Abstract
Modification of the silica surface leads to the change in chemical composition of the surface which can be modified either by physical treatment (thermal or hydrothermal) or by chemical treatment. Such modifications significantly affect the adsorption properties of the materials and especially mechanical stability and water insolubility, increasing the efficiency, sensitivity and selectivity of the analytical application. A variety of types of organic polymers can be employed in the synthesis of hybrids with silica. One of them is chitosan. Chitosan and silica as well as their composites have attracted a great attention as effective hybrid biopolymeric sorbents due to high sorption capacity, cost-effectiveness, renewability and high stability. Owing to the presence of amino groups, chitosan is cationic and capable of heavy metal ions bonding. Several studies have reported on the metal ions removal of using chitosan or chitosan adsorbed onto conventional silica. Their short characterization is presented in this chapter. Moreover different ways of silica–chitosan composites are also discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Introduction
Organic–inorganic hybrid materials, usually known as ceramers or ormocers, have received considerable attention as a new class of composite materials through the novel properties that can arise from the combination of organic polymer and inorganic material. The design and synthesis of organic–inorganic hybrid materials have attracted considerable interest over the past decades not only for the structural diversity, but also due to their multifunctional applications in different areas, such as adsorption, magnetism, photochemical areas, ion-exchange and catalysis. Organic–inorganic hybrid materials synthesized at room temperature by a sol–gel method are particularly attractive for obtaining: micro-optical elements, optical coatings as well as sorbents as they combine some typical properties of organic (elasticity organic functionalities, flexibility, ductility, tightness and chemical inertness) and inorganic (hardness, thermal and chemical stability, transparency and a large refractive index) constituents [1–3]. If the inorganic species is a silica, derivatives are formed by the sol–gel process and the change from base to acid catalysis makes a large difference because base catalysis leads to a more particle-like microstructure while acid catalysis leads to a polymer-like microstructure. In this process small molecules are used as precursors (alkoxysilanes, metal alkoxides as well as metal halides in organic solvents) for the formation of cross-linked materials. As a medium water and organic solvents are used. Applying acid-catalyzed reactions (HCl) an open network structure is formed in the first steps of the reaction leading to condensation of small clusters and forming dense structures. The base-catalyzed reaction (NaOH or NH4OH) leads to highly cross-linked sol particles already in the first steps. Formed materials are porous and loose. This can lead to variations in the homogeneity of the final hybrid materials. Therefore the role of the catalyst and its concentration is very important. The pH used therefore has an effect on the kinetics which is usually expressed by the gel point of the sol–gel reaction. The structure of the precursor is also important. Larger substituents decrease the reaction time due to steric effects and play a significant role in the solubility of the precursor in the solvent. Another factor is solvent. Water is required for the reaction and if the organic substituents are quite large (usually the precursor becomes immiscible in the solvent). Moreover the solvent concentration and its polarity have a great role. Therefore the choice of solvent should be very well thought out. Another used solvent can interfere in the hydrolysis reaction, for example alcohols can undergo trans-esterification reactions leading to quite complicated equilibria in the mixture. The transition from a sol to a gel—gelation point occurs when links between the sol particles are formed. At this point the reaction has not finished, but condensation reactions can go on for a long time until a final stage is reached. This process is called ageing. During this reaction the material shrinks and stiffens. Reaction parameters such as drying rate, gelation time, pH, etc. can have a major influence on the cracking of the gels and have therefore to be optimized. The water to precursor ratio is also a major parameter in the sol–gel process (for tetraalkoxysilanes used as precursors, two water molecules per starting compound are necessary to form completely condensed SiO2). Transition-metal alkoxides also show a lower electronegativity compared with silicon which causes them to be more electrophilic and therefore less stable towards hydrolysis in the sol–gel reactions.
9.2 Method for Sol–Gel Hybrid Materials Preparation
9.2.1 Basics of the Sol–Gel Reactions
Sol–gel reaction has been extensively studied for several decades as a method to prepare ceramic precursors and inorganic glasses at the relatively low temperatures. The major advantage of the process is that mild conditions, such as relatively low temperature and pressure, are used in this type of ceramics processing. Within the past years, the sol–gel process was widely used to create novel hybrid nanoscaled materials based on organic and inorganic components [4–6]. For obtaining such composite materials, the sol–gel reaction is carried out in the presence of macromolecules, which contain functional groups and could be immobilized on the inorganic component. Simplicity and possibility of variation of the quantitative ratio of reagents, as well as the nature of initial materials, provide a wide range of applications of the materials [7].
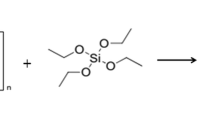
The sol–gel process can be viewed as a two-network forming process, the first step being the hydrolysis of silicon alkoxide and the second consisting in a polycondensation reaction. Most interest in this method is focused on silicon and metal-containing alkoxides, since they can form an oxide network in organic matrices. The sol–gel reactions of alkoxysilane can be described as follows (Eqs. 9.1–9.4) [3, 4]:
hydrolysis:

polycondensation:

and/or

If these sol–gel reactions are complete, full condensed silica is obtained in this process that can be summarized by the following equation:

Hydrolysis of alkoxysilanes could take place under both base- and acid-catalyzed mechanisms. However, base or acid catalysts for alkoxysilane hydrolysis are mostly catalysts used in the condensation process. Effects of substituents were studied for better understanding the peculiarities of alkoxysilane hydrolysis [8–11]. Under basic conditions, the hydrolysis of alkoxy groups usually takes place in a stepwise manner. Carbon-bonded substituents can have profound effects on the rate of hydrolysis. With the exception of aminosilanes, most silanes are employed in the surface treatment applications under acid-catalyzed conditions. The rate of acid hydrolysis is significantly greater than that of base hydrolysis and is minimally affected by other carbon-bonded substituents. The hydrolysis is preceded by protonation of the O-R group. The rates of hydrolysis of the alkoxy groups are generally related to their steric bulk and increase with the increasing carbon number in the molecule. It was found that the rate of methoxysilane hydrolysis was 6–10 times greater than that of ethoxysilane. In the case of base condensation, the hydrolysis of alkoxy groups is stepwise and the difference in the hydrolysis rate between the first alkoxy group and the second one is greater. Thus, the increased organic substitution enhances the hydrolysis rate, i.e. Me3SiOMe > Me2Si(OMe)2 > MeSi(OMe)3 [12].
There are many different synthetic techniques used in the sol–gel process to generate polymer–silica hybrid materials. One of them is the in situ formation of an inorganic network in the presence of a preformed organic polymer. These hybrid materials possess strong chemical bonds (covalent or ionic) between the organic and inorganic phases. Also, physical or weak interactions can be observed between phases, for example, hydrogen bonding or van der Waals attractions. The technique where the simultaneous formation of both organic polymer and silica matrix occurred also exists. Those two methods of preparation of organic–inorganic materials are distinguished by the sequence of formation of the organic and inorganic components.
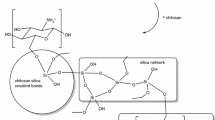
Polysaccharides, such as chitosan, are polyhydroxy compounds because they are composed of numerous monosaccharide residues. Their hydroxyl groups could form hydrogen bonds or get into the condensation reaction with silanol groups produced in the course of hydrolysis of the precursor, thus providing silica nucleation on macromolecules. Presence of amino groups in a molecule of chitosan facilitates the hydrolysis of tetraethoxysilane (TEOS) and condensation of created silanol groups as well as reaction of silanol groups of silica with carbonyl groups of polymer formatting Si–O–C bonds. Another factor which influences the formation of such a network is pH which is chosen for materials preparation. Under the acidic conditions employed, the amine group of chitosan is protonated and forms a charged NH3 + group which, in turn, is an even better hydrogen bonding partner than an uncharged NH2 group.
9.2.2 Application of Nonfunctional Silica Precursors
In [13] the possibility of cross-linked chitosan–silica beads to be used as an adsorbent to adsorb the lignosulfonates, water-soluble anionic polyelectrolyte polymer was investigated. The surface morphologies of chitosan and chitosan–silica beads at ×5000 magnification are shown in Fig. 9.1. It was observed that the surface of chitosan prior to the cross-linking process was smooth and less porous. However, after crosslinking, the surface morphology became coarser and more porous. The silica particles are in the form of white round beads, and their dispersion within the matrix is clearly visible.
A simple microfluidic method to fabricate chitosan–silica hybrid microspheres in one step was described in [14]. TEOS was used as a silica precursor into the chitosan/acetic acid aqueous solution to form the chitosan–silica sol, and then emulsified it in an organic phase mainly containing n-octanol and an organic base trioctylamine by a coaxial microfluidic device (Fig. 9.2). Usage of such device allows one to prepare aqueous droplets with further solidification because of the extraction of acetic acid and water to the organic phase. Obtained hybrids were characterized by such advantages, as a controllable sphere diameter, narrow size distribution and good sphericity of the particles. It is found that the inner and surface structures of the microspheres can be controlled by adjusting the solidification reagent component. It was discussed that described hybrid microspheres could find application in protein immobilization by chemical grafting a model protein, bovine serum albumin, onto the hybrid microspheres. Loading capacity of the hybrid microspheres is much higher than that of pure chitosan and increases with the TEOS concentration. Moreover, the existence of silica in the chitosan spheres can enhance both of mechanical intensity and protein loading capacity of the microspheres.
Chitosan–silica composites were obtained by the sol–gel method through hydrolysis of TEOS in the chitosan solution in the acidic medium with different time of sol maturing (5, 10 and 14 days). The scheme of the synthesis is presented on the Fig. 9.3.
The synthesis time by the sol–gel method influences the textural characteristics of the surface. It was found that the surface area increased with increasing the time of sol maturing. Thus, the BET surface area of composites increased with the increasing the number of sol–gel reaction days: 359 m2/g in the case of the composite prepared within 5 days, 476 m2/g—10 days, and 490 m2/g—14 days. It was found that all the obtained composites correspond to the microporous materials (Fig. 9.4).
The strength and thermal properties of hybrid materials made of tetraethoxysilane/vinyltriethoxysilane (TEOS/VTES) and chitosan at different weight ratios were studied in [15]. It was confirmed that hydrogen bonds were formed between chitosan and SiO2 in hybrid materials. With the addition of more VTES and TEOS, the surface of the hybrid material appears as thick granules. In addition, mechanical performance and thermostability of both types of hybrids are better than those of pure chitosan.
By the introduction of urethane groups into phthalated chitosan the highly homogeneous composite materials of chitosan and silica gel were obtained. The homogeneity of the polymer hybrids was shown to be dependent on the initial contents of tetramethoxysilane (TMOS) and the acid catalyst. With the increase in TMOS or the acid catalyst, the homogeneity of the polymer hybrids was improved due to the enhanced interactions of the hydrogen bonding and/or the increasing rate of gelation. The homogeneity of the polymer hybrids was found to be improved in a similar manner. Chitosan was initially treated with phthalic anhydride to improve its solubility. This phthalated chitosan was further reacted with n-propyl isocyanate in N,N–dimethylformamide (DMF) at 90 °C. Comparing the results of the chitosan with and without the urethane groups, the affinity of the organic polymer to silica gel was improved by the introduction of the urethane group that should work as a good hydrogen bonding accepting group. The carbamated chitosan was mixed with TMOS in DMF in the presence of HCl as a catalyst for the sol–gel reaction of TMOS, and the resulting reaction mixture was heated at 90 °C for 200 h. The homogeneous and transparent composites were obtained when the ratio of the chitosan and TMOS was less than 1:4. The dispersity of the chitosan domain in the polymer hybrid was verified by the nitrogen porosimetry method. A porous silica obtained by charring the chitosan polymer hybrids was found to have nano-pores at a nanometer scale. The results indicate that the chitosan was dispersed in the polymer hybrids at a molecular level. The present method may provide a general method for the preparation of homogeneous polymer hybrids starting from natural organic polymers that have hydroxyl groups [16].
The sol–gel process has potential application in sensor coatings application [17]. In paper [18] the authors combine advantages of chitosan, such as the high content of a basic amino group, the high hydrophilicity and the ability to form a transparent thin film with valuable characteristics of sol–gel method: a simple process which can be used to prepare gel materials that can immobilize different functional reagents in working electrodes. The electrochemiluminescence-optic oxalic acid sensor was created with Pt-electrode coated with chitosan modificated by Ru(bpy) 2+3 complex moieties [19].
The chitosan–silica aerogels were obtained through preparation of primary aqueous sol. It was shown that it is necessary to use a mixture of HCl and HF acids at the ratio 3:1 to achieve reasonable gelation times [20]. The silica gel/chitosan composite was prepared by the sol–gel method by mixing silica gel and chitosan and cross-linking with bifunctional cross-linker glutaraldehyde [21].
The hybrid xerogels were produced by combination of chitosan with siloxane oligomers via sol–gel generating. It was described that these xerogels can be used to produce silica samples with a high surface area and controlled porosity by choosing a convenient siloxane/chitosan ratio for the hybrid precursor. Moreover, the morphology of the hybrid xerogels and the final silica after elimination of the organic residue depends on the method of preparation, giving either flat irregular shaped silica particles with a layered morphology or porous spherical silica particles. The results have shown that chitosan readily associates with siloxane oligomers, precursors of silica by sol–gel, to form nanocomposites acting as templating agents for the morphology of the inorganic network [22].
A new method for immobilizing α-amylase in a hybrid inorganic–organic membrane has been developed for the purpose of potential applications as a membrane bioreactor in aqueous phase [23]. It was shown that chitosan can act as an enzyme-stabilizing host and a dispersant which can homogeneously mix an inorganic (SiO2) phase and an organic (polymer) phase. It was found that the enzyme-incorporated membrane showed a very stable activity for the period of our experimental setup (30 days).
9.2.3 Application of Functional Organosilanes as a Silica Precursor
Functional silanes are broadly used for providing additional functionality to hybrids materials. Application of such modifiers leads to reduction of synthesis stages and ability to control the number of additional functional groups. Thus, 3–aminopropyltriethoxysilane (APTES) is broadly used for the involvement or increase of a number of amino groups on the hybrid surface [24]. In preparing the hybrid membranes [25], the amino-contained precursor (10 wt%) was first hydrolyzed in the presence of the acid catalyst, e.g. HCl, resulting in formation of silanol groups. Then, the hydroxyl groups in one organosilanol formed siloxane bonds with those in another organosilanol or with the amine groups in chitosan through a reaction with water or alcohol elimination during the membrane drying. Also, the hydroxyl and amine groups in the silanol can form hydrogen bonds with the dissociated hydroxyl groups or amine groups in the chitosan amorphous region, and these hydrogen and siloxane bonds are the cross-linking sites in the hybrid membranes. The synthesized membranes were applied for pervaporation (PV) of the methanol/dimethyl carbonate mixture. The results of PV separation show that the hybrid exhibits a higher permeation flux and separation factor than those of the pure chitosan membrane under the same operating conditions, and the surface reconstruction occurred to both the pure chitosan membrane and the hybrid during PV processes. It was found that the time for the surface reconstruction of the hybride is longer than that of the pure chitosan. The effects of feed methanol (MeOH) concentration and temperature on the rate of surface reconstruction of the chitosan membrane and the hybride is similar. The rate of surface reconstruction increases with the increasing MeOH concentration or temperature. The feed concentration affects not only the hydrophilicity of the membrane, but also the rate of surface reconstruction. However, under the same feed concentration, the feed temperature only affects the rate of surface reconstruction.
In [26] it was reported that chitosan/silica hybrids are of benefit as suitable environmental biomaterials and adsorbents under a wide range of conditions. There were investigated thermal, optical, adsorption and biodegradability properties of chitosan/silica hybrids prepared by the sol–gel process in a wide range of in situ silica content from 0 to 100%. To prevent the dissolution of chitosan and silica in the acid and alkaline solutions, respectively, all prepared hybrids were capable of chelating Cu(II) as well as Fe(III) to a large extent. Biodegradability of hybrids was confirmed by lysozyme treatment.
The sol–gel method has been effectively used for the synthesis of homogenous and flexible films using chitosan and silica for optic application. In [27] it was found that 2 wt% chitosan is optimum concentration for obtaining a homogeneous and transparent coating. This amount was investigated by varying the experimental conditions. A higher concentration of chitosan will increase the sol viscosity to a greater extent and will interfere with the uniformity of coating. Functionalization of the hybrid was effected through in situ hydrolysis–condensation reaction of methyltrimethoxysilane (MTMS) and vinyltri-methoxysilane (VTMS) in the reaction medium. The process yields highly transparent and hydrophobic silica–chitosan hybrids.
In the paper [28] transparent chitosan–silica hybrid films with various silica contents were prepared by in situ incorporation of silica by the sol–gel process at room temperature. These composites were characterized for their viscoelastic nature, thermal stability and optical sensitivity. Study of morphology using a scanning electron micrograph and the high resolution transmission electron microscopy images of the nanocomposite films suggests that the SiO2 nanoparticles are within the range of 2–7 nm in diameter and are uniformly dispersed in the polymeric matrix. It was found that the glass transition temperature and storage modulus determined by the dynamic mechanical thermal analysis indicate better cohesion between the phases, and the shift to higher temperature suggests the increased interfacial interaction. It was shown that thermal stability of the chitosan-based film was enhanced and the thermal stability of the composites increased with the increasing silica concentration and the residue corresponds to the content used for reinforcement suggesting that the sol–gel reaction is complete.
The hybrid material was synthesized in a one-pot way, based on synergic grafting polymerization, sol–gel reaction and amphiphilic self-assembly. The core organosilica was formed by hydrolysis and condensation of 3-(trimethoxysilyl)-propylmethacrylate precursor that was simultaneously polymerized using aminogroups contained tert-butyl hydroperoxide, as a redox-pair initiator [29]. Through applied the proper ratio of 3-(trimethoxysilyl)-propylmethacrylate to chitosan, the size of nanospheres were adjusted below 100 nm. This synthesis method simplified the preparation of polymer–silica nanospheres by eliminating the previous core-forming step, and employing biopolymers as shell material. Since a large number of biomolecules contain −NH2 groups (e.g. gelatin and casein), this method sheds a new light on the preparation of a variety of silica–biomolecule hybrids. It was reported that the obtained organosilica–biopolymer nanospheres could be applied in heterogeneous catalysis, gene deliveries and antibacterial technologies.
Polysaccharide–inorganic hybrids based on low molecular weight chitosan attracts researchers’ attention because of their chemical versatility, which allows tailoring novel functionalities accomplished by linkages between different polymer groups and inorganic components. Novel chitosan–siloxane hybrids were synthesized by a sol–gel derived carboxylic acid solvolysis (Fig. 9.5).
Investigation of structural characteristics confirmed that the derivatives are bifunctional hybrids, in which urea and urethane bridges covalently bond chitosan to the polysiloxane network (Fig. 9.6). It was shown that synthesized siloxane–chitosan hybrids exhibit interesting photoluminescence characteristics that may be suitable for their use as optical probes for applications in vivo [30].
The authors [31] suggested a new method to prepare macroporous chitosan macrospheres used for enzyme immobilization. In contrast to chitosan, silica particles, which are commonly used as porogen, are insoluble in acidic media but soluble in alkaline solutions. It was shown that these contrary properties allow use of silica particles to control the number of pores in the chitosan macrospheres. Through the adjusting the weight ratio of silica to chitosan the porous chitosan macrospheres were obtained. In order to show the effect of silica on the surface structure of the chitosan macrospheres, the scanning electron micrographs were registered (Fig. 9.7). It can be seen that the carrier without silica as porogen had a much more porous surface structure than hybrid macrosphere.
3-Glycidoxypropyltrimethoxysilane (GPTMS) is broadly used as a precursor for organic–inorganic hybrids preparation. Application of such siloxanes enables easy preparation of porous composite sorbents. The method described in [32] allows obtaining a new hybrid sorbent without organic solvents. Moreover, cost-effectiveness and high stability make this approach attractive in biosorption. The porous sorbent was synthesized by covalent grafting of molecularly imprinted organic–inorganic hybrid on silica gel. With sucrose and polyethylene glycol 4000 being synergic imprinting molecules, covalent surface coating on the silica gel was obtained by using polysaccharide-incorporated sol–gel process starting with the functional biopolymer, chitosan and an inorganic epoxy-precursor, GPTMS at room temperature.
Ion-imprinting concept and polysaccharide-incorporated sol–gel process were applied for preparation of a new silica-supported organic–inorganic hybrid sorbent for selective separation of Cd(II) from aqueous solution. In the prepared shell/core composite sorbent [33], covalent bound surface coating on the supporting silica gel was obtained using Cd(II)-imprinting sol–gel process starting with an inorganic precursor, GPTMS and chitosan. The sorbent was prepared by self-hydrolysis of GPTMS, self-condensation and co-condensation of silanol groups from siloxane and silica gel surface, in combination with in situ covalent crosslinking of chitosan with partial amine shielded by Cd(II) complexation. Extraction of the imprinting molecules left a predetermined arrangement of ligands and tailored binding pockets for Cd(II). The ion-imprinted composite sorbent offered fast kinetics for Cd(II) sorption and the maximum capacity was 1.14 mmol/g. The uptake capacity of the imprinted sorbent and the selectivity coefficient were much higher than that of the non-imprinted sorbent. The imprinted sorbent exhibited a high reusability. The prepared functional sorbent was shown to be promising for the preconcentration of cadmium in the environmental and biological samples.
A new and effective strategy was proposed for the preparation of an organic–inorganic composite matrix using spherical silica as a supporting core and porous chitosan hybrid layer as shell, based on the sol–gel reaction and simple treatment with ammonia solution [34]. After metal ion loading, an immobilized metal exhibits affinity for protein. In the prepared composite matrix, the coating layer was covalently bonded on the supporting silica by the polysaccharide-incorporated sol–gel process starting with chitosan and an inorganic precursor GPTMS. This siloxane possessed an epoxide group to cross-link amine groups of chitosan. The scanning electron microscopy investigation showed that the wet phase inversion of chitosan in ammonia solution endowed the coated chitosan hybrid layer with a porous surface. X-ray diffraction investigations revealed significant drop in chitosan crystallization, indicating the availability of active amine groups.
9.3 Removal of Heavy Metal Ions by Organic–Inorganic Hybrid Materials
Most of types of industrial wastes are accompanied by disposal of toxic heavy metal ions such as As, Sb, Cr, Cu, Pb, Zn, Co, Ni, Cd and Hg. The presence of toxic heavy metal ions, compounds or species in the aquatic systems or other environmental samples poses serious health risks and problems to humans as well as other living organisms [35, 36]. Many technologies and methods for heavy metal ions removal from waste waters have been developed, such as ion-exchange, evaporation and concentration, chemical precipitation, reverse osmosis, adsorption and electrodialysis. Considering from economy and efficiency point of view, adsorption is regarded as one of the most promising and widely used methods [37]. These methods have been proposed not only for the separation and recovery of heavy metal ions but also CO2, H2S and ammonia. As for mercury adsorption, the efforts have been mostly directed towards incorporation of thiol (–SH) groups in the pores of mesoporous silicas and their hybrid materials.
9.3.1 Heavy Metal Ions Removal on Silica–Chitosan Hybrid Materials
Modified by different ways, silica composites can be used for Cu(II) removal. For example, batch adsorption experiments of Cu(II) removal were conducted using silica, grafted silica with amine, silica coated with chitosan and silica coated with low molecular weight chitosan as adsorbents [38–43]. The kinetic curves reveal that Cu(II) adsorption is rather rapid. The equilibrium data match well with both Langmuir and Freundlich isotherms with high maximum sorption capacities, for example for SiO2 + NH2, SiO2 + CS and SiO2 + CSLMW 83.3 mg/g, 296.6 mg/g and 87 mg/g, respectively [43]. The sorption kinetics follows the pseudo-second-order equation. Adsorption capacity of Cu(II) on CS–SiO2 composite was significantly increased with the increasing CS content coated on the silica gel. Since chitosan coordinate Cu(II) ions the higher amount of chitosan was coated on the adsorbent, the higher amount of Cu(II) could be adsorbed.
Silica gel–chitosan composites (SiCS) can be prepared by the sol–gel method using silica gel and chitosan well as cross-linker glutaraldehyde for the chelation of Cu(II) and Pb(II) from aqueous solutions using the batch method. A maximum sorption capacity was observed at pH 5 for both Cu(II) and Pb(II). In the acidic medium the protonation of amine groups takes place, which restricts the number of binding sites for the sorption of Cu(II) and Pb(II). On the other hand, at pH > 6 precipitation of Cu(II) as Cu(OH)2 and Pb(II) as Pb(OH)2 was observed [41]. It was proved that 870 mg/g of Cu(II) and 330 mg/g Pb(II) can be sorbed and other ions such Cl−, NO3 − and Ca(II) (3000 mg/L) do not have much effect on the efficiency. SiCS removes Cu(II) and Pb(II) by means of ion-exchange, adsorption as well as chelation.
According to Escoda et al. [42] colloidal silica particles were functionalized by grafting by carboxylic functional groups SiO2(COOH) and encapsulation with chitosan and N-carboxylated carboxymethyl–chitosan. The maximum uptake capacity for Cu(II) was obtained with N-carboxylated carboxymethyl–chitosan (172.4 mg/g). The removal of Cu(II) was further improved by coupling adsorption with ultrafiltration allowing an efficient separation of the silica composites and adsorbed Cu(II) ions from the treated solution. Synthesized silica-supported porous sorbent by organic–inorganic hybridization combined with chitosan and polyethylene glycol imprinting was also applied for Cu(II) ions removal in wastewater treatment.
Different types of composites obtained by both encapsulation of modified colloidal silica of submicronic size by chitosan (SiO2 + CS) or by surface-condensation of 4-triethoxysilylbutyronitrile and transformation to carboxyl-grafted silica (SiO2(COOH) + CS) by hydrolysis of the nitrile groups were used for Ni(II) removal from aqueous solutions [44]. The zeta potentials (ζ) of silica particles change from 0 to −8 mV in the pH range 2–4. At pH higher than 4 they decreased to about −45 mV. However, after grafting of the carboxylic groups, it was equal to −60 mV. The isoelectric point of the composite was reached for a pH between 6 and 7. The zeta potentials of the composite were positive at pH < 7 and then the zeta potential turned to negative values at higher pH. The behaviour of the obtained adsorbents show that the adsorption of Ni(II) is more extensive than at pH 5 due to the competition between the adsorption of Ni(II) and protonation of amine sites of CS. The maximum adsorption capacity is found to be 182 mg/g for SiO2 + CS and 210 mg/g for SiO2(COOH) + CS. In paper [45] the results of Ni(II) on chitosan coated silica SiO2-CS were also described. Maximum removal of Ni(II) on biopolymer sorbents was at pH 5.0. The equilibrium adsorption data were correlated well by the Langmuir and Freundlich isotherm equations. The maximum monolayer adsorption capacity was equal to 254 mg/g. Besides, the batch studies the sorbent was used in the dynamic mode. Column adsorption studies were carried out under the optimum conditions with a flow rate of 2 ml/min and the inlet Ni(II) concentration of 100 mg/L. The determined breakthrough curve profiles show that Ni(II) removal was fast and highly effective. Its concentration in the effluent was almost zero up to about 220 mL. Moreover, the maximum desorption occurred in 30 min with 60 mL volume of 0.1 M EDTA.
The study by Mohmed et al. [46] deals with the competitive adsorption of Cd(II) ion onto silica grafted by chitosan. The batch adsorption experiments were performed for different initial Cd(II) concentrations −5, 10, 15, 20 and 25 mg/dm3 and different ratio of silica to chitosan (100, 95, 85, 75 and 65%) and pH 5. It was found that the recovery decreased with the increasing initial concentration of Cd(II). The adsorption capacity of Cd(II) in regard to the ratio of silica and chitosan hybrid adsorbents changes and its capacity was 1.95 mg/g with 100% of silica and increases to 2.46 mg/g, 3.14 mg/g, for silica–chitosan 95 and 85%, respectively. Decrease of adsorption percentages with the mass increase from 0.05 to 0.25 mg was also observed because all the active groups on the adsorbent interacted with Cd(II) ions. Elution of Cd(II) from the silica–chitosan sorbent was investigated using nitric(V) acid at the concentration 0.01 M and it was proved not to be efficient for Cd(II) removal.
Carboxymethyl chitosan (CMCS) grafted with β-cyclodextrin (CMCS-g-CD) modified silica gel as a new solid phase extraction (SPE) material has been developed and used for Cd(II) removal [47]. It was found that such parameters as pH, amount of adsorbent, contact time, concentration and volume of elution solution and coexisting ions affect the separation and determination of Cd(II). As pH of the solution increases from 3.0 to 6.0 the extraction percentage of Cd(II) also increases. The same was observed in the case of the adsorbent dose increase from 0.01 to 0.12 g and the dose 0.12 g was sufficient for Cd(II) retention. In 10 min. the extraction percentage reached above 95%. The adsorption capacity reached a saturation value at the initial Cd(II) ion concentration of 120 mg/L (saturation of the active binding cavities on the sorbents). Additionally, the effect of different cations 1000-fold Mg(II) and Ca(II), 100-fold Pb(II) and Co(II), 10-fold Zn(II) and Cu(II) on the adsorption of was studied. The results showed that these ions did not affect the separation process and not interfere with the separation and determination of the analytes. As for the Cd(II) determination in the tap water and lake water samples by the FAAS method recoveries were in the range of 96–102%.
The silicate–chitosan composite biosorbents: silica (S), silica–chitosan (SC), silica–chitosan–silica (SCS), silica–chitosan–silica–chitosan–silica (SCSCS) and chitosan (Ch) were studied for removal of Cd(II), Cr(III) and Cr(VI) from aqueous solutions [48]. For the SCS and SCSCS films incubated with Cd(II) or Cr(III) the maximum adsorption was achieved at pH 7 whereas for Cr(VI) at pH 4.0. Above pH 7 Cd(II) and Cr(III) precipitate in the form of Cd(OH)2 or Cr(OH)3. The SCSCS films reached equilibrium within 4 h for the studied ions and for the SCS films the equilibrium was obtained within 8 h. The maximum sorption capacities were higher for SCSCS compared to the SCS films for Cd(II) 50.58 and 67.44 mg/g and for Cr(VI) 35.37 and 46.28 mg/g, respectively. 80% of the adsorbed metal ions were eluted by 5% HNO3. This non-covalent immobilization method allowed chitosan surface retention and did not affect its adsorption properties.
In the paper by Gandhi [49] silica, silica gel–chitosan hybrid composite (SiCS) and La(III) loaded SiCS composite (LaSiCS) were compared as for Cr(VI) removal. It was proved that these sorbents reached saturation at 60 min. The maximum sorption capacities at pH 4 of LaSiCS and SiCS composite are 5.31 and 3.5 mg/g, respectively, whereas Si and CS has 1.08 and 0.7 mg/g, respectively, at 60 min of the phase contact time. The studies were also carried out in the presence of common ions like Ca(II), Mg(II), Cl−, SO 2−4 , HCO3 − and NO3 − (initial concentration 400 mg/L). There was no remarkable influence on the maximum sorption capacities in the presence of anions which is due to large charge density of chromate(VI). However, the presence of Ca(II) and Mg(II) ions resulted in the decrease of that value from 5.6 to 4.0 mg/g.
Glutaraldehyde cross-linked silica gel/chitosan-g-poly(butyl acrylate) (Cs-g-PBA/SG) nanocomposite obtained by the sol–gel method was used for removal of Cr(VI) ions [50]. The effect of contact time was studied by varying time (1–6 h) at a constant temperature (25 °C), adsorbent dose (1 g/100 ml), pH (7) and initial concentration (100 mg/L). It was found that the optimal conditions were as follows: 4 g of the optimum adsorbent dose, 120 min. of the contact time and pH 7. The functional groups played a significant role in the adsorption of Cr(VI) on Cs-g-PBA/SG composite and the Langmuir isotherm model characterized the process properly. The monolayer adsorption capacity was 55.71 mg/g. The kinetic studies showed that the adsorption followed pseudo-second-order kinetics.
Silica–chitosan hybrid materials can also be used for As(III,V) removal [51, 52]. In [51] a hybrid process is described for the removal of As(III) and As(V) from drinking water. For this aim two ceramic membrane modules connected in a series were used. The first was functionalized by trichloromethylsilane (triClMS) and the second by the in situ generation of Fe3O4 nanoparticles in the interior of pore structure. Iron oxide has been selected due to its affinity for As(V). Water containing 0.07 mg/L of arsenic (50% As(III) and 50% As(V) species) was purified during 35 min of operation.
9.3.2 Heavy Metal Ions Removal on Clay and Zeolite Chitosan Hybrid Materials
Sorbents obtained from assembling or dispersion of nanobuilding blocks are prepared as phase separated, intercalated and exfoliated structures. The typical examples of such materials are nanocomposites of chitosan with clays and zeolites [53–55]. Chitosan has a low surface area with poor chemical and mechanical properties. Thus, physical and chemical modifications are necessary to overcome these limitations. Clay has a lamellar structure with negatively charged surface that interacts with polycationic chitosan. Chitosan functionalized cloisite, bentonite, perlite, clinoptilolite, montmorillonite, alumina and calcium alginates were used for the adsorption of heavy metals from water, for example such as Cr(VI). In these cases electrostatic interactions between chitosan and the negatively charged surface of their layers resulted in the formation of nanocomposites. In the case of the CS-montmorillonite composites, pH of the solution affects the adsorption capacity because Cr(VI) will form different kinds of anions at different pH values of the solution [54].
The exfoliated structure of hybrid material was also obtained in the case of chitosan and Cloisite 10A sorbent. The zeta potential of chitosan–cloisite surface at pH below eight was positive because the amine groups of chitosan were protonated. It is well-known that Cr(VI) ions existed as Cr2O7 2−, CrO4 2− and HCrO4 − ions in the pH range of 2–6. The optimum pH for the adsorption process was found to be 3.0, qmax value of chitosan–cloisite for Cr(VI) was 357.14 mg/g [55].
Poly(methacrylic acid)-grafted chitosan/bentonite (CS-PMAA-B) composite was also synthesized for the adsorption of U(VI). They used N,N′-methylenebisacrylamide as a cross-linking agent. XRD patterns showed that bentonite was exfoliated during the formation of composite. The adsorption process was carried out at pH 5.5 in which the CS-PMAA-B surface charge was negative and UO2(OH)+ was the predominant species. XPS spectra showed that –COOH groups of composite interacted with UO2 2+ [56].
9.3.3 Heavy Metal Ions Removal on Magnetite Silica–Chitosan Hybrid Materials
Recently magnetite (Fe3O4) nanoparticles coated by the various inorganic and organic materials forming stable shells on nano/microparticles have been the most popular. Among them, silica and chitosan are used [57, 58]. For example, superparamagnetic iron oxide modified by chitosan and coated with silica can be applied in engineering and biomedicine areas. Moreover, the efficient removal of Cu(II) from aqueous solutions by Fe3O4@ hexadecyl trimethoxysilane@chitosan composites (Fe3O4@C16@CTS) was also described in [59]. Adsorption of Cu(II) on Fe3O4@C16@CTS composites increases slowly at pH ranging from 2.0 to 5.0, then increases rapidly at pH 5.0–8.0 and at last maintains a high level adsorption at pH 8.0. The result is dependent on the species of Cu(II) and the surface properties of Fe3O4@C16@CTS at different pH values. The maximum adsorption capacity of Cu(II) on Fe3O4@C16@CTS was found to be 261.78 mg/g at 298 K and higher than those of Cu(II) adsorption on other sorbents such as amino-functionalized magnetic nanosorbents, chitosan-bound Fe3O4 magnetic nanoparticles, sulfonated magnetic GO, magnetic maghemite nanotubes, chitosan–tripolyphosphate beads or modified active carbons.
9.3.4 Heavy Metal Ions Removal on Silica–Chitosan Hybrid Materials with Chelating Agents
The group of Repo et al. [60] proposed the application of chitosan–silica hybrid materials functionalized with EDTA (ethylenediaminetetraacetic acid) for Co(II), Ni(II), Pb(II) and Cd(II) removal. The sorbents were obtained by reacting EDTA anhydride with chitosan amino groups in the acetic acid methanol solution Three different adsorbents were obtained: Chi:TEOS 2:60, Chi:TEOS 2:30, Chi:TEOS 2:15. They were also modified by EDTA: EDTA-Chi:TEOS 2:60, EDTA-Chi:TEOS 2:30, EDTA-Chi:TEOS 2:15. The optimal pH for all the adsorbents was 3.0 with varying adsorption efficiency from 93 to 99% for 0.8 mM single metal solutions. The maximum adsorption capacities ranged from 0.25 to 0.63 mmol/g. The material with the highest chitosan content (EDTA-Chi:TEOS 2:15) and thus the highest surface coverage of EDTA showed the best adsorption properties. Analogous studies were carried out for applicability of DTPA functionalized silica gel and chitosan [61] to the adsorption of Co(II) in the presence of EDTA. An excess of oxalate ions or Fe(II) did not influence the Co(II) adsorption by DTPA-chitosan due to its high ligand loading. However, adsorption of Co(II) was enhanced by oxalate in the case of DTPA-silica gel, which was attributed to the oxalate binding on the surface creating more adsorption sites for Co(II).
By grafting of chitosan onto the surface of ordered mesoporous silica, the material was synthesized and used for on-line flow injection micro-column separation/preconcentration coupled with ICP-OES determination of trace heavy metals in the environmental water samples such as Cu(II), Pb(II), Cd(II), Hg(II) and V(V) [62]. The following sorption capacities were obtained: 16.3, 21.7, 22.9, 12.2, 13.5 mg/g for V(V), Cu(II), Pb(II), Cd(II) and Hg(II), respectively. The studied ions were poorly adsorbed at pH < 4, however, in the pH range of 5–9 the adsorption was quantitative (>90%) for all the studied metals. As for Cu(II), Pb(II), Cd(II) and Hg(II) the adsorption mechanism could be attributed to the chelation of the amino groups in the chitosan with the studied metal ions whereas V(V) anion could be retained on the surface of sorbent by electrostatic effect.
9.4 Summary
Preparation of organic–inorganic hybrid materials is one of the most attractive fields of sol–gel chemistry. Such materials have attracted much attention from material scientists and chemists in recent years due to the possibility of combination at the nanosize level of inorganic and organic or even bioactive components in a single hybrid composite. The mechanism of metal adsorption on the silica–chitosan derivatives involves electrostatic interactions (ion-exchange), metal chelation (coordination) and ion pair formation. Several parameters influence this reaction. These are ionic charge of the adsorbent, solution pH and the chemistry of the metal ion (ionic charge, ability to be hydrolyzed and form). Heavy metal ion removal has attracted a considerable attention for beneficial water usage due to their long-term environmental toxicity as well as short-term public health damage. The design and synthesis of organic–inorganic hybrid materials have developed over the last two decades as chemists and materials engineers paid their attention to these materials. In the synthetic process of organic–inorganic hybrid materials, the organic components usually act as templates for directing the connectivity and arrangement of inorganic building blocks. Specifically, according to the size and scale of inorganic building blocks, these organic–inorganic hybrid materials can be divided into molecular scale and nanoscale organic–inorganic hybrid materials.
9.5 Future Scenario
New materials based on the modified chitosan–silica can be used. Chitosan as a biopolymer represents an attractive alternative to other biomaterials because of its significant physicochemical (reactive hydroxyl and amino groups, high positive charge in acidic conditions, good film formation) and biological behaviours [63]. Their application in analytical determination is favoured by rather not complicated synthesis and simple, fast, sensitive analysis. In most cases they are selective and can be applied for the determination of trace heavy metals in the environmental water samples. It is believed that this tendency of using the above mentioned materials will be dynamically developed in the next years. Another way is application of mesoporous silica nanoparticles (MSNs) with chitosan which enables pH responsive drug release and demonstrates great potential for drug delivery [32] or biocomposite scaffolds containing chitosan/alginate/nano-silica (CS/Alg/nSiO2) for bone tissue engineering applications [64–66]. Mesoporous chitosan/silica bioinorganic hybrid materials can be also applied as electrochemical sensors and biosensors and catalysts such as silica/chitosan-supported nanosized palladium catalysts [67–69].
Abbreviations
- APTES:
-
3-aminopropyltriethoxysilane
- CS-PMAA-B:
-
Poly(methacrylic acid)-grafted chitosan/bentonite
- GPTMS:
-
3-Glycidoxypropyltrimethoxysilane
- MeOH:
-
Methanol
- MTMS:
-
Methyltrimethoxysilane
- MSNs:
-
Mesoporous silica nanoparticles
- PV:
-
Pervaporation
- TEOS:
-
Tetraalkoxysilanes
- TMOS:
-
Tetramethoxysilane
- VTES:
-
Vinyltriethoxysilane
- VTMS:
-
Vinyltrimethoxysilane
References
Sanchez C, Ribot F, Lebeau B (1999) J Mater Chem 9:35–44
Jal PK, Patel S, Mishra BK (2004) Talanta 62(1005–102):8
Collinson MM (1999) Crit Rev Anal Chem 29:289–311
Huang HH, Orler B, Wilkes GL (1985) Polym Bull 14:557–564
Schmidt H, Non-Cryst J (1985) Solids 73:681–691
Zou H, Wu S, Shen J (2008) Chem Rev 108:3893–3957
Budnyak TM, Pylypchuk IV, Tertykh VA, Yanovska ES, Kolodynska D (2015) Nanoscale Res Lett 10:87
McNeil KJ, DiCaprio JA, Walsh DA, Pratt RF (1980) J Am Chem Soc 102:1859–1865
Osterholtz FD, Pohl ER (1992) J Adhes Sci Technol 6:127–149
Smith KA (1986) J Org Chem 51:3827–3830
Voronkov MG, Mileshkevich VP, Yuzhelevskii YA (1978) The siloxane bond. Consultants Bureau, New York
Arkles B, Steinmetz JR, Zazyczny J, Mehta P (1992) J Adhes Sci Technol 6:193–206
Zulfikar MA, Wahyuningrum D, Lestari S (2013) Sep Sci Technol 48:1391–1401
Lan W, Li S, Xu J, Luo G (2010) Biomed Microdevices 12:1087–1095
Yeh JT, Chen CL, Huang KS (2007) Mater Lett 61:1292–1295
Tamaki R, Chujo Y (1998) Synthesis. Compos Interfaces 6:259–272
Miao Y, Tan SN (2001) Anal Chim Acta 437:87–93
Zhao CZ, Egashira N, Kurauchi Y, Ohga K (1998) Anal Sci 14:439–441
Zhao CZ, Egashira N, Kurauchi Y, Ohga K (1997) Anal Sci 13:333–336
Ayers MR, Hunt AJ (2001) J Non Cryst Solids 285:123–127
Hu H, Xin JH, Hu H, Chan A, He L (2013) Carbohydr Polym 91:305–313
Retuert J, Quijada R, Arias V, Yazdani-Pedram M (2003) J Mater Res 18:487–494
Cho G, Moon IS, Lee JS (1997) Chem Lett 26:577–578
Fuentes S, Retuert PJ, Ubilla A, Fernandez J, Gonzalez G (2000) Biomacromolecules 1:239–243
Chen JH, Liu QL, Fang J, Zhu AM, Zhang QZ (2007) J Colloid Interface Sci 316:580–588
Lai SM, Yang AJM, Chen WC, Hsiao JF (2006) Polym Plast Technol Eng 45:997–1003
Smitha S, Shajesh P, Mukundan P, Warrier KGK (2008) J Mater Res 23:2053–2060
F. Al-Sagheer, S. Muslim (2010) Ites. J Nanomater (ID 490679). doi:10.1155/2010/490679
Fei B, Lu H, Xin JH (2006) Polymer 47:947–950
Silva SS, Ferreira RAS, Fu L, Carlos LD, Mano JF, Reis RL, Rocha J (2005) J Mater Chem 15:3952–3961
Sun S, Zhang Y, Dong L, Shen S (2010) Kinet Catal 51:771–775
Li F, Du P, Chen W, Zhang S (2007) Anal Chim Acta 585:211–218
Li F, Jiang H, Zhang S (2007) Talanta 71:1487–1493
Xu X, Dong P, Feng Y, Li F, Yu H (2010) Anal Methods 2:546–551
Vunain E, Mishra AK, Mamba BB (2016) Int J Biol Macromol 86:570–586
D. Kołodyńska, Z. Hubicki (2012) Investigation of sorption and separation of lanthanides on the ion exchangers of various types. In: Ion Exchange Technologies, (ed. Ayben Kilislioğlu), InTech, Publishers, pp 101–154. ISBN 980–953-307-139-3
Kumar P, Guliants VV (2010) Micropor Mesopor Mater 132:1–14
Zheng L, Jiang FH, Dong PJ, Zhuang QF, Li F (2010) Chem Res Chin Univ 26:355–359
Zhao H, Xu J, Lan W, Wang T, Luo G (2013) Chem Eng J 229:82–89
Airoldi C, Monteiro OAC Jr (2000) App Polym Sci 77:797–804
Gandhi MR, Meenakshi S (2012) Int J Biol Macromol 50:650–657
Escoda A, Euvrard M, Lakard S, Husson J, Mohamed AS, Knorr M (2013) Sep Purif Technol 118:25–32
Budnyak T, Tertykh V, Yanovska E (2014) Mater Sci (Medžiagotyra) 20:177–182
Singhon R, Husson J, Knorr M, Lakard B, Euvrard M (2012) Colloids Surf B: Biointerfaces 93:1–7
Vijaya Y, Popuri SR, Boddu VM, Krishnaiah A (2008) Carbohyd Polym 72:261–271
Mohmed MA, Mulyasuryani A, Sabarudin A (2013) J Pure App Chem Res 2:62–66
Lü H, An H, Wang X, Xie Z (2013) Int J Biol Macromol 61:359–362
Copello GJ, Varela F, Vivot RM, Díaz LE (2008) Bioresour Technol 99:6538–6544
Gandhi MR, Meenakshi S (2012) J Hazard Mater 203–204:29–37
Nithya R, Gomathi T, Sudha PN, Venkatesan J, Anil S, Kim SK (2016) Int J Biol Macromol 87:545–554
Sklari S, Pagana A, Nalbandian L, Zaspalis V (2015) J Water Process Eng. 5:42–47
Mohan D, Pittman ChU Jr (2007) J Hazard Mater 142:1–53
Lewandowska K, Sionkowska A, Kaczmarek B, Furtos G (2014) Internat Int J Biol Macromol 65:534–541
Ngah WSW, Teong LC, Hanafiah MAKM (2011) Carbohyd Polym 83:1446–1456
Pandey S, Mishra SB (2011) J Colloid Interface Sci 361:509–520
Anirudhan TS, Rijith S (2012) J Environ Radioact 106:8–19
Hakami O, Zhang Y, Banks ChJ (2012) Water Res 46:913–3922
Rahbar N, Jahangiri A, Boumi S, Khodayar MJ (2014) Jundishapur J Nat Pharm Prod 9(2):e15913
Liu Y, Chen L, Yang Y, Li M, Li Y, Dong Y (2016) J Mol Liq 219:341–349
Repo E, Warchoł JK, Bhatnagar A, Sillanpää M (2011) J Colloid Interface Sci 358:261–267
Repo E, Malinen L, Koivula R, Harjula R, Sillanpää M (2011) J Hazard Mater 187:122–132
Chen D, Hu B, Huang Ch (2009) Talanta 78:491–497
Lewandowska-Łańcucka J, Staszewska M, Szuwarzyński M, Kępczyński M, Romek M, Tokarz W, Szpak A, Kania G, Nowakowska M (2014) J Alloys Comp 586:45–51
J.A. Sowjanya, J. Singh, T. Mohita, S. Sarvanan, A. Moorthi, N. Srinivasan, N. Selvamurugan (2013) Colloids surfaces B: Biointerfaces 109: 294–300
Toskas G, Cherif Ch, Hund RD, Laourine E, Mahltig B, Fahmi A, Heinemann Ch, Hanke T (2013) Carbohyd Polym 94:713–722
Ramos JVH, de Matos Morawski F, Costa TMH, Dias SLP, Benvenutti EV, de Menezes EW, Arenas LT (217) Micropor Mesopor Mater 217:109–118
da Costa Note BP, da Mata ALML, Lopes MV, Rossi-Bergmann B, Ré MI (2014) Powder Technol 255:109–119
Zhao P, Liu H, Deng H, Xiao L, Qin C, Du Y, Shia X (2014) Colloids Surf B: Biointerfaces 123:657–663
Lu J, Zhang W, Zhang Y, Zhao W, Hu K, Yu A, Liu P, Wu Y, Zhang S (2014) J Chromatogr 1350:61–67
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kołodyńska, D., Budnyak, T.M., Hubicki, Z., Tertykh, V.A. (2017). Sol–Gel Derived Organic–Inorganic Hybrid Ceramic Materials for Heavy Metal Removal. In: Mishra, A. (eds) Sol-gel Based Nanoceramic Materials: Preparation, Properties and Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-49512-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-49512-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49510-1
Online ISBN: 978-3-319-49512-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)