium significantly impeded the growth of common bean seedlings, resulting in a 35% reduction compared to the control in the first stage and a more pronounced 75% decrease in the second stage. In contrast, plants treated with nano-WHP exhibited enhanced growth under normal conditions, surpassing the control by 45% and 55% in the first and second stages, respectively. Remarkably, under cadmium stress, nano-WHP-treated plants demonstrated growth increments of approximately 35% and 50% in the first and second stages, respectively, compared to the control.
Effect of nano-water hyacinth protein treatment on growth parameters (leaf relative water content (RWC) (A), leaf moisture (LM) (B), leaf dry matter content (LDMC) (C), growth change percent (D), and shoot live fine fuel moisture (LFFM) (E)) after a week (first stage) and 3 weeks (second stage) after Cd treatment. Group I: Control plants (plants grown in non-treated soil without Cd treatment); Group II: Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment); Group III: Cd-Plants (plants grown in non-treated soil with Cd treatment); and Group IV: Cd-Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment). Columns followed by different letters are significantly different according to a two-way ANOVA test (P = 0.05). The bars represent the standard deviation
In addition to assessing growth changes, we investigated shoot live fine fuel moisture (LFFM), a reliable indicator of plant health and combustibility that reflects shoot water content relative to its dry mass. Figure 3E demonstrates a significant enhancement in shoot LFFM with nano-WHP treatment. In the first stage, it increased from approximately 120% in the control to 150% under both normal and cadmium stress conditions, and in the second stage, it rose from 150 to 180%. This indicates that nano-WHP treatment contributes to increased shoot moisture, thereby aiding in biomass formation. Moreover, nano-WHP treatment significantly improved leaf growth parameters, including leaf relative water content (RWC), leaf moisture (LM), and leaf dry matter content (LDMC), under both normal conditions and cadmium contamination (Fig. 3A-C).
3.4 Effect of nano-WHP on antioxidant machinery and oxidative stress markers
The present study reveals a significant increase in oxidative stress markers H2O2, malondialdehyde (a product of lipid peroxidation), dityrosine (a product of protein oxidation), and 8-hydroxydeoxyguanosine (an indicator of DNA oxidation) under cadmium stress, observed in both nano-Water Hyacinth Protein (nano-WHP)-treated and non-treated plants during the first stage. However, in the second stage, these oxidative stress markers substantially decreased in nano-WHP-treated plants while significantly increasing in non-treated plants. The application of nano-WHP led to a remarkable reduction in lipid peroxidation (by 90.63%), DNA oxidation (by 85.13%), protein oxidation (by 79.35%), and H2O2 levels (by 81.85%) compared to plants without nano-WHP treatment (Table 1, Fig. 4).
Effect of nano-water hyacinth protein treatment on oxidative stress markers (hydrogen peroxide (H2O2) (A), protein oxidation marker, dityrosine (B), lipid peroxidation marker (MDA) (C), nucleic acid oxidation marker (8-OHdG) (D)) in common bean shoots after a week (first stage) and 3 weeks (second stage) after Cd treatment. Group I: Control plants (plants grown in non-treated soil without Cd treatment); Group II: Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment); Group III: Cd-Plants (plants grown in non-treated soil with Cd treatment); and Group IV: Cd-Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment). Columns followed by different letters are significantly different according to a two-way ANOVA test (P = 0.05). The bars represent the standard deviation
The treatment with Nano-WHP significantly boosted the levels of antioxidant enzymes (as shown in Fig. 5) and non-enzymatic antioxidant compounds, including radical scavenging capacity, phenols, and flavonoids (as illustrated in Fig. 6) during both the initial and subsequent stages. Conversely, the untreated group exhibited a minor increase in antioxidant enzyme levels during the first stage compared to the control, but during the second stage, the activity of antioxidant enzymes dramatically decreased, dropping significantly lower than that of the control (as depicted in Fig. 5).
Effect of nano-water hyacinth protein treatment on antioxidant enzymes (superoxide dismutase (SOD) (A), polyphenol oxidase (PPO) (B), and peroxidase (POX) (C) in common bean shoots after a week (first stage) and 3 weeks (second stage) after Cd treatment. Group I: Control plants (plants grown in non-treated soil without Cd treatment); Group II: Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment); Group III: Cd-Plants (plants grown in non-treated soil with Cd treatment); and Group IV: Cd-Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment). Columns followed by different letters are significantly different according to a two-way ANOVA test (P = 0.05). The bars represent the standard deviation
Effect of nano-water hyacinth protein treatment on non-enzymatic antioxidants (DPPH radical scavenging% (A), total phenols (B), and total flavonoids (C)) in common bean shoots after a week (first stage) and 3 weeks (second stage) after Cd treatment. Group I: Control plants (plants grown in non-treated soil without Cd treatment); Group II: Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment); Group III: Cd-Plants (plants grown in non-treated soil with Cd treatment); and Group IV: Cd-Nano-WHP plants (plants grown in nano-WHP-treated soil without Cd treatment). Columns followed by different letters are significantly different according to a two-way ANOVA test (P = 0.05). The bars represent the standard deviation
4 Discussion
Protein immobilization is a well-known technique for improve the protein use and stability [71]. Nano-particles are promising immobilization material due to its high surface area. Optimization the different conditions of the immobilization is an important point determines the immobilization efficiency [110]. The immobilization efficiency of water hyacinth protein on the nano-chitosan increased with the increase in incubation time until 48 h. Beyond this timeframe, no further increase in immobilization efficiency occurred. This indicates saturation of the nanoparticles with immobilized materials. This observation aligns with existing literature, as reported by Dwevedi [30].
One of the applications of the immobilized protein is its use as adsorbent to various environmental pollutants such as heavy metals. The amount of adsorbent used is a crucial factor influencing the effectiveness of the metal adsorption process [62]. Notably, nano-water hyacinth protein (nano-WHP) demonstrated a Cd removal efficiency of about 96% at 5 g/L, with no significant change in efficiency beyond this concentration. Higher adsorbent masses resulted in the saturation of active sites due to interference between these sites, as reported by Poonam et al. [76]. However, the adsorption capacity decreased with an increase in nano-WHP concentration, attributed to insufficient solutes to occupy all active adsorption sites. This reduction is consistent with findings by Masoumi et al. [66] and Poonam et al. [76], emphasizing the impact of the unsaturation of active sites.
Nano-WHP exhibited a remarkable adsorption capacity of approximately 150 mg Cd+2 g−1 (Fig. 1B). This is surpassing various adsorbent materials (Table 2). Also, nano-WHP showed high stability and reusability as it save more than 75% of its removal efficiency after 50 treatment cycle (Fig. 1C). This heightened adsorption capacity can be attributed to the synergistic effects of WHP and nano-chitosan, as reported by Sobral et al. [88]. The intrinsic heavy metal removal capabilities of water hyacinth, documented by Mahmood et al. [64], Zheng et al. [112], Cao et al. [17], Liu et al. [61], and Hemalatha et al. [44], further contribute to the impressive adsorption capacity of nano-WHP. The extensive surface area of nanoparticles enhances their adsorption effectiveness compared to bulk materials, as highlighted by Roberto et al. [79].
The heavy metal pollution is not only a danger in the aquatic environments but also, it represents a high danger in soil pollution, as heavy metals negatively affect the soil health and so plant growth [28]. Heavy metals render the soil enzymes activity [89]. Various soil enzymes have microbial origins and are intricately linked to essential cycles such as carbon, nitrogen, and phosphorus. Enzyme activity is widely measured in microbiology, biochemistry, and agricultural sciences, often serving as a crucial indicator of soil health [34, 48, 50, 92]. These enzymes are highly sensitive to changes in soil conditions, whether natural or anthropogenic. Notably, soil pollution with heavy metals, including Cu, Pb, Zn, Cd, V, and Ni, represents a significant alteration to soil ecosystems, with Cd posing the highest ecological risk among these metals [93].
The present study results ensured the negative effect of cadmium on soil enzymes activity (Fig. 2). This aligns with the findings by Lin et al. [60], who reported enzyme reduction under heavy metal pollution. Sharma et al. [86] similarly documented negative impacts on soil enzyme activity due to heavy metal pollution in the soil near the Yamuna River in Delhi. Tang et al. [94] demonstrated a significant decrease in soil enzymes with elevated Cd and Zn levels in heavy metal-polluted soil. Subsequently, Tang and colleagues associated the decline in soil enzyme activity with increased heavy metal concentrations in lead–zinc tailing pond soils [93].
The observed decline in soil enzyme activity can be attributed to the adverse effects of heavy metals on the growth and metabolism of soil microorganisms, which serve as the primary source of soil enzymes [12]. Tang et al. [94] proposed that the decrease in soil enzyme activity is linked to heavy metals interacting with enzyme proteins, leading to denaturation, chelation with enzyme substrates, or interfering with the formation of enzyme reaction products. Additionally, the accumulation of heavy metals in the soil can reduce available nutrients, further contributing to a decline in soil enzyme activity [24, 25].
On the other hand, with soil treatment with nano-Water Hyacinth Protein (nano-WHP), the soil enzymes activity significantly increased under either the normal conditions or Cd contamination condition (Fig. 2). This heightened enzyme activity is attributed to the activation of soil microbiota stimulated by the presence of nano-WHP. Similar findings were reported by Chaudhary et al. [19], who observed the activation of microbial diversity and improved soil health with the application of water hyacinth biochar. Additionally, Hammam et al. [41] demonstrated the positive impact of water hyacinth biochar on soil enzymes through the activation of the soil microbiota, further supporting the beneficial effects on soil health.
The enhancement in soil enzyme activity with nano-WHP application can also be linked to another component, namely nano-chitosan. Khati et al. [55] documented the positive influence of nano-chitosan application on soil health. Furthermore, the increase in soil enzyme activity with nano-WHP treatment under Cd pollution can be attributed to the adsorption of Cd on nano-WHP, as illustrated in Fig. 1, where 5g of nano-WHP removed 96% of Cd in a 100 mL solution. This adsorption renders Cd less available in the soil, minimizing or eliminating its negative effects. Tang et al. [94] highlighted that the adverse impact of heavy metals on soil enzymes depends primarily on their availability in the soil. The more available the heavy metal, the greater its negative effect [36].
Furthermore, improving soil enzyme activity in the presence of nano-WHP can be related to the ability of nano-WHP to provide a suitable environment for the stability and activity of soil enzymes. Similarly, Yasin and his colleagues recently reported that the application of biochar-modified nanoparticles improved soil enzyme activity by providing suitable conditions for stability and activity [103].
The decrease in soil enzymes activity results in the decrease in the available nutrients in the soil, which negatively affect the plant growth [33]. The deleterious impact of cadmium on plant growth is extensively documented, prompting a comprehensive exploration of strategies to enhance plant tolerance to cadmium stress [117]. This study provides evidence for the efficacy of nano-Water Hyacinth Protein (nano-WHP) in alleviating the adverse effects of cadmium on common bean growth. Cadmium significantly decreased the growth of common bean seedlings (Fig. 3D). On the other, plants treated with nano-WHP showed enhanced growth under normal conditions, or under cadmium stress. These findings underscore the potential of nano-WHP in promoting common bean growth and mitigating the inhibitory effects of cadmium stress, highlighting its application as a promising strategy for enhancing plant tolerance to heavy metal-induced stress.
In addition to assessing growth changes, we investigated shoot live fine fuel moisture (LFFM), a reliable indicator of plant health and combustibility that reflects shoot water content relative to its dry mass [29]. The results showed a significant enhancement in shoot LFFM with nano-WHP treatment under both normal and cadmium stress conditions. This indicates that nano-WHP treatment contributes to increased shoot moisture, thereby aiding in biomass formation. Moreover, nano-WHP treatment significantly improved leaf growth parameters, including leaf relative water content (RWC), leaf moisture (LM), and leaf dry matter content (LDMC), under both normal conditions and cadmium contamination (Fig. 3A-C). This growth increase is attributed to the positive impact of nano-WHP on soil enzyme activity, consistent with findings by Hammam et al. [41], who observed enhanced corn growth with water hyacinth biochar application due to its positive effects on soil enzymes. Increased soil enzyme activity enhances nutrient availability, benefiting plant growth parameters [113]. Similarly, Attaran Dowom et al. [8] reported that chitosan enhanced plant growth by improving soil health, leading to increased nutrients and improved physiological and biochemical status under stress. The significant growth increase observed with nano-WHP treatment suggests a synergistic effect between nano-chitosan and water hyacinth protein.
One of the most perilous consequences of heavy metal stress is the initiation of oxidative stress, characterized by the production of reactive oxygen species (ROS) that oxidize crucial cellular components such as lipids, proteins, and nucleic acids, causing oxidative stress. The products of the lipids, proteins, and nucleic acids oxidation are considered as oxidative stress markers which content directly proportion with ROS content in the cells [38, 52, 115]. The study finds that exposure to cadmium stress increases oxidative stress markers in plants. Initially, both nano-Water Hyacinth Protein (nano-WHP)-treated and untreated plants show elevated levels of these markers. However, in the second stage, plants treated with nano-WHP demonstrate a significant decrease in oxidative stress markers, while levels continue to rise in untreated plants. The initial surge in oxidative stress markers plays a crucial role as signaling molecules, inducing plant defense mechanisms against various stresses [85, 97]. Cadmium, being a non-redox metal, rapidly induces the production of ROS, leading to oxidative stress in plants exposed to cadmium pollution. This rapid ROS production occurs indirectly by disrupting the electron transport chain and cellular metabolism. Additionally, cadmium can displace redox-active metals, such as ferrous and copper, triggering the Fenton and Haber–Weiss reactions—common mechanisms for ROS production in living cells, thus contributing to oxidative stress [26]. The oxidative stress markers were significantly higher in the non-treated plants than in those treated with nano-WHP. This difference can be attributed to the adsorption capacity of nano-WHP, which reduces the availability of Cd in the soil. Consequently, the toxic effects on the treated plants' proteins are diminished. This aligns with findings from Haider et al. [39, 40], who observed that increased Cd availability in the soil led to heightened oxidative stress, causing peroxidation of proteins and lipids as well as DNA damage. Additionally, Shaari et al. [84] reported that the extent of oxidative stress damage resulting from Cd toxicity is contingent upon its availability in the soil.
Plants exhibit tolerance to increased reactive oxygen species (ROS) and the consequent oxidative stress by employing highly effective antioxidant machinery, which is induced to detoxify excess ROS and maintain cellular oxidative homeostasis. This antioxidant machinery includes various molecules, such as enzymes (peroxidases, polyphenol oxidases, catalase, and superoxide dismutase), and non-enzymatic compounds like phenols and flavonoids [15]. Nano-WHP treatment significantly increased both antioxidant enzymes (Fig. 5) and non-enzymatic antioxidant compounds (radical scavenging capacity, phenols, and flavonoids) (Fig. 6) in both the first and second stages. Consequently, nano-WHP-treated plants demonstrated enhanced tolerance to oxidative stress under cadmium exposure, leading to a significant decrease in oxidative stress markers comparable to control plants. In contrast, non-treated plants failed to sustain antioxidant machinery activity, resulting in a significant decrease in the second stage and a subsequent increase in oxidative stress markers. This observation aligns with the findings of Cuypers et al. [26], emphasizing the importance of balanced redox biology for plant adaptation to oxidative stress induced by cadmium pollution. Additionally, reports by Paithankar et al. [73] highlight the role of signaling molecules in activating the antioxidant machinery to suppress oxidative stress induced by heavy metal stress. Many studies have underscored the activation of the antioxidant machinery as a crucial biochemical change necessary for inducing heavy metal tolerance. For instance, melatonin has been shown to induce cadmium tolerance in soybean and strawberry plants through the activation of antioxidant signaling cascades [46, 81]. Nano-selenium has also been reported to counteract Cd, Pb, and Hg toxicity in Brassica chinensis by improving its antioxidant system [114].
4.1 Conclusion and future prospective
In conclusion, our study represents a pioneering effort to evaluate the efficacy of nano-water hyacinth protein as a soil amendment for cadmium (Cd) phytoremediation and its impact on plant growth under Cd stress. We highlight the economic feasibility and effectiveness of nano-Water Hyacinth Protein (nano-WHP) when applied as soil amendment at a minimal rate of 5 g per 1 kg of soil. The application of nano-WHP activates soil enzymes, thereby improving soil health and enhancing common bean plant growth under both normal and Cd stress conditions. Nano-WHP not only promotes growth but also induces Cd tolerance in plants by activating the antioxidant machinery, mitigating oxidative stress.
This research opens avenues for further exploration into the potential of nano-water hyacinth protein as soil amendment to mitigate Cd stress. Future studies could delve into understanding various factors influencing the Cd adsorption and desorption capacity, providing valuable insights for optimizing its application. Additionally, assessing the Cd content in soil and plants, as well as investigating different factors influencing the protein's efficiency would contribute substantially to the existing knowledge on its role in Cd removal and improving plant tolerance. Our findings pave the way for the development of sustainable and economically viable strategies for addressing Cd contamination in agricultural settings.
Data availability
No datasets were generated or analysed during the current study.
References
Abdella, M.A.A., El-Sherbiny, G.M., El-Shamy, A.R., Atalla, S.M.M., Ahmed, S.A.: Statistical optimization of chemical modification of chitosan-magnetic nano-particles beads to promote Bacillus subtilis MK1 α-amylase immobilization and its application. Bull. Natl. Res. Cent. 44, 40 (2020). https://doi.org/10.1186/s42269-020-00301-3
Abd-Elrahman, S.H., El-Gabry, Y.A.E.-G., Hashem, F.A., Ibrahim, M.F.M., El-Hallous, E.I., Abbas, Z.K., Darwish, D.B.E., Al-Harbi, N.A., Al-Qahtani, S.M., Taha, N.M.: Influence of nano-chitosan loaded with potassium on potassium fractionation in sandy soil and strawberry productivity and quality. Agronomy 13(4), 1126 (2023). https://doi.org/10.3390/agronomy13041126
Ajayi, E.I., Oladele, J.O., Babalola, A.D.: Chapter 17 - Application of nanochitosan in enzyme immobilization. In: Adetunji, C., Hefft, D., Jeevanandam, J., Danquah, M. (eds.) Next Generation Nanochitosan, pp. 235–272. Academic Press (2023)
Alexieva, V., Sergio, I., Mapelli, S., Karanov, E.: The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 24, 1337–1344 (2001). https://doi.org/10.1046/j.1365-3040.2001.00778.x
Amin, K.F., Gulshan, F., Asrafuzzaman, F.N.U., Das, H., Rashid, R., Hoque, S.M.: Synthesis of mesoporous silica and chitosan-coated magnetite nanoparticles for heavy metal adsorption from wastewater. Environ. Nanotech. Monit. Manag. 20, 100801 (2023). https://doi.org/10.1016/j.enmm.2023.100801
Amin, M.A., Haider, G., Rizwan, M., Schofield, H.K., Qayyum, M.F., Zia-ur-Rehman, M., Ali, S.: Different feedstocks of biochar affected the bioavailability and uptake of heavy metals by wheat (Triticum aestivum L.) plants grown in metal contaminated soil. Environ. Res. 217, 114845 (2023). https://doi.org/10.1016/j.envres.2022.114845
Arul, A., Kavitha, S., Christus, A.A.B., Surya, V.J., Ravikumar, A., Sivalingam, Y.: Enhanced removal of Pb (II) and Cd (II) ions from aqueous systems using coated magnetic nanoparticles in activated carbon derived from corncob waste. Surf. Interf. 40, 103095 (2023). https://doi.org/10.1016/j.surfin.2023.103095
Attaran Dowom, S., Karimian, Z., Mostafaei Dehnavi, M., Samiei, L.: Chitosan nanoparticles improve physiological and biochemical responses of Salvia abrotanoides (Kar.) under drought stress. BMC Plant Biol. 22, 364 (2022). https://doi.org/10.1186/s12870-022-03689-4
Ayub, M.A., Ahmad, H.R., Zia-ur-Rehman, M., et al.: Comparative investigation of cd adsorption on alkaline sandy clay loam soil treated with cerium oxide nanoparticles, organic and inorganic amendments. Eurasian Soil Sc. (2023). https://doi.org/10.1134/S1064229323601555
Badawy, N.M., Naguib, D.M.: Nano metallothionein for lead removal from battery industry wastewater. Biocat Agric Biotech 38, 102201 (2021). https://doi.org/10.1016/j.bcab.2021.102201
Bahmani, R., Modareszadeh, M., Bihamta, M.R.: Genotypic variation for cadmium tolerance in common bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Safety 190, 110178 (2020). https://doi.org/10.1016/j.ecoenv.2020.110178
Bai, D.-S., Wang, Y.-W., Yang, X., Lai, J.-L., Luo, X.-G.: Effects of long-term (10 years) remediation of Caragana on soil enzyme activities, heavy metals, microbial diversity and metabolic spectrum of coal gangue. Ecol. Engin. 181, 106679 (2022). https://doi.org/10.1016/j.ecoleng.2022.106679
Beyer, Jr, Fridovich, W.F.: Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 161, 559–566(1987).https://doi.org/10.1016/0003-2697(87)90489-1
Blois, M.: Antioxidant determinations by the use of a stable free radical. Nature 181, 1199–1200 (1958). https://doi.org/10.1038/1811199a0
Borges, C.V., Orsi, R.O., Maraschin, M., Lima, G.P.P.: Chapter 27 - Oxidative stress in plants and the biochemical response mechanisms. In: Ghorbanpour, M., Shahid, M.A. (eds.) Plant Stress Mitigators, pp. 455–468. Academic Press (2023). https://doi.org/10.1016/B978-0-323-89871-3.00022-7
Campbell, M.M., Ellis, B.E.: Fungal elicitor-mediated responses in pine cell cultures. Planta 186, 409–417 (1992). https://doi.org/10.1007/BF00195322
Cao, F., Lian, C., Yu, J., Yang, H., Lin, S.: Study on the adsorption performance and competitive mechanism for heavy metal contaminants removal using novel multi-pore activated carbons derived from recyclable long-root Eichhornia crassipes. Bioresour. Technol. 276, 211–218 (2019). https://doi.org/10.1016/j.biortech.2019.01.007
Chandra, D., Molla, M.T.H., Bashar, M.A., Islam, S., Ahsan, S.: Chitosan-based nano-sorbents: synthesis, surface modification, characterisation and application in Cd (II), Co (II), Cu (II) and Pb (II) ions removal from wastewater. Sci. Rep. 13, 6050 (2023). https://doi.org/10.1038/s41598-023-32847-3
Chaudhary, P., Sharma, A., Chaudhary, A., Khati, P., Gangola, S., Maithani, D.: Illumina based high throughput analysis of microbial diversity of maize rhizosphere treated with nanocompounds and Bacillus sp. Appl. Soil Ecol. 159, 103836 (2021). https://doi.org/10.1016/j.apsoil.2020.103836
Chen, L., Li, F., Wei, Y., Li, G., Shen, K., He, H.-J.: High cadmium adsorption on nanoscale zero-valent iron coated Eichhornia crassipes biochar. Environ. Chem. Lett. 17, 589–594 (2019). https://doi.org/10.1007/s10311-018-0811-y
Chen, Q., Cao, X., Liu, B., Nie, X., Liang, T., Suhr, J., Ci, L.: Effects of functional carbon nanodots on water hyacinth response to Cd/Pb stress: Implication for phytoremediation. Environ. Manag. 299, 113624 (2021). https://doi.org/10.1016/j.jenvman.2021.113624
Cheng, Y., Wen, T., Zhou, W., Yuan, Y., Sun, R.: Surface-loaded magnesium and phosphorus-modified lignite adsorbents: Efficient adsorption and immobilization for remediation of Cd-contaminated water and soil. Environ. Techn. Innov. 32, 103442 (2023). https://doi.org/10.1016/j.eti.2023.103442
Chouhan, D., Mandal, P.: Applications of chitosan and chitosan based metallic nanoparticles in agrosciences-A review. Inter. J. Biol. Macromol. 166, 1554–1569 (2021). https://doi.org/10.1016/j.ijbiomac.2020.11.035
Ciarkowska, K.: Organic matter transformation and porosity development in non-reclaimed mining soils of different ages and vegetation covers: a field study of soils of the zinc and lead ore area in SE Poland. J. Soils Sediments 17, 2066–2079 (2017). https://doi.org/10.1007/s11368-017-1678-4
Ciarkowska, K., Gargiulo, L., Mele, G.: Natural restoration of soils on mine heaps with similar technogenic parent material: a case study of long-term soil evolution in Silesian-Krakow upland Poland. Geoderma 261, 141–150 (2016). https://doi.org/10.1016/j.geoderma.2015.07.018
Cuypers, A., Vanbuel, I., Iven, V., Kunnen, K., Vandionant, S., Huybrechts, M., Hendrix, S.: Cadmium-induced oxidative stress responses and acclimation in plants require fine-tuning of redox biology at subcellular level. Free Radic. Biol. Med. 199, 81–96 (2023). https://doi.org/10.1016/j.freeradbiomed.2023.02.010
Datta, K., Roychoudhury, A.: Chapter 17 - Cadmium-induced oxidative stress and remediation in plants. In: Naeem, M., Aftab, T., Ansari, A.A., Gill, S.S., Macovei, A. (eds.) Hazardous and Trace Materials in Soil and Plants, pp. 247–261. Academic Press (2022). https://doi.org/10.1016/B978-0-323-91632-5.00017-3
Deng, S., Zhang, X., Zhu, Y., Zhuo, R.: Recent advances in phyto-combined remediation of heavy metal pollution in soil. Biotechnol. Adv. 72, 108337 (2024). https://doi.org/10.1016/j.biotechadv.2024.108337
Dey, R., Raghuwanshi, R.: Comprehensive assessment of growth parameters for screening endophytic bacterial strains in Solanum lycopersicum (Tomato). Heliyon 6(10), e05325 (2020). https://doi.org/10.1016/j.heliyon.2020.e05325
Dwevedi, A.: Basics of Enzyme Immobilization. In: Enzyme Immobilization. Springer, Cham, pp 21–44 (2016) https://doi.org/10.1007/978-3-319-41418-8_2
Elias, M.M.C., Soares, L.C., Maia, L.C., Taylor, J.G., Adarme, O.F.H., Ferreira, G.M.D., de Azevedo, E.R., de Siervo, A., Silva, L.H.M., Gurgel, L.V.A.: Batch and continuous adsorption of Cd(II) and Pb(II) on polycarboxylated sugarcane bagasse. J. Water Proc. Eng. 54, 103947 (2023). https://doi.org/10.1016/j.jwpe.2023.103947
Feki, K., Tounsi, S., Mrabet, M., Mhadhbi, H., Brini, F.: Recent advances in physiological and molecular mechanisms of heavy metal accumulation in plants. Environ. Sci. Pollut. Res. 28, 64967–64986 (2021). https://doi.org/10.1007/s11356-021-16805-y
Fu, H., Ma, S., Wang, L., Xue, W., Xiong, S., Sui, F., Liu, H., Li, C., Li, G., Duan, R., Zhao, P.: Hierarchically porous magnetic biochar as an amendment for wheat (Triticum aestivum L.) cultivation in alkaline Cd-contaminated soils: Impacts on plant growth, soil properties and microbiota. Chemosphere 352, 141295 (2024). https://doi.org/10.1016/j.chemosphere.2024.141295
Furtak, K., Gałązka, A.: Enzymatic activity as a popular parameter used to determine the quality of the soil environment. Polish J. Agro. 37, 22–30 (2019). https://doi.org/10.26114/pja.iung.385.2019.37.04
Gaurav, G.K., Mehmood, T., Cheng, L., Klemeš, J.J., Shrivastava, D.K.: Water hyacinth as a biomass: A review. J. Clean. Prod. 277, 122214 (2020). https://doi.org/10.1016/j.jclepro.2020.122214
Ghassemi-Golezani, K., Farhangi-Abriz, S.: Biochar related treatments improved physiological performance, growth and productivity of Mentha crispa L. plants under fluoride and cadmium toxicities. Indus. Crop. Prod. 194, 116287 (2023). https://doi.org/10.1016/j.indcrop.2023.116287
Ghorbani, A., Emamverdian, A., Pehlivan, N., Zargar, M., Razavi, S.M., Chen, M.: Nano-enabled agrochemicals: mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J. Nanobiotechnol. 22, 91 (2024). https://doi.org/10.1186/s12951-024-02371-1
Ghuge, S.A., Nikalje, G.C., Kadam, U.S., Suprasanna, P., Hong, J.C.: Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 450, 131039 (2023). https://doi.org/10.1016/j.jhazmat.2023.131039
Haider, F.U., Coulter, J.A., Cheema, S.A., Farooq, M., Wu, J., Zhang, R., Shuaijie, G., Liqun, C.: Co-application of biochar and microorganisms improves soybean performance and remediate cadmium-contaminated soil. Ecotoxicol. Environ. Safety 214, 112112 (2021b). https://doi.org/10.1016/j.ecoenv.2021.112112
Haider, F.U., Liqun, C., Coulter, J.A., Cheema, S.A., Wu, J., Zhang, R., Wenjun, M., Farooq, M.: Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Safety 211, 111887 (2021a). https://doi.org/10.1016/j.ecoenv.2020.111887
Hammam, A.A., Mohamed, E.S., El-Namas, A.E., Abd-Elmabod, S.K., Badr Eldin, R.M.: Impacted application of water-hyacinth-derived biochar and organic manures on soil properties and barley growth. Sustainability 14, 13096 (2022). https://doi.org/10.3390/su142013096
Hasnain, M., Munir, N., Abideen, Z., Zulfiqar, F., Koyro, H.W., El-Naggar, A., Caçador, I., Duarte, B., Rinklebe, J., Yong, J.W.H.: Biochar-plant interaction and detoxification strategies under abiotic stresses for achieving agricultural resilience: A critical review. Ecotoxicol. Environ. Safety 249, 114408 (2023). https://doi.org/10.1016/j.ecoenv.2022.114408
He, X., Wang, Y., Tai, M.H., Lin, A., Owyong, S., Li, X., Leong, K., Yusof, M.L.M., Ghosh, S., Wang, C.-H.: Integrated applications of water hyacinth biochar: A circular economy case study. J. Clean. Prod. 378, 134621 (2022). https://doi.org/10.1016/j.jclepro.2022.134621
Hemalatha, D., Narayanan, R.M., Sanchitha, S.: Removal of Zinc and Chromium from industrial wastewater using water hyacinth (E. crassipes) petiole, leaves and root powder: Equilibrium study. Mater Today: Proc. 43(2), 1834–1838 (2021). https://doi.org/10.1016/j.matpr.2020.10.725
Huang, J., Xiao, H., Li, B., Wang, J., Jiang, D.: Immobilization of Pycnoporus sanguineus laccase on copper tetra-aminophthalocyanine–Fe3O4 nanoparticle composite. Biotechnol. Appl. Biochem. 44, 93–100 (2006). https://doi.org/10.1042/BA20050213
Imran, M., Khan, A., Mun, B.-G., Bilal, S., Shaffique, S., Kwon, E.-H., Kang, S.-M., Yun, B.-W., Lee, I.-J.: Melatonin and nitric oxide: Dual players inhibiting hazardous metal toxicity in soybean plants via molecular and antioxidant signaling cascades. Chemosphere 308, 136575 (2022). https://doi.org/10.1016/j.chemosphere.2022.136575
Irawan, C., Refki, M.F., Hidayat, R., Mu’minah, R., Nata, I.F., Putra, M.D., Triantoro, A.: Synthesis of magnetic nanoparticles coated zirconia using one-pot solvothermal processes as adsorbent for Pb(II) and Cd(II) removal. South Afri. J. Chem. Eng. 45, 247–255 (2023). https://doi.org/10.1016/j.sajce.2023.06.003
Jain, R., Pandey, A.: Soil enzymes and microbial endophytes as indicators of climate variation along an altitudinal gradient with respect to wheat rhizosphere under mountain ecosystem. Rhizosphere 2, 75–84 (2016). https://doi.org/10.1016/j.rhisph.2016.07.007
Kar, M., Mishra, D.: Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiol. 57, 315–319 (1976). https://doi.org/10.1104/pp.57.2.315
Karaca, A., Cetin, S.C., Turgay, O.C., Kizilkaya, R.: Soil Enzymes as Indication of Soil Quality. In: Shukla, G., Varma, A. (eds.) Soil Enzymology. Soil Biology, vol 22. Springer, Berlin, Heidelberg (2010). https://doi.org/10.1007/978-3-642-14225-3_7
Kato, Y., Wu, X., Naito, M., Nomura, H., Kitamoto, N., Osawa, T.: Immunochemical detection of protein dityrosine in atherosclerotic lesion of apoEdeficient mice using a novel monoclonal antibody. Biochem. Biophys. Res. Commun. 275(1), 11–15 (2000). https://doi.org/10.1006/bbrc.2000.3265
Kaur, H., Goyal, N.: Chapter3 - Biochemical adaptations in plants under heavy metal stress: A revisit to antioxidant defense network. In: Aftab, T., Hakeem, K. (eds.) Metals Metalloids Soil Plant Water Systems, pp. 51–90. Academic Press (2022). https://doi.org/10.1016/B978-0-323-91675-2.00001-9
Kaur, J., Kaur, G.: Dehydrogenase activity as a biological indicator of soil health. Chem. Sci. Rev. Lett. 10(39), 326–329 (2021). https://doi.org/10.37273/chesci.cs205205338
Khan, Z., Fan, X., Khan, M.N., Khan, M.A., Zhang, K., Fu, Y., Shen, H.: The toxicity of heavy metals and plant signaling facilitated by biochar application: Implications for stress mitigation and crop production. Chemosphere 308, 136466 (2022). https://doi.org/10.1016/j.chemosphere.2022.136466
Khati, P., Chaudhary, P., Gangola, S., Bhatt, P., Sharma, A.: Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech 7, 81 (2017). https://doi.org/10.1007/s13205-017-0668-y
Kujawska, J.: Content of heavy metals in various biochar and assessment environmental risk. J. Ecol. Engin. 24(8), 287–295 (2023). https://doi.org/10.12911/22998993/166557
Li, F., He, X., Srishti, A., Song, S., Tan, H.T.W., Sweeney, D.J., Ghosh, S., Wang, C.-H.: Water hyacinth for energy and environmental applications: A review. Biores. Technol. 327, 124809 (2021). https://doi.org/10.1016/j.biortech.2021.124809
Li, H.S.: Principles and techniques of plant physiological biochemical experiment, 2nd edn., pp. 260–263. Higher Education Press, Beijing, China (2000)
Limmun, W., Limmun, W., Borkowski, J.J., Ishikawa, N., Pairintra, R., Chungcharoen, T., Phanchindawan, N., Maneesri, W., Pewpa, O., Ito, A.: Eco-friendly magnetic biochar from Leb Mu Nang banana peel: Response surface methodology optimization for Cd(II) adsorption from synthetic wastewater. Biores. Tech. Rep. 2023, 101743 (2023). https://doi.org/10.1016/j.biteb.2023.101743
Lin, H., Liu, C., Li, B., Dong, Y.: Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Haz. Mater. 402, 123829 (2021). https://doi.org/10.1016/j.jhazmat.2020.123829
Liu, C., Ye, J., Lin, Y., Wu, J., Price, G.W., Burton, D., Wang, Y.: Removal of Cadmium (II) using water hyacinth (Eichhornia crassipes) biochar alginate beads in aqueous solutions. Environ. Pollution 264, 114785 (2020). https://doi.org/10.1016/j.envpol.2020.114785
Liu, Y., Xin, Z., Tian, L., Villa-Gomez, D., Wang, W., Cao, Y.: Fabrication of peptide-encapsulated sodium alginate hydrogel for selective gallium adsorption. Inter. J. Biol. Macromol. 263, 130436 (2024). https://doi.org/10.1016/j.ijbiomac.2024.130436
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193(1), 265–75 (1951). (https://pubmed.ncbi.nlm.nih.gov/14907713/)
Mahmood, T., Malik, S.A., Hussain, S.T.: Biosorption and recovery of heavy metals from aqueous solutions by Eichhornia crassipes (water hyacinth) ash. BioRes 5(2), 1244–1256 (2010). (https://bioresources.cnr.ncsu.edu/resources/biosorption-and-recovery-of-heavy-metals-from-aqueous-solutions-by-eichhornia-crassipes-water-hyacinth-ash/)
Masood, N., Irshad, M.A., Nawaz, R., Abbas, T., Abdel-Maksoud, M.A., AlQahtani, W.H., AbdElgawad, H., Rizwan, M., Abeed, A.M.A.: Green synthesis, characterization and adsorption of chromium and cadmium from wastewater using cerium oxide nanoparticles; reaction kinetics study. J. Mol. Struc. 1294, 136563 (2023). https://doi.org/10.1016/j.molstruc.2023.136563
Masoumi, H., Ghaemi, A., Gilani, H.G.: Experimental and RSM study of hypercrosslinked polystyrene in elimination of lead, cadmium and nickel ions in single and multi-component systems. Chem. Engin. Res. Design. 182, 410–427 (2022). https://doi.org/10.1016/j.cherd.2022.04.014
Merker, D., Bertinetti, D., Merz, R., Kopnarski, M., Herberg, F.W., Popov, C.: Enhanced protein immobilization efficacy by nanostructuring of ultrananocrystalline diamond surface. Diamond Rel. Mat. 136, 109898 (2023). https://doi.org/10.1016/j.diamond.2023.109898
Mohamed, A., Atta, R.R., Kotp, A.A., Abo El-Ela, F.I., Abd El-Raheem, H., Farghali, A., Alkhalifah, D.H.M., Hozzein, W.N., Mahmoud, R.: Green synthesis and characterization of iron oxide nanoparticles for the removal of heavy metals (Cd2+ and Ni2+) from aqueous solutions with Antimicrobial Investigation. Sci. Rep. 13, 7227 (2023). https://doi.org/10.1038/s41598-023-31704-7
Nassef, H.M., Al-Hazmi, G.A.A.M., Alayyafi, A.A., El-Desouky, M.G., El-Bindary, A.A.: Synthesis and characterization of new composite sponge combining of metal-organic framework and chitosan for the elimination of Pb(II), Cu(II) and Cd(II) ions from aqueous solutions: Batch adsorption and optimization using Box-Behnken design. J. Mol. Liq. 394, 123741 (2024). https://doi.org/10.1016/j.molliq.2023.123741
Neris, J.B., Luzardo, F.H.M., Santos, P.F., de Almeida, O.N., Velasco, F.G.: Evaluation of single and tri-element adsorption of Pb2+, Ni2+ and Zn2+ ions in aqueous solution on modified water hyacinth (Eichhornia crassipes) fibers. J. Environ. Chem. Engin. 7, 102885 (2019). https://doi.org/10.1016/j.jece.2019.102885
Nezhad, N.G., Rahman, R.N.Z.R.A., Normi, Y.M., Oslan, S.N., Shariff, F.M., Leow, T.C.: Recent advances in simultaneous thermostability-activity improvement of industrial enzymes through structure modification. Inter. J. Biol. Macromol. 232, 123440 (2023). https://doi.org/10.1016/j.ijbiomac.2023.123440
Ou, C., Cheng, W., Wang, Z., Yao, X., Yang, S.: Exogenous melatonin enhances Cd stress tolerance in Platycladus orientalis seedlings by improving mineral nutrient uptake and oxidative stress. Ecotoxicol. Environ. Safety 252, 114619 (2023). https://doi.org/10.1016/j.ecoenv.2023.114619
Paithankar, J.G., Saini, S., Dwivedi, S., Sharma, A., Chowdhuri, D.K.: Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 262, 128350 (2021). https://doi.org/10.1016/j.chemosphere.2020.128350
Pallab, K., Tapan, B.K., Tapas, K.P., Ramen, K.: Estimation of total flavonoids content (TPC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. J. Drug Deliv. Therapeut. 3, 33–37 (2013). https://doi.org/10.22270/jddt.v3i4.546
Peng, H., Wang, Y., Tan, T.L., Chen, Z.: Exploring the phytoremediation potential of water hyacinth by FTIR Spectroscopy and ICP-OES for treatment of heavy metal contaminated water. Int. J. Phytoremediation 22, 939–951 (2020). https://doi.org/10.1080/15226514.2020.1774499
Poonam, Bharti, S.K., Kumar, N.: Kinetic study of lead (Pb+2) removal from battery manufacturing wastewater using bagasse biochar as biosorbent. Appl Water Sci 8, 119 (2018). https://doi.org/10.1007/s13201-018-0765-z
Qi, J., Zhu, H., Yang, T., Wang, X., Wang, Z., Lei, X., Li, B., Qian, W.: Biomass-derived carbon/iron composite (FexOy-BC (RM)) with excellent Cd(II) adsorption from wastewater – Red mud resource utilization. Arab. J. Chem. 17, 105411 (2024). https://doi.org/10.1016/j.arabjc.2023.105411
Qu, J., Zhang, X., Liu, S., Li, X., Wang, S., Feng, Z., Wu, Z., Wang, L., Jiang, Z., Zhang, Y.: One-step preparation of Fe/N co-doped porous biochar for chromium(VI) and bisphenol a decontamination in water: Insights to co-activation and adsorption mechanisms. Biores. Tech. 361, 127718 (2022). https://doi.org/10.1016/j.biortech.2022.127718
Roberto, S.-C.C., Andrea, P.-M., Andrés, G.-O., Norma, F.-P., Hermes, P.-H., Gabriela, M.-P., Fabián, F.-L.: Chapter 9 - Phytonanotechnology and environmental remediation. In: Thajuddin, N., Mathew, S. (eds.) In Micro and Nano Technologies Phytonanotechnology, pp. 159–185. Elsevier (2020). https://doi.org/10.1016/B978-0-12-822348-2.00009-7
Sanchez-Hernandez, J.C., Sandoval, M., Pierart, A.: Short-term response of soil enzyme activities in a chlorpyrifos-treated mesocosm: use of enzyme-based indexes. Ecol. Indicat. 73, 525–535 (2017). https://doi.org/10.1016/j.ecolind.2016.10.022
Saqib, M., Shahzad, U., Zulfiqar, F., Tiwari, R.K., Lal, M.K., Naz, S., Jahan, M.S., Awan, Z.A., El-Sheikh, M.A., Altaf, M.A.: Exogenous melatonin alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in strawberry. South Afr. J. Bot. 157, 10–18 (2023). https://doi.org/10.1016/j.sajb.2023.03.039
Saroop, S., Chanda, S.V., Singh, Y.D.: Changes in soluble and ionically bound peroxidase activities during brassica juncea seed development. Bulg. J. Plant Physiol. 28, 26–34 (2002). (http://www.bio21.bas.bg/ipp/gapbfiles/v-28/02_3-4_26-34.pdf)
Senniappan, S., Palanisamy, S., Mani, V.M., Umesh, M., Govindasamy, C., Khan, M.I., Shanmugam, S.: Exploring the adsorption efficacy of Cassia fistula seed carbon for Cd (II) ion removal: Comparative study of isotherm models. Environ. Res. 235, 116676 (2023). https://doi.org/10.1016/j.envres.2023.116676
Shaaria, N.E.M., Tajudina, M.T.F.M., Khandakera, M.M., Majrashib, A., Alenazic, M.M., Abdullahia, U.A., Mohd, K.S.: Cadmium toxicity symptoms and uptake mechanism in plants: a review. Braz. J. Biol. 84, e252143 (2024). https://doi.org/10.1590/1519-]6984.252143
Sharma, P., Sharma, P., Arora, P., Verma, V., Khanna, K., Saini, P., Bhardwaj, R.: Chapter 8 - role and regulation of ROS and antioxidants as signaling molecules in response to abiotic stresses. In: Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A. (eds.) Plant Signaling Molecules, pp. 141–156. Woodhead Publishing (2019). https://doi.org/10.1016/B978-0-12-816451-8.00008-3
Sharma, R., Singh, N.S., Singh, D.K.: Impact of heavy metal contamination and seasonal variations on enzyme’s activity of Yamunariver soil in Delhi and NCR. Appl. Water Sci. 10, 83 (2020). https://doi.org/10.1007/s13201-020-1166-7
Sharma, S., Kesharwani, A.K., Kulshreshtha, A.: Bioengineered Microbes for Restoration of Soil Health. In: Bhatia, R.K., Walia, A. (eds.) Advancements in Microbial Biotechnology for Soil Health. Microorganisms for Sustainability, vol 50. Springer, Singapore, pp 33–47 (2024) https://doi.org/10.1007/978-981-99-9482-3_3
Sobral, M.C.M., Martins, I.M., Sobral, A.J.F.N.: Chapter 13 - Role of chitosan and chitosan-based nanoparticles against heavy metal stress in plants. In: Kumar, S., Madihally, S.V. (eds.) Nanomaterial-Plant Interactions, Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences, pp. 273–296. Academic Press (2022). https://doi.org/10.1016/B978-0-323-85391-0.00011-3
Song, B., Xue, Y., Yu, Z., He, Y., Liu, Z., Fang, J., Wang, Y., Adams, J.M., Hu, Y., Razavi, B.S.: Toxic metal contamination effects mediated by hotspot intensity of soil enzymes and microbial community structure. J. Haz. Mater. 466, 133556 (2024). https://doi.org/10.1016/j.jhazmat.2024.133556
Sulej, J., Osińska-Jaroszuk, M., Jaszek, M., Olszewska, A., Belcarz, A., Piątek-Gołda, W.: Chitosan as a promising support of a CDH activity preservation system for biomedical and industrial applications. Inter. J. Mol. Sci. 24(5), 4535 (2023). https://doi.org/10.3390/ijms24054535
Sun, Q., Zhang, Y., Ming, C., Wang, J., Zhang, Y.: Amended compost alleviated the stress of heavy metals to pakchoi plants and affected the distribution of heavy metals in soil-plant system. J Environ. Manag. 336, 117674 (2023). https://doi.org/10.1016/j.jenvman.2023.117674
Sun, X., Ye, Y., Ma, Q., Guan, Q., Jones, D.L.: Variation in enzyme activities involved in carbon and nitrogen cycling in rhizosphere and bulk soil after organic mulching. Rhizosphere 19, 100376 (2021). https://doi.org/10.1016/j.rhisph.2021.100376
Tang, B., Xu, H., Song, F., Ge, H., Yue, S.: Effects of heavy metals on microorganisms and enzymes in soils of lead–zinc tailing ponds. Environ. Res. 207, 112174 (2022). https://doi.org/10.1016/j.envres.2021.112174
Tang, J., Zhang, L., Zhang, J., Ren, L., Zhou, Y., Zheng, Y., Luo, L., Yang, Y., Huang, H., Chen, A.: Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total. Environ. 701, 134751 (2020). https://doi.org/10.1016/j.scitotenv.2019.134751
Tomczyk, A., Sokołowska, Z., Boguta, P.: Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 19, 191–215 (2020). https://doi.org/10.1007/s11157-020-09523-3
Verma, M.L., Kumar, S., Das, A., Randhawa, J.S., Chamundeeswari. M.: Enzyme Immobilization on Chitin and Chitosan-Based Supports for Biotechnological Applications. In: Crini, G., Lichtfouse, E. (eds.) Sustainable Agriculture Reviews 35. Sustainable Agriculture Reviews, vol 35. Springer, Cham (2019). https://doi.org/10.1007/978-3-030-16538-3_4
Verma, S., Verma, P.K., Chakrabarty, D.: Chapter 19 - Plant transcription factors: important factors controlling oxidative stress in plants. In: Srivastava, V., Mishra, S., Mehrotra, S., Upadhyay, S.K. (eds.) Plant transcription factors, pp. 383–417. Academic Press (2023). https://doi.org/10.1016/B978-0-323-90613-5.00006-6
Wang, Y., Lin, G., Li, X., Tai, M.H., Song, S., Tan, H.T.W., Leong, K., Yip, E.Y.B., Lee, G.Y.C., Dai, Y., Wang, C.-H.: Meeting the heavy-metal safety requirements for food crops by using biochar: An investigation using sunflower as a representative plant under different atmospheric CO2 concentrations. Sci. Total. Environ. 867, 161452 (2023). https://doi.org/10.1016/j.scitotenv.2023.161452
Wang, Z., Wu, Q., Zhang, J., Zhang, H., Feng, J., Dong, S., Sun, J.: In situ polymerization of magnetic graphene oxide-diaminopyridine composite for the effective adsorption of Pb(II) and application in battery industry wastewater treatment. Environ. Sci. Pollut. Res. 26, 33427–33439 (2019). https://doi.org/10.1007/s11356-019-06511-1
Yaashikaa, P.R., Kumar, P.S., Varjani, S., Saravanan, A.: A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotech. Rep. 28, e00570 (2020). https://doi.org/10.1016/j.btre.2020.e00570
Yan, Y., Qi, F., Zhang, L., Zhang, P., Li, Q.: Enhanced Cd adsorption by red mud modified bean-worm skin biochars in weakly alkali environment. Sep. Puri. Tech. 297, 121533 (2022). https://doi.org/10.1016/j.seppur.2022.121533
Yang, H., Huang, X., Thompson, J.: Biochar: Pros must outweigh cons. Nature 518, 483 (2015). https://doi.org/10.1038/518483f
Yasin, M.U., Hannan, F., Munir, R., Muhammad, S., Iqbal, M., Yasin, I., Khan, M.S.S., Kanwal, F., Chunyan, Y., Fan, X., Gan, Y.: Interactive mode of biochar-based silicon and iron nanoparticles mitigated Cd-toxicity in maize. Sci. Total. Environ. 912, 169288 (2024). https://doi.org/10.1016/j.scitotenv.2023.169288
Yi, Y., Shi, K., Ding, S., Hu, J., Zhang, C., Mei, J., Ying, G.: A general strategy for protein affinity-ligand oriented-immobilization and screening for bioactive compounds. J. Chromatography B 1218, 123591 (2023). https://doi.org/10.1016/j.jchromb.2023.123591
Yifru, A., Mekonnen, N., Mehretie, S., Admassie, S.: Polypyrrole–polyaniline-water hyacinth leaf protein concentrate composite for the removal of Cr(VI) from aqueous solution: kinetics, isotherm and thermodynamic studies. Bull. Chem. Soc. Ethiop. 36(3), 571–584 (2022). https://doi.org/10.4314/bcse.v36i3.7
Yin, G., Chen, X., Sarkar, B., Bolan, N.S., Wei, T., Zhou, H., Wang, H.: Co-adsorption mechanisms of Cd(II) and As(III) by an Fe-Mn binary oxide biochar in aqueous solution. Chem. Engin. J. 466, 143199 (2023). https://doi.org/10.1016/j.cej.2023.143199
Yin, X., Wang, Y., Wei, L., Huang, H., Zhou, C., Ni, G.: Reduced cadmium(Cd) accumulation in lettuce plants by applying KMnO4 modified water hyacinth biochar. Heliyon 8(11), e11304 (2022). https://doi.org/10.1016/j.heliyon.2022.e11304
Younes, E.A., El-Sheikh, A.H., Alsmadi, R.B.: The use of new class α-amino nitrile modified magnetic adsorbents for removal of Cd(II) from aqueous medium: Sorbent modification vs. α-amino nitrile addition to the adsorption medium. Emer. Contamin. 10, 100261 (2024). https://doi.org/10.1016/j.emcon.2023.100261
Zhao, Z.W., Wu, Y., Chen, W.J., Sun, W., Wang, Z.H., Liu, G.B., Xue, S.: Soil enzyme kinetics and thermodynamics in response to long-term vegetation succession. Sci. Total. Environ. 882, 163542 (2023). https://doi.org/10.1016/j.scitotenv.2023.163542
Zhang, J., Lovell, J.F., Shi, J., Zhang, Y.: Nanomaterials for co-immobilization of multiple enzymes. BMEMat 2024, e12080 (2024). https://doi.org/10.1002/bmm2.12080
Zhang, K., Cen, R., Moavia, H., Shen, Y., Ebihara, A., Wang, G., Yang, T., Sakrabani, R., Singh, K., Feng, Y., Lian, F., Ma, C., Xing, B.: The role of biochar nanomaterials in the application for environmental remediation and pollution control. Chem. Engine. J. 492, 152310 (2024). https://doi.org/10.1016/j.cej.2024.152310
Zheng, J.-C., Liu, H.-Q., Feng, H.-M., Li, W.-W., Lam, M.H.-W., Lam, P.K.-S., Yu, H.-Q.: Competitive sorption of heavy metals by water hyacinth roots. Environ. Pollution 219, 837–845 (2016). https://doi.org/10.1016/j.envpol.2016.08.001
Zhou, Y., Staver, A.C.: Enhanced activity of soil nutrient-releasing enzymes after plant invasion: a meta-analysis. Ecol. 100(11), e02830 (2019). https://doi.org/10.1002/ecy.2830
Zhu, Y., Dong, Y., Zhu, N., Jin, H.: Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Safety 240, 113681 (2022). https://doi.org/10.1016/j.ecoenv.2022.113681
Zhu, Y., Wang, K., Jia, X., Fu, C., Yu, H., Wang, Y.: Antioxidant peptides, the guardian of life from oxidative stress. Med. Res. Rev. 44, 275–364 (2024). https://doi.org/10.1002/med.21986
Zohrabi, Y., Ghazi, M.E., Izadifard, M.: Valipour A (2023) Synthesis, structural, magnetic property, and Cd(II) adsorption behavior of Ca-substituted MgFe2O4 nanomaterials in aqueous solutions. Environ. Sci. Pollut. Res. (2023). https://doi.org/10.1007/s11356-023-31326-6
Zulfiqar, U., Ayub, A., Hussain, S., Waraich, E.A., El-Esawi, M.A., Ishfaq, M., Ahmad, M., Ali, N., Maqsood, M.F.: Cadmium toxicity in plants: recent progress on morpho-physiological effects and remediation strategies. J. Soil Sci. Plant Nutr. 22, 212–269 (2022). https://doi.org/10.1007/s42729-021-00645-3
Funding
This paper is self-funding and we did not take any funds from any organization or person.
Author information
Authors and Affiliations
Contributions
Abdulrahman A Alzandi, Layla Yousif Abdullah Al Hijab, Zarah I. Alzahrani, and Deyala M. Naguib contributed to the study conception and design, Material preparation, data collection and analysis. Deyala M. Naguib wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This research article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Consent for participation
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alzandi, A.A., Al Hijab, L.Y.A., Alzahrani, Z.I. et al. Nano-water hyacinth protein adsorbent as soil amendment alleviates cadmium stress in common bean seedlings by improving soil enzymes and mitigating oxidative stress. Adsorption 30, 1419–1435 (2024). https://doi.org/10.1007/s10450-024-00511-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-024-00511-5