Abstract
Silica-supported chitosan adsorbent was synthesized by sol–gel process and evaluated for desulfurization of sulfur compounds. Removal of thiophene from thiophene/ n-heptane solutions has been investigated on silica-chitosan hybrid as a new adsorbent. The reduction of sulfur compound happens with strong adsorption between sulfur and the surface of the silica/ chitosan hybrid. This adsorption is the result of the interaction between sulfur atoms in thiophene and NH2 groups in chitosan as well as, the penetration of thiophene molecules into the pores of silica. Recyclability and reusability of this composite is a main advantage of this adsorbent. The preparation method as well as sulfur removal and adsorption characteristics of this adsorbent are described in this paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fossil fuels are those energy sources that were formed by the remains of once-living organisms. They include oil, natural gas, coal, and fuels derived from oil. Historically, oil and gas exploration and production of petroleum were among the important sources, and crude oil represents a main part of the current fossil fuels energy mixture [1]. World oil and gas resource consumption is expected to grow from 104 to 153 trillion ft3 from 2006 to 2030, and thus, it will grow more strongly than any other fossil fuels, becoming one of the most important energy sources for the future [2]. This fuel is usually obtained directly from gas resources with a wide range of pressures (normally between 2030 and 7090 kPa) and compositions [3]. Thousands of different chemical compounds have been identified in crude oils and natural gas; some of these are saturates, alkenes, aromatics, and polar compounds containing sulfur atoms such as thiophene [4]. The sulfur-removing operation from oil and natural gas is usually called sweetening and is a vital step in refineries [5]. Sulfur is the most abundant element in petroleum after carbon and hydrogen. In addition, the importance of sulfur in chemical, biological, and industrial, sulfur-containing compounds been widely studied [6,7,8,9,10]. The concentration of sulfur in crude oil is typically between 0.05 and 5.0% (by weight) [11] and since the containing sulfur-containing compounds in fuels, even at in ppm scales could be poison, the concentration of thiophene often needs to be reduced to very low levels for most applications [12].
Thiophene is an organosulfur compound in liquid hydrocarbon streams produced in a petroleum refinery. Several desulfurization technologies such as adsorptive desulfurization (ADS), charge-transfer complex formation, extraction using ionic liquids, bio-catalytic treatment, etc., have been proposed recently for the desulfurization of liquid fuels. The ADS has been studied by metal oxide, metal–organic framework, Zeolite and mesoporous material as effective and reliable methods for the removal of organosulfur compounds like benzothiophene, dibenzothiophene, and thiophene etc. Sweetening methods used for removing sulfur impurities include chemical absorption by amines or physical adsorption using polymer membranes and other adsorbents. On the other hand, physical adsorption is a more superior compared to chemical absorption. Adsorbents used for chemical adsorption were not regenerated and caused environmental problems, whereas sweetening by physical adsorption allows regeneration and reuse of the adsorbent [13]. Mesoporous materials are usually more appropriate for physical adsorption. They have abundant adsorption sites with the possibility of easy separation and recovery. They have been intensively studied recently because of their applicability in removing pollutants and impurities through adsorption [5].
Silica as a mesoporous material can be functionalized to improve the adsorption capacities. In the present work, desulfurization by selective adsorption on a new adsorbent has been investigated. Silica/ chitosan hybrid is a natural adsorbent that is prepared by the functionalizing of the silica with chitosan. The development of bio-hybrid materials has become of great interest in recent years. Therefore, the adsorption of sulfur compounds via bionanocompositional silica/ chitosan sorbent can be counted as a novel procedure.
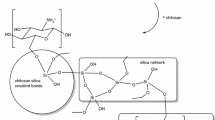
Chitin, a naturally occurring mucopolysaccharide, has been found in a wide range of natural sources such as crustaceans, fungi, insects, annelids, and molluscs. However, chitin and chitosan are only commercially extracted from crustaceans primarily because a large amount of the crustacean’s exoskeleton is available as a by-product of marine food processing [14] (Scheme 1).
Chitosan has reactive amino and hydroxyl functional groups that with high potential for adsorbing a wide range of compounds. This biopolymer represents an attractive alternative to other biomaterials because of its low cost, physico-chemical characteristics, chemical stability, high reactivity, excellent chelation behavior, and high selectivity toward pollutants [15,16,17,18]. On the other hand, the bio-degradability, non-toxicity, and naturally abundance of chitosan make it a green material. It has attracted attention in the medical research community, for orthopedic applications and targeted drug delivery weight management supplements and etc. Generally, biological treatment is often the most economical alternative when compared with other physical and chemical processes [19, 20].
Chitosan can be attached to the silica surface through CH2-OH functional groups and increases its capability of adsorption. Silica/ chitosan hybrid has a high adsorption capacity to remove sulfur compounds and heavy materials compared to many other adsorbents. Being recyclable and reusable are the most important features of this adsorbent.
In this work, a silica/ chitosan hybrid was used as an absorbent to remove thiophene from n-heptane/thiophene solution and the absorption of this process was compared with the absorption of pure silica (MCM-41).
2 Experimental
2.1 Materials
Tetraethyl orthosilicate (TEOS) (FW: 208.33 g mol−1, Merck, Germany), acetic acid, aqueous ammonia and chitosan (deacetylation degree: 98%, FW: 600,000- 800,000 g mol−1, density: 0.15 – 0.30 g mol−1, pKa: 6.3, color: light yellow powder) (ACROS ORGANICS, CN) were used.
2.2 Synthesis of silica/ chitosan hybrid
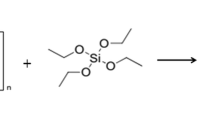
Preparation procedure for silica/ chitosan hybrid [21]: 10 ml of TEOS was added drop-wisely to a mixture of 1 g chitosan in 100 ml of acetic acid 2% and stirred. After stirring overnight at room temperature, the resulting homogeneous mixture was poured out into a flask, containing 200 ml of 3% ammonia solution. After 24 h the resulting precipitate was filtered, washed by water, ethanol, and hexane, and dried first in air, and then on heating at 80°C.
2.3 Characterizations
The structure and crystallinity of solid products were confirmed by the technique of powder X-ray diffraction (XRD) using a Seifer PTS 3000, West Germany diffractometer. A PHILIPS XL30 Series, 30 kV scanning electron microscope (SEM) was used for the investigation of the morphology and particle size of chitosan, pure silica and silica/ chitosan hybrid.
2.4 Desulfurization by adsorption on silica/ chitosan hybrid
A solution of thiophene/ n-heptane with a sulfur concentration of 500 ppm was prepared as a fuel model. Equilibrium adsorption of thiophene from the solution was carried out in a flask equipped with a magnetic stirrer with a solid/liquid weight ratio of 0.05 and under reflux conditions. The experiment was performed with a known quantity of the adsorbent (0.5 g) in 10 ml of thiophene/ n-heptane solution at 60°C for 24 h. At the end of the adsorption process, the adsorbent was separated by centrifuge, and the liquid phase was collected for quantitative analysis of remaining sulfur compounds with an OMEGAWAX 250 (max temperature) gas chromatograph (GC) equipped with a Fused silica capillary column, 0.25 μm film thickness, and FID detector.
3 Results and discussion
3.1 Characterization of silica/ chitosan hybrid
When the silica precursor TEOS was mixed with an aqueous chitosan solution, no phase separation or precipitation was observed, which showed good compatibility [22]. In this work, we have investigated the sol–gel process of hydrolytically polycondensation of TEOS in the presence of chitosan, and the optimal conditions to precipitate the silica/ chitosan nanocomposite adsorbent was determined experimentally. The sol–gel process could proceed at ambient temperature without the addition of any catalysts and it seems that chitosan has a catalytic effect on the sol–gel process of TEOS. The resulting silica/ chitosan hybrid was obtained as a porous powder consisting of microspherical particles (Scheme 2).
The Silica surface is full of Silanol groups and these groups can be replaced by CH2-OH functional groups in chitosan. Chitosan is attached to the silica surface and increases its performance (Scheme 3).
The X-ray diffraction analysis of silica, chitosan, and composite is shown in Fig. 1. According to this figure the differences of these structures are obvious. Chitosan into silica network leads to decreased crystallinity. The silica/ chitosan hybrid is amorphous in analogy with silica and chitosan. This difference suggests that strong hydrogen bonds are formed between silanol groups of the silica network and functional groups of chitosan.
The surface morphology of silica, chitosan, and silica/ chitosan hybrid were observed using SEM and shown in Fig. 2. These images have shown that resulting samples of silica/chitosan hybrid have different properties than the pure silica and the chitosan. A rough structure was created with the dispersion of chitosan flakes in a silica network in comparison with silica. So that, the successful hybridization was found in Fig. 2c which is the irregular distribution of the interfacial interaction between organic and inorganic phase of chitosan and silica attributes respectively [23].
The FTIR spectrum of the silica/ chitosan hybrid showed the characteristic peaks of silica and chitosan. For the hybrid sample, the broad absorption peak at 3450 cm−1 is assigned to hydrogen-bonded Si–OH groups, the increased frequency resulting from the interaction between Si–OH groups and the functional groups of chitosan. For the chitosan sample absorption peaks at 1652 and 1616 cm−1 are observed and assigned to the stretching of Amid groups, while for silica/ chitosan hybrid the shift of these peaks in 1645 and 1562 cm−1 is observed. In addition, for chitosan absorption peak at 2900 cm−1 area is related to C-H groups of alkanes this peak is also observed in the spectrum of the silica/ chitosan hybrid (Fig. 3).
3.2 Desulfurization by adsorption on silica/ chitosan hybrid
Chitosan functionalized on silica particles was used as an adsorbent and to evaluate the efficiency of the prepared adsorbents, the adsorption of the thiophene was studied. The reason for using silica/ chitosan in this method is the formation of hydrogen bonds of thiophene through its sulfur atoms with NH2 groups in chitosan. In the other hand, thiophene can be captured within the porous silica surface.
To investigate, n-heptane/ thiophene solution with an initial concentration of 500 ppm was prepared and was in contact with the pure silica and silica/chitosan hybrid as an adsorbent. Then, adsorption was examined by a gas chromatograph (GC). The data obtained from GC are shown in Figs. 4 and 5.
The thiophene concentration in the presence of adsorbent was calculated by some standard solutions with different concentrations. These standard solutions have the 10, 50, 100, 200, 300, 400, 500 ppm concentrations. The resulting data of GC is shown in Fig. 5. Using the equation y = 20383x – 136018 thiophene concentration is calculated after adsorption by pure silica and silica/ chitosan hybrid as an adsorbent (Fig. 6).
The results of GC showed that in the presence of a silica/ chitosan hybrid, thiophene concentration is reduced from 500 to 43 ppm, while in the presence of pure silica, thiophene concentration is reduced to 197 ppm. Desulfurization by silica/chitosan hybrid was done with good efficiency. Continuous adsorption of the thiophene on the adsorbent led to an increase in its concentration on the adsorbent surface. Then, thiophene molecules are connected through α-positions and oligothiophene is formed (Scheme 4). Oligomerization of thiophene on silica/ chitosan occurs for longer exposures and these processes are relatively slow [24,25,26,27,28,29].
The removal ability increased when the adsorbent/ solution ratio was increased. This adsorbent can be regenerated by heating or washing with solvent. The SEM micrograph of adsorbent after adsorption and then washing with n-heptane and ethyl acetate as a solvent is shown in Fig. 7.
4 Conclusions
Chitosan is abundant in nature, so the synthesis of silica/ chitosan is not expensive in the industry, on the other hand, this bio-adsorbent is not harmful to the environment. Silica/ chitosan hybrid has an adsorption potential for the removal of refractory sulfur compounds such as thiophene and this should be the result of an increase in the interaction between sulfur atoms in thiophene and NH2 groups in chitosan and higher surface area and average pore volume in silica. Decreasing the solution/ adsorbent ratio and increasing the adsorption temperature enhance the desulfurization. The advantages of Silica/ chitosan hybrid are recyclable, reusable, and easy work-up. However, the ADS performance in the large scale is mainly dependent on improving the selectivity, stability, reusability of the process and is crucial to commercialize ADS, while this report is not exceptional as well.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
National research council: Oil in the sea III: Inputs, fates and effects, p. 280. The National Academies Press, Washington, D.C. (2003)
Aparicio, S., Atilhan, M.: Computational study of hexamethylguanidinium lactate ionic liquid: a candidate for natural gas sweetening. Energy Fuels 24, 4989–5001 (2010)
Safari, M.H., Ghanizadeh, A., Montazer-Rahmati, M.M.: Optimization of membrane-based CO2-removal from natural gas using simple models considering both pressure and temperature effects. Int. J. Greenh. Gas Cont. 3, 3–10 (2009)
Marshall, A.G., Rodgers, R.P.: Petroleomics: the next grand challenge for chemical analysis. Acc. Chem. Res. 37, 53–59 (2004)
Zhou, L., Zhong, L., Yu, M., Zhou, Y.: Sorption and desorption of a minor amount of H2S on silica gel covered with a film of triethanolamine. Ind. Eng. Chem. Res. 43, 1765–1767 (2004)
Abass, A.K., Hart, J.P., Cowell, D.: Development of an amperometric sulfite biosensor based on sulfite oxidase with cythchrome c, as electron acceptor, and a screen-printed transducer. Sens. Actuators B 62, 148–153 (2000)
Li, M., Yuan, D.X., Li, Q.L., Jin, X.Y.: Sequential analysis of dimethyl sulfur compounds in seawater. Chin. Chem. Lett. 18, 99–102 (2007)
ASTM D5542–16. :Standard test method for trace anions in high-purity water by ion chromatography. ASTM International, 1-13 (2016)
Li, Y., Zhao, M.: Simple methods for rapid determination of sulfite in food products. Food Cont. 1, 975–980 (2006)
Diao, X., Sumi, K.: Determination of iron monosulfide and iron disulfide in suspensions by an electrochemical method using a platinum-silver twin-electrode. Anal. Sci. 26, 681–687 (2010)
Aribike, D.S., Susu, A.A., Nwachukwu, S.C.U., Kareem, S.A.: Biodesulfurization of Kerosene by Desulfobacterium indolicum. Nat. Sci. 7, 28–35 (2009)
Reut, S., Prakash, A.: Evaluation of sorbents for thiophene removal from liquid hydrocarbons. Fuel Process. Technol. 87, 217–222 (2006)
Saha, B., Vedachalam, S., Dalai, A.K.: Review on recent advances in adsorptive desulfurization. Fuel Process. Technol. 2014, 106685 (2021)
Ahmaruzzaman, Md.: Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv. Colloid Interf. Sci. 143, 48–67 (2008)
Schiffman, J.D., Schauer, C.L.: A review: electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 48, 317–352 (2008)
Veerapur, R.S., Gudasi, K.B., Aminabhavi, T.M.: Pervaporation dehydration of isopropanol using blend membranes of chitosan and hydroxypropyl cellulose. J. Membr. Sci. 304, 102–111 (2007)
Guibal, E.: Interactions of metal ions with chitosan-based sorbents: a review. Sep. Purif. Technol. 38, 43–74 (2004)
Varma, A.J., Deshpande, S.V., Kennedy, J.F.: metal complexation by chitosan and its derivatives: a review. Carbohydr. Polym. 55, 77–93 (2004)
Kurita, K., Ikeda, H., Shimojoh, M., Yang, J.: N-phthaloylated chitosan as an essential precursor for controlled chemical modifications of chitosan: synthesis and evaluation. Polym. J. 39, 945–952 (2007)
Al-Sagheer, F.A., Merchant, S.: Visco-elastic properties of chitosan–titania nano-composites. Carbohyd. Polym. 85, 356–362 (2011)
Crini, G., Badot, P.: Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog. Polym. Sci. 33, 399–447 (2008)
Rashidova, S.S., Shakarova, D.S., Ruzimuradov, O.N., Satubaldieva, D.T., Zalyalieva, S.V., Shpigun, O.A., et al.: Bionanocompositional chitosan-silica sorbent for liquid chromatography. J. Chromatogr. B 800, 49–53 (2004)
Roosen, J., Spooren, J., Binnemans, K.: Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. J. Mater. Chem. A 45, 19415–19426 (2014)
Wang, G., Zhang, L.: Using novel polysaccharide-silica hybrid material to construct an amperometric biosensor for hydrogen peroxide. J. Phys. Chem. B 110, 24864–24868 (2006)
Garcia, C.L., Lercher, J.A., Phys, J.: Adsorption and surface reactions of thiophene on ZSMS zeolites. Chem 96, 2669–2675 (1992)
Chica, A., Strohmaier, K., Iglesia, E.: Adsorption, desorption, and conversion of thiophene on H-ZSM5. Langmuir 20, 10982–10991 (2004)
Bauerle, P.: End-capped oligothiophenes-new model compounds for polythiophenes. Adv. Mater. 4, 102–107 (1992)
Mena-Osteritz, E., Zhang, F., Gotz, G., Reineker, P., Bauerle, P.: Optical properties of fully conjugated cyclo[n]thiophenes – an experimental and theoretical approach. Beilstein J. Nanotechnol. 2, 720–726 (2011)
Tasaka, S., Katz, H.E., Hutton, R.S., Orenstein, J., Fredrickson, G.H., Wang, T.T.: Electrical conductivity of α-quinquethiophene/stearic acid langmuir-blodgett films doped with iodine. Synth. Met. 16, 17–30 (1986)
Acknowledgements
The authors gratefully acknowledge Iran Polymer and Petrochemical Institute for laboratory equipment, material and instrument support.
Funding
This research received no specific grant from any funding agency. However, we wish to express our gratitude to Iran Polymer and Petrochemical Institute for laboratory equipment, material, and instrument support.
Author information
Authors and Affiliations
Contributions
Investigation, chemical methodology, data curation, Formal analysis and writing the original draft have been done by Leila Motlagh and Soroush Shabani.
Dr. Saman Ghaderzadeh contributed to project administration, supervision, validation, review and editing.
Corresponding author
Ethics declarations
Although the ethical approval declaration is not applicable, the authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
The authors report financial support and equipment, chemical material, or supplies were provided by Iran Polymer and Petrochemical Research Institute. They declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Motlagh, L., Shabani, S. & Ghaderzadeh, S. Adsorption of thiophene using chitosan functionalized silica as a biopolymer composite. Adsorption 30, 1153–1160 (2024). https://doi.org/10.1007/s10450-024-00484-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-024-00484-5