Abstract
Aortic valve (AV) disease involves stiffening of the AV cusp with progression characterized by inflammation, fibrosis, and calcification. Here, we examine the relationship between biomechanical valve function and proteomic changes before and after the development of AV pathology in the Emilin1−/− mouse model of latent AV disease. Biomechanical studies were performed to quantify tissue stiffness at the macro (micropipette) and micro (atomic force microscopy (AFM)) levels. Micropipette studies showed that the Emilin1−/− AV annulus and cusp regions demonstrated increased stiffness only after the onset of AV disease. AFM studies showed that the Emilin1−/− cusp stiffens before the onset of AV disease and worsens with the onset of disease. Proteomes from AV cusps were investigated to identify protein functions, pathways, and interaction network alterations that occur with age- and genotype-related valve stiffening. Protein alterations due to Emilin1 deficiency, including changes in pathways and functions, preceded biomechanical aberrations, resulting in marked depletion of extracellular matrix (ECM) proteins interacting with TGFB1, including latent transforming growth factor beta 3 (LTBP3), fibulin 5 (FBLN5), and cartilage intermediate layer protein 1 (CILP1). This study identifies proteomic dysregulation is associated with biomechanical dysfunction as early pathogenic processes in the Emilin1−/− model of AV disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aortic valve (AV) disease affects over 2% of the world population.33 Aging is an independent risk factor for AV disease, and as the general population ages, the incidence of disease increases.5 AV disease is progressive, typically characterized by inflammation, fibrosis and calcification. These pathologic changes result in stiffening of the valve cusps obstructing forward blood flow (stenosis), and/or faulty closure of the valve cusps leading to backward blood flow (insufficiency). Patients with moderate to severe aortic valve stenosis develop cardiac dysfunction and heart failure, yet treatment is currently restricted to surgical or transcatheter aortic valve replacement.30 Identification of mechanisms that may provide therapeutic targets to treat early stages of AV disease are of critical importance; however, the regulation of AV disease initiation and progression is largely unknown.
Protein dysregulation is a key event in the pathological progression of AV disease. Changes in the content and organization of the extracellular matrix (ECM) structure are a primary cause of valve stiffening, and the resulting biomechanical abnormalities contribute to valve dysfunction.28 In healthy valve tissue, ECM is organized in a trilayer structure composed of collagens, glycosaminoglycans and elastic fibers as the result of a fetal developmental process.1 In diseased valve tissue, ECM loses its compartmentalization, becoming inflamed and calcified, and studies in mice have shown that these structural changes result in altered biomechanical properties.14,22,49 In human AV disease, proteomics on valve interstitial cells (VIC) demonstrated increased content of actin binding proteins.3 Likewise, proteomic evaluation of diseased human AV tissue showed significant changes in a number of proteins, including those involved in immune responses and inflammation.27 However, in humans, proteomic analyses are limited to end stage AV disease because of a lack of tissue availability, confounding analysis. Proteomic alterations associated with the progression of AV disease have not been explored.
Recently, we reported a new model of AV disease, the Elastin Microfibril Interfacer 1 deficient (Emilin1−/−) mouse, which mimics the natural history of human AV disease.29,31 EMILIN1 is necessary for proper elastogenesis, and Emilin1 deficiency results in latent hemodynamic AV disease in the majority of mice between 10–12 months of age, characterized in part by an early increase in p-Erk1/2 activation and late ECM disorganization and inflammation.31,53 Late-onset hemodynamic disease allows the distinction between early features of disease processes, before 6 months of age before hemodynamic disease, and advanced features of disease, after the manifestation of overt disease as heart failure. EMILIN1 inhibits TGFB1 signaling,52 and Emilin1−/− AVs demonstrate both canonical (Smad2/3) and non-canonical (Erk1/2) TGFB1 activation, as well as fibrosis and inflammation, but not calcification.31 Emilin1−/− mice demonstrate a dynamic pathologic process that is characterized by early ECM changes and provides the opportunity to study disease progression. Understanding early disease processes is a priority in the heart valve community.35
The objective of this study was to examine AV biomechanics and protein networks before and after disease onset to further elucidate structure–function processes contributing to the progression of AV disease using the Emilin1−/− mouse model. We hypothesized that Emilin1 deficiency results in early proteomic alterations that precede AV disease and subsequent biomechanical dysfunction that occurs with disease. Global proteomic expression from mutant mice was interrogated before and after the manifestation of disease, and these findings were correlated with micro and macro biomechanical functional studies. This study provides new insight into mechanisms of early processes of AV disease and may contribute to the identification of new diagnostic and therapeutic approaches.
Methods
Animal Groups
All animal use and handling was performed in accordance with protocols approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee. Mixed gender Emilin1−/− mice and wild type (WT) littermates (Emilin1+/+) were studied at adult (4–6 months) and aged (12–16 months). These time points were chosen as an early stage that preceded any disease and a late stage that manifested advanced disease, as described in previous work.31 This resulted in four experimental groups: adult wild type (AdWT), aged wild type (AgWT), adult Emilin1−/− (AdEM), and aged Emilin1−/− (AgEM). We have presented genes and proteins according to the standards of nomenclature developed and maintained by the International Committee on Standardized Genetic Nomenclature for Mice (http://www.informatics.jax.org/mgihome/nomen/).
Dissection, Histochemistry and Immunohistochemistry
Dissection, histochemistry using a modified pentachrome stain, and immunohistochemistry were used to assess ECM content and organization, as previously described.14,22 See Supplemental Methods for details.
Macro Biomechanical Testing Using Micropipette Aspiration
Regional biomechanical properties of ex vivo AV tissue were quantified using the micropipette approach that we developed recently.21 Ten mice per genotype per stage were studied. Briefly, the AV tissue was dissected to reveal cusp and annulus regions in situ, and mounted on a microscope slide. The valve surface was accessed from the ventricular side using a micromanipulator and glass micropipette with an inner diameter of 50–60 μm; upon contact, aspiration pressure was applied in a step-wise manner, and an aspiration length was determined using image analysis of video frames.19 Pressure vs. aspiration length data were fit using linear regression. Young’s modulus (E) as a measure of tissue stiffness was determined using a half-space model equation.12,44
Microscale Biomechanical Testing Using Atomic Force Microscopy (AFM)
To quantify the microscale elastic modulus of the AVs, AFM testing was performed, as previously described.39 Five mice per genotype per stage were studied. Briefly, adult and aged mice were sacrificed and whole hearts were excised into cold PBS. Samples were flash frozen without fixation in optimal cutting medium (OCT) and sectioned at 10 µm. Sections were prepared for AFM analysis by rinsing off the OCT in PBS, blocking with 10% FBS for 30 min, washing in PBS 3X, rinsing with diH2O, and immediately subjected to AFM analysis. Scanning was completed on 30 µm X 30 µm areas on valve cusp and annulus regions using borosilicate glass particle tips with a nominal diameter of 3 µm and spring constant of 0.03 N/m. The tip was calibrated to a 2.5 MPa poly(dimethysiloxane) standard prior to sample analysis. For each animal, scans were analyzed on each of five adjacent sections and a median modulus value was calculated for each scan. Median values were aggregated to calculate an average modulus for each animal, which was then used for statistical comparison.
Statistical Analyses for Biomechanical Measurements
Multivariate ANOVA and post hoc Bonferroni multiple comparison tests (SPSS Inc.) were used to determine effects by region (cusp, annulus), genotype (Emilin1−/−, WT) and stage (adult, aged) on measures of tissue stiffness. All variables were reported as means ± standard deviation. Differences were considered significant at p ≤ 0.05.
Proteomic Analysis
Protein was extracted from a pool of twelve AV cusps per experimental group and examined by proteomic methods, as described previously.4 Proteomics was performed on the excised cusp only. Annulus tissue is difficult to isolate by the microdissection methods needed for proteomic studies without introducing aortic wall tissue contamination. Excised cusps were decellularized using SDS detergent buffers, quantified using a bicinchoninic acid assay. A total of 60 µg loaded onto a 12% Bis-Tris minigel (Invitrogen, Carlsbad, CA) and electrophoresed 15 minutes at 150 volts using MOPS–SDS running buffer, until high molecular weight marker (250 kDa) was approximately 0.4 cm below the well. Gel plugs were briefly stained (SimplyBlue SafeStain, Invitrogen, Grand Island NY) for protein visualization and each lane was minced into 1 mm cubes and treated by in-gel digestion, followed by peptide extraction using established protocols.4 Peptides were analyzed by inline multidimensional chromatography coupled to ion trap tandem mass spectrometry (LTQ, Thermo Scientific, Inc., San Jose, CA). Peptides were identified through database searching followed by clustering to proteins at ≤1% false discovery rate using ProteoIQ. Proteins that had greater than five spectral counts were used in quantitative comparisons. Comparative protein ratios were further filtered by significant ANOVA testing (p value ≤0.05) and ratios of ≥2-fold. Further details are provided in the Supplemental Methods.
Bioinformatic Analysis
Protein function and pathway enrichment were calculated by WebGestalt program comparing against KEGG and Wikipathways using a threshold of two fold enrichment and a corrected p value ≤1.0E−3 as a cutoff point.11,54 The term protein group enrichment corresponds to the ratio of observed to expected number of genes from each proteome using the mouse genome as a reference set. Enrichment was calculated as the number of observed proteins compared to the expected number of proteins using the mouse genome as a reference. We have adopted the following approach to evaluating enrichment data: When a function or pathway is altered in the aged WT, but not the adult WT, it indicates the possible effect of aging. Further, a function or pathway may be altered in the adult mutant only (suggesting it is not an “aging” function), the aged mutant only (suggesting it is an “aging” function) or both the adult and aged mutant (suggesting it may or may not be an “aging” function). Significance of the ratio was calculated using a hypergeometric statistical method and was adjusted for multiple testing using the Benjamini–Hochberg correction.8 Protein processes were evaluated using Ingenuity Pathways Analysis (IPA, QIAGEN Redwood City) v24390178 build 346717. A Comparison Analysis was used to calculate functions for each total proteome, filtered using a negative log p value cutoff ≥3 as a threshold. Data were visualized using IPA or the TM4 Microarray Software Suite.37
Results
Biomechanical Testing Shows Macroscale AV Tissue-Level Effects in Aged Emilin1−/− Mice
Emilin1−/− mice are a model of latent fibrotic AVD, exhibiting elastin fiber fragmentation and fibrosis, but do not demonstrate gross calcification or histopathologic mineralization.22 To examine the effect of the altered ECM on valve biomechanical properties contributing to valve dysfunction, we evaluated stiffness of the cusp and annulus during aging in Emilin1−/− mice. A micropipette aspirate approach was used to determine Young’s modulus in the cusp and annulus regions of Emilin1−/− and WT AVs at adult and aged stages (Fig. 1), showing differential effects of aging by genotype. Between 4 and 12 months of age, Emilin1−/− AV annulus stiffness increased 1.7 fold. Similarly, there was a significant 1.3 fold increase in Emilin1−/− cusp stiffness measurements with age. In contrast, there was no significant difference in WT annulus or cusp stiffness values. Whole mount testing of AV stiffness therefore indicated that Emilin1 deficiency results in increased stiffness in both annulus and cusp valve regions at the aged stage only.
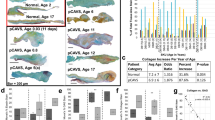
Determination of mechanical stiffness by genotype and age using micropipette biomechanical testing. Micropipette biomechanical characterization (MBC) of the Emilin1−/− (EM) on whole mount AVs compared to WT litter mates. (a) Valve tissue regions studied by different modalities. (b) Emilin1 deficiency significantly increased AV stiffness in the annulus compared to aged WT littermates and during aging of the Emilin1−/− mouse; (b) Emilin1 deficiency increased stiffness in the cusp during aging, with no significant change in WT due to aging. Significant differences were determined using one-way ANOVA p value ≤0.03. Asterisks denote significant differences when comparing ages; Hashtags denote significant differences when comparing genotypes. AdWT, adult wild type; AgWT, aged wild type; AdEM, adult Emilin1−/−, AgEM, aged Emilin1−/−.
AFM Biomechanical Microscale Testing Shows Intrinsic ECM Stiffening in the Adult and Aged Emilin1 Deficient AV, as Well as the Aged WT AV
Biomechanical testing using AFM was utilized to assess microstructural intrinsic properties of AV ECM (Fig. 2). There was no significant difference in microstructural ECM properties between the WT and Emilin1−/− AV annulus regions at either adult or aged stages (Fig. 2a), although aged Emilin1−/− trended towards increasing stiffness with high variation from animal to animal. In contrast, the AV cusp region of Emilin1−/− mice showed a significant 1.4 fold increase in the microenvironmental stiffness between 4 and 12 months (Fig. 2b). Emilin1 deficiency resulted in a significant increase in microscale cusp stiffness in both adult and aged groups as compared to WT. Aging was associated with significant increases in microscale AV cusp stiffness in both Emilin1−/− and WT mice (p value ≤0.01). Cross sectional topography illustrated the subtle variation between the adult and aged WT, as well as a marked difference between the aged WT and aged Emilin1−/− AV (Fig. 2c). Therefore, Emilin1 deficiency results in AV microstructure biomechanical stiffening in the cusp before 4 months, prior to the development of macroscopic scale stiffening, which occurs between the ages of 4 and 12 months, and before hemodynamic AV disease.
Determination of mechanical stiffness by genotype and age using atomic force microscopy. AFM of AV cusp cross sections (30 × 30 µm2 measurement areas). (a) Annulus region did not show a significant difference in microscale cross sectional measurements. (b) AFM showed a significant increase in microenvironmental cusp stiffness between WT and Emilin1−/− at 4 and 12 months, and when comparing Emilin1−/− mice at 4 and 12 months. (c) AFM topography of 30 × 30 µm cross sections, where z-axis shows thickness distribution, and color represents stiffness distribution used to calculate the average values presented in (b). Significant differences were determined using one-way ANOVA p value ≤0.01. Asterisks denote significant differences when comparing ages; Hashtags denote significant differences when comparing genotypes.
Emilin1 Deficient AV Proteomes Show Increased Proportions of Proteins Involved in Inflammation, Angiogenesis and Fibrosis
In order to assess alterations in protein composition, we utilized a mass spectrometry based proteomics analysis. We first examined functions and pathways reported by global proteomes of adult and aged WT and Emilin1−/− AV. When a function or pathway is reported altered in the aged WT, but not the adult WT, this function or pathway may be altered in the adult mutant only (suggesting it is not a function of aging), the aged mutant only (suggesting it is a function of aging) or both the adult and aged mutant (suggesting it may or may not be a function of aging). Clustering proteomes to functions revealed that whereas adult WT AVs showed no significant detection of fibrogenesis, the adult Emilin1−/− AVs showed pro-fibrotic aberration of ECM, as did aged WT and aged Emilin1−/− AVs (Fig. 3a; Supplementary Table 1A). This is taken to illustrate that fibrogenesis is a normal process of aging and that ECM aberrations caused by Emilin1 deficiency accelerate these processes This is consistent with prior observations that ECM aberrations accelerate the effects of aging.34 Enrichment of angiogenesis-related proteins was detected in all proteomes, with subtle changes in aged wild type and both Emilin1−/− AVs (−log p value of enrichment 21.2 AdWT; 25.5 AgWT; 25.4 AdEM; 23.3 AgEM, where Bonferroni corrected p value ≤1.0E−3 was considered significant). We have previously reported that TGFB1 mediates increases in angiogenesis during aging in the Emilin1−/− mouse.31 The current data may be interpreted that at the global proteome level as follows: a subset of angiogenesis proteins are detectable at both stages, and another set of angiogenesis proteins are expressed at high levels in the aged Emilin1 deficient mouse indicating similarities in disease development due to aging and in the Emilin1−/− model. Interestingly, while “inflammatory response” appeared uniform across all proteomes, an increase with proteins involved in immune related processes of “adhesion of immune cells” and “cell movement of neutrophils” was observed in Emilin1−/− valves. Immune cell infiltration, as evidenced by Mac-3 immunohistochemistry, has been previously demonstrated in the Emilin1−/− aortic valve.31,32 Further investigation of proteins showed that ~ 15% overlap in proteins grouped to inflammation and immune response (Supplemental Table 1A), suggesting that these processes may be intertwined at the protein level in this model. Additionally, the function “connective tissue” was enriched in the Emilin1−/− mice but not in the WT counterparts, and “morphology of cardiovascular tissue” was enriched in the aged Emilin1−/− proteome (−log p value 8.3) only, potentially reflecting the altered ECM protein content of increased tissue remodeling and increased cusp stiffness. Therefore, at the translational level, Emilin1 deficiency results in increased fibrogenesis accompanied by alterations in immune function and age-associated remodeling, and these alterations occur at the same time as micro level and before macro level biomechanical dysfunction.
Heat maps for overall proteomic comparison of Emilin1−/− and WT aortic valves before and after disease manifestation. Heat maps of functions and pathways from overall proteomic comparison of adult and aged Emilin1−/− and wild type AVs. (a) Differential biological functions and processes across each of the 4 proteomes; (b) pathway enrichment from each proteome. All functions and pathways utilize negative log Fisher’s exact p value ≤1.0E−3 for statistical significance as shown on the heat map. Red is highest enrichment, blue indicates lower but significant enrichment. Black colored functions are not significant. AdWT, adult wild type; AgWT, aged wild type; AdEM, adult Emilin1−/−; AgEM, aged Emilin1−/−.
Emilin1 Deficiency Results in Enrichment of Unique Pathways Associated with AV Disease
Pathway enrichment was utilized to earmark pathways influenced by Emilin1 deficiency (Fig. 3b, gene annotation and statistics in Supplementary Table 1B). Multiple changes in signaling pathways occurred coincident with the progressive stiffening observed by biomechanical testing. The EGFR1 signaling pathway, associated with endothelial dysfunction and cardiac remodeling,16,26 showed increased pathway enrichment in Emilin1−/− mice vs. WT (Supplemental Table 1B). The PPAR signaling pathway, known to protect against cardiovascular calcification,9 showed an increase in enrichment in the aged Emilin1−/− AV relative to other valve proteomes. “Apoptosis” was not significantly enriched in the adult WT mice but appeared enriched in all other groups, suggesting that apoptosis is a process of normal aging that is accelerated by Emilin1 deficiency. While AV tissue in adult WT showed a low level of enrichment for VEGF and adult Emilin1−/− showed no significant enrichment for VEGF pathways, AV tissue in the aged WT and aged Emilin1−/− showed significant enrichment of VEGF signaling, which is consistent with previous observations.31 The mTOR pathway, critical to the regulation of inflammation and complex immune responses during hemodynamic stress,47,50 was significantly enriched in the aged Emilin1−/− AV only. Overall, these findings report that Emilin1 deficiency influences changes in multiple signaling pathways simultaneous with microscale AV stiffening that precedes macroscale biomechanical alterations.
Altered Protein Networks in Emilin1−/− Mice Indicate an Early AV Disease Process and Demonstrate Increased Aberrancy Over time Coincident with Disease Progression
Significantly altered protein expression was further analyzed for primary biological functions, comparing WT and Emilin1−/− during aging. Bioinformatics analysis showed that altered proteins affected filament and myofibril formations, morphology of cells, and apoptosis (Fig. 4a; Supplemental Table 3). Proteins involved in both the formation of myofibrils (MYH6, MYL2, PKP2, TTN) and filaments (MYOMI, PKP2, TTN) decreased with the exception of STAT3, which increased. Cell death and apoptosis were increased in adult Emilin1−/− mice compared to adult WT. Importantly, EMILIN1 protein was obliterated at both ages, consistent with null expression of the gene. In previous work (Munjal et al.31), we showed that several myofibril components are altered in the Emilin1 deficient aortic valve, including upregulation of the markers SMA, SMemb and SM22. Likewise, we documented increased apoptosis in the Emilin1−/− mutant aortic valve using cleaved caspase-3 immunohistochemistry. The current proteomic data reports that multiple proteins are altered by the absence of EMILIN1, proteins that contribute to the formation of myofibrils and filaments, as well as the regulation of programmed cell death (apoptosis). Thus perturbation of myofibril processes and multiple maladaptive compensatory effects are observed at the translational level due to aging in the Emilin1−/− AV.
Protein network of significant functions regulated due to Emilin1 deficiency. (a) Ratio of adult Emilin1−/− (AdEM) to adult wild type (AdWT). (b) Ratio of aged Emilin1−/− (AgEM) to aged WT (AgWT). EMILIN1 was not detected in the Emilin1−/− AV. Protein alterations were detected as greater than twofold change, p value ≤0.05. IPA analysis log threshold of p value ≤3.0.
In the aged Emilin1−/− valves, protein networks impacting cell proliferation were notably changed compared to the adult Emilin1−/− AV (Fig. 4b). A majority of the related proteins (22/30, 73%) were increased in aged Emilin1−/− AVs. In this subset of altered proteins, there were significant differences showing dysregulated adhesion and immune responses. In addition, there was derangement of proteins and processes involved in cytoskeletal organization, consistent with previous observations describing the role of VIC activation in AV disease pathogenicity.31 Immune cell adhesion proteins (PECAM1, MYO1G, MRC1) were elevated compared to WT littermates, with decreases in proteins SRC and MYADM. Interestingly, FBLN5, associated with inhibition of angiogenesis,43 was decreased in the aged Emilin1−/− compared to WT, suggesting that EMILIN1–FBLN5 interactions may have a role in the increased angiogenesis detected at the proteomic level, consistent with the increase in aberrant angiogenesis detected in AV disease progression.51 Overall, the adult Emilin1−/− AV showed dysregulation related primarily to cellular organization and fibril maintenance, while the aged Emilin1−/− AV shows differences in protein expression related to increased proliferation, aberrant angiogenesis and immune dysregulation.
Cell Adhesion and Fibronectin Binding are Altered in the Context of Emilin1 Deficiency and Aging
A small subset of proteins was altered when comparing the adult and aged Emilin1−/− mutants (Figs. 5a and 5b). ECM organization was the main process interrupted. Collagens 4a3 and 6a5 were increased in the aged compared to adult Emilin1−/− mice, and were linked to the Gene Ontology function of “ECM organization.” The aged Emilin1−/− AV was characterized also by abnormal cell adhesion, through increased COL4A3 and COL6A5, and decreased CCDC80 and PPAP2B. FN1 was not significantly altered in any group comparison. These changes likely reflect early primary structural changes to the valve scaffold in response to Emilin1 deficiency and different secondary changes in the older mouse.
Comparison of aging processes in Emilin1 deficient or wild type AV tissue. (a) Protein changes in the aging Emilin1−/− represented as a ratio aged Emilin1−/− (AgEM) to adult Emilin1−/− (AdEM). (b) Functional processes of altered proteins for ratio AgEM/AdEM. (c) Function analysis of ratio aged WT (AgWT) to adult WT (AdWT). Functions are calculated using toppcluster.org and reported as −log bonferroni adjusted p values ≤3.0 and drawn in ingenuity pathways analysis.
Aged WT AVs (AgWT/AdWT) showed increased cell activation, immune response, and adhesion (Fig. 5c), including increases in the proteins vitronectin (VTN, secreted protein associated with maintenance of valve integrity),2,48 VCAM1 (marker of inflammation), and FBLN1 (modulator of OFT endothelial mesenchymal transition),13 identifying multiple processes involved in alteration of AV structure. Overall, these findings are consistent with the observation that age is an independent risk factor for developing AV disease and that normal aging processes contribute to the pathogenesis of AV disease.
Emilin1 Deficiency Affects ECM Proteins Downstream of TGFB1 Signaling
Since previous work demonstrated that non-canonical TGFB1 signaling contributes to AVD pathogenesis,31 we examined proteins interactive with TGFB1 signaling. Calculation of the primary local network surrounding EMILIN1, ELASTIN and TGFB1 showed complex interactions using proteomics (Fig. 6A). Proteins within this network contribute to structural integrity of valve tissue and therefore impact organization and durability, ultimately effecting valve function and resulting in disease. Trend analysis showed which proteins paralleled or diverged from EMILIN1 levels (Fig. 6B). Proteins that were simultaneously decreased in the Emilin1 deficient mouse included cartilage intermediate layer protein (CILP1), growth differentiation factor 11 (GDF11, also known as BMP11), and FIBULIN1. Conversely, ECM proteins that increased with Emilin1 deficiency over time included WNT9B and COL6A5. The latent transforming growth factor beta binding protein 3 (LTBP3) and fibulin-5 (FBLN5) protein were decreased in aged Emilin1−/− mice. EMILIN1 protein is primarily localized to the ventricularis layer of the WT valve cusp, and protein expression is obliterated in Emilin1−/− mice (Fig. 7a). EMILIN1 protein content and localization has not been reported in healthy or diseased human heart valves. Additional experiments confirmed that Emilin1 deficiency results in decreased LTBP3, FBLN5 and CILP1 in aged AV tissue (Fig. 7). Overall, these findings identify new downstream effects of EMILIN1 signaling that link TGFB1 signaling and aberrant ECM expression, further showing that EMILIN1 has a broad and diverse role in maintaining the structural integrity of AVs.
TGFB1–ECM interactive proteins altered by Emilin1 deficiency or by normal aging. (A) Altered proteins interactive network with TGFB1, ELASTIN and EMILIN1, green lines highlight potential interactions with EMILIN1 as shown by the current study; (B) trend analysis of log transformed spectral counts of ECM proteins showing significant alterations in ratios as tested by p value ≤0.05: a—Emilin1−/− adult/WT adult; b—Emilin1−/− aged/WT aged; c—Emilin1−/− aged/Emilin1−/− adult; d—WT aged/WT adult.
Immunofluorescence confirms that Emilin1 deficiency reduces expression of multiple ECM proteins in the EMILIN1–TGFB1 interaction network. Comparisons are WT aged AV to Emilin1−/− aged AV. (a, b) Confirmation of Emilin1 deficiency in aged AV; LTBP3 (c, d) and FBLN5 (e, f) are significantly decreased in the aged Emilin1−/−; CILP1 expression is negligible in aged Emilin1−/−.
Discussion
In the current study, proteomic composition was examined in relationship to biomechanical properties in the context of Emilin1 deficiency and aging. The Emilin1 deficient mouse has been characterized as a model of latent AV disease with pathogenesis primarily related to ECM defects and altered non-canonical TGFB1 signaling.31 We report that biomechanical measurements are associated with translational level changes, and patterns of misexpression of specific biologic processes and signaling pathways are associated with early AV disease processes, prior to the manifestation of overt disease. Strengths of this study are the analyses of aggregate protein profile in a model of aortic valve disease before and after disease onset, and the correlation of this comprehensive description to measures of valve function. These findings provide important information about pathologic changes that occur in early stages of disease, and provide patterns of disease progression that may provide insight into human AV disease and mechanisms of aging.
To our knowledge this is the first time that biomechanical functional studies have been performed with proteomic expression analysis during AV disease progression. Biomechanical measurements by two modalities indicate that EMILIN1 deficiency is associated with significant changes in tissue stiffness at both the macro and micro scales in the aged mouse, correlating with the manifestation of hemodynamically significant disease.31 Aging in the context of Emilin1 deficiency resulted in macro level stiffening of both cusp and annulus regions, as well as proteomic processes, including inflammation, immune infiltration and fibrosis. Interestingly, testing at the micro level also shows biomechanical perturbations due to Emilin1 deficiency in the adult cusp before pathological signs of AV disease. In the adult AV cusp, proteomic changes in pathway signaling and biological function were concomitant with altered biomechanical properties. We suggest that EMILIN1 is necessary for biomechanical stability of the healthy AV cusp and that loss of EMILIN1 accelerates the maladaptive biomechanical effects of the aging process primarily through premature ECM abnormalities. Importantly, this study shows that two modalities of measuring tissue stiffness are in agreement, but that AFM has increased sensitivity to identify more subtle changes. This study identifies that altered protein expression is associated with significant biomechanical changes that worsen at the microscale level and contribute to progressive degeneration of valve tissue.
Previous work demonstrated that Emilin1−/− AV structure deteriorates prior to dysfunction, and advanced AV disease processes, including inflammation and fibrosis, occur later in the context of hemodynamic disease.31 In our current work, we focused on proteomic analyses of the AV cusp as there is no reliable way to dissect the amount of annulus material needed for proteomic studies. Here, proteomic analysis showed similar enrichment of inflammatory response among all four proteomes, but with differentiating immune processes (Fig. 3a). This suggests that at the global protein level, normal aging of the valvular structure involves similar inflammatory regulation that is not affected by Emilin1 deficiency. We suggest that proteins clustered to “inflammatory response” may collectively participate in fundamental AV homeostasis, including proteins that contribute to normal AV development but also intersect with injury-induced inflammatory processes, and may or may not be sufficient to cause pathological changes. Instead, pathological AV structural changes at the global proteomic level appear to involve increased immune processes in combination with specific inflammatory processes that are present in the aged Emilin1 deficient mouse. This is further supported by the new pathway information involved in EMILIN1 expression (Fig. 3b), namely the mTOR, EGFR1 and the PPARγ pathways, suggesting complex regulation of homeostasis and pathogenesis.
The mTOR pathway has multiple roles in cardiac adaptability by controlling protein turnover through targets of rapamycin,6,15 and modifies inflammatory response through regulation of immunoproteasomal degradation under hemodynamic stress.50 We hypothesize that the proteomic enrichment of the mTOR pathway in the aged Emilin1−/− AV suggests that mTOR signaling may regulate an immune mechanism that modifies inflammatory processes and ultimately contributes to the progression of AV disease. The role of mTOR in resident immune cells is complex and activation of this pathway may have variable inflammatory responses.45 The EGFR1 and PPAR pathways showed increased enrichment in both adult and aged Emilin1−/− AVs compared to WT, suggesting these molecular changes occurred before biomechanical dysfunction or the effects of aging. Both pathways are known to have a fundamental role in the structural integrity of valve tissue. The EGFR tyrosine kinase receptor is required for appropriate AV development, and regulates proliferation of mesenchymal cells during cushion remodeling.16 EGFR works to suppress BMP signaling, and primary osteoblasts from Egfr−/− mice show increased formation of calcific bone nodules relative to controls, showing a role for EGFR in limiting processes of calcification.41 In valve tissue, loss of EGFR causes strain specific AV hyperplasia and AV structure thickening leading to stenosis and calcification.7 Emilin1−/− mice do not show development of calcific nodules, therefore, the Emilin1−/− proteomic increases in enrichment of EGFR signaling may reflect activation of mechanisms surrounding the lack of calcification in this model. This is a novel insight provided by the proteomic data and further work is needed to completely delineate these mechanisms. PPARγ has protective anti-calcific effects in valve tissue through upstream regulation of osteopontin.23,42 Increases in EGFR1 and PPARγ pathways may contribute to disease progression but paradoxically prevent calcification in Emilin1−/− valves. The results from the current study suggest multiple inflammatory pathways may contribute to disease and these pathways could provide new therapeutic targets by blocking maladaptive inflammation prior to advance disease states.
Emilin1 deficiency has been linked to both canonical and non-canonical TGFB1 signaling in aged AV tissue.31 We have expanded these observations by demonstrating that Emilin1 inactivation reduces expression of multiple ECM proteins in the EMILIN1–TGFB1 network, better defining the downstream structural and functional effects of Emilin1 deficiency (Fig. 6). All direct methods for protein-to-protein interaction annotation use the common principle that proteins that interact with each other are more likely to show similar expression patterns upon phenotypic modulation.40 This is referred to as the modularity of disease.17 Here, the data from the ECM proteins LTBP3, FBLN5, CILP1, established factors in AVD progression, suggests that these proteins have functional protein-to-protein interactions with EMILIN1 as defined by similarities in expression patterns at all time points (CILP1) or in aged Emilin1−/− AV (LTBP3 and FBLN5). Emilin1 deficiency in the aged AV resulted in marked decreases of LTBP3, FBLN5, and CILP1. LTBP3 is capable of binding all three TGFB isoforms, but its relationship to TGFB1 is less well known.36 LTBP3 deficiency in mice is associated with decreased TGFB1 signaling,10 but the valves of these mice have not been studied. LTPB3 increased in the adult Emilin1−/− AV but decreased in the aged Emilin1−/− AV, suggesting that LTBP3–TGFB1 interactions are dynamically modulated. This is supported by reports that LTBP3 is capable of differentially regulating TGFB1 signaling.20,46 FBLN5 is essential for elastic fiber development through sequestration of TGFB1, and patients homozygous for a missense mutation in FBLN5 have cutis laxa, a skin disease, and show thickened aortic valves with supravalvular aortic stenosis.25 The current data suggest that EMILIN1 interactions may play a significant role in FBLN5 modulation of TGFB1. Finally, CILP1 was effectively eliminated in both adult and aged Emilin1−/− AVs. CILP1 acts to suppress IGF1-induced proliferation18 and inhibits transcriptional activation of cartilage genes through TGFB1.38 Emiliin1 deficiency has been shown to have increased proliferative effects.31 The combination of proteomic and biomechanical data suggests that excessive proliferation may be the result of loss of CILP1. Taken together, the data suggest that the EMILIN1-TGFB1 interactions influence a variety of proteins and protein networks that regulate valve structure and function and contribute to disease progression.
This study has notable limitations. Emilin1 deficiency results in systemic hypertension, which may result in cardiac remodeling over time. The Emilin1−/− mouse has been carefully characterized with highly penetrant (100% of mice in the seminal study) systemic hypertension as measured both invasively and non-invasively.52 We have confirmed the same penetrance and severity of hypertension in our colony of mice, but did not measure blood pressure in the mice used in this study. While AV dysfunction also results in cardiac remodeling, it is unknown if mechanisms of cardiac dysfunction could cause or exacerbate valve dysfunction. It is possible that proteomic effects in Emilin1−/− AV could involve some compensation for global loss of EMILIN1. Proteomics was performed on the cusp and hinge and did not include a large amount of annulus. Annulus tissue alone is difficult to isolate by the microdissection methods needed for proteomic studies and may be associated with large amounts of aortic wall tissue, which would contaminate the valvular proteome. Some limitations in the proteomic work are due to processes inherent with shotgun proteomic sampling. Proteomic studies capture only the top ~ 35% of the entire proteomes due to dynamic sampling by mass spectrometry.24 Increasing the number of technical replicates per biological sample increases the detection of low count proteins,24 but performing extensive replicates is limited by sample availability and cost. Scaffold ECM proteins may be missing from the analysis, including various predictable elastic fiber proteins and proteoglycans. This is because the discussed proteomes were obtained by decellularization processes,4 which leaves behind bound scaffold proteins. However, these studies provide information that will direct future studies to target specific scaffold proteins to further elucidate ECM modifications occurring during AV disease progression.
EMILIN1 is necessary for maintenance of normal AV structure and function. Emilin1 deficiency results in early ECM structural disorganization and disruption of ECM-cell signaling, which can be detected functionally at the micro level before hemodynamic disease. Late activation of protein networks results in increased proliferation, inflammation and fibrosis, as well as a complex immune response, which collectively results in both micro and macro biomechanical dysfunction at the time of overt hemodynamic AV disease (Fig. 8). This is shown in established histopathological changes characterizing this model.31,32 Multiple signaling pathways are altered before overt disease manifestation, including mTOR, EGFR1 and PPARγ pathways, suggesting a complex role for inflammatory and immune mediated responses. EMILIN1 interacts with TGFB1, a primary controller of AV disease progression, and the protein network associated with this interaction has identified new protein relationships that may inform AV structure and function. EMILIN1 interaction networks have a significant impact on ECM composition and intrinsic biomechanical properties that influence valve function over time. This study increases our understanding of markers of aging in the context of early AV disease processes, a necessary step to elucidating the contribution of aging to AV disease pathogenesis. EMILIN1 is an important modifier of AV disease progression. Taken together, EMILIN1 impacts proteins involved in disease initiation and progression, as well as processes involved in advanced disease. Defining early patterns of protein changes during early stages of disease may provide new predictive biomarkers to monitor disease progression or therapeutic targets.
Summary model of the effects of Emilin1 deficiency in AV disease. EMILIN1 is an antagonist of TGFB1.52 Emilin1 deficiency results in increased TGFB1 signaling through canonical pathways involving phosphorylation of Smad2/3 and non-canonical phosphorylation of Erk1/2. This leads to ECM structural disorganization, disruption of ECM-cell networks, and increased protein interactions of inflammation, fibrosis and immune responses in the adult Emilin1−/− AV before the manifestation of overt AV disease (early), which is associated with microscale cusp stiffening. In the aged Emilin1−/− AV (late), ongoing activation of protein networks results in increased proliferation, ECM production and immune recruitment. Increased ECM production leads to overall macroscale tissue-level valve stiffening at the same time that valve dysfunction develops. Observations of early processes improve our understanding of AV disease progression, a necessary step to improve diagnostic and therapeutic tools.
Abbreviations
- AFM:
-
Atomic force microscopy
- AV:
-
Aortic valve
- ECM:
-
Extracellular matrix
- TGFB1:
-
Transforming growth factor beta 1
- VIC:
-
Valve interstitial cell
- WT:
-
Wild type
References
Aikawa, E., et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113(10):1344–1352, 2006.
Akhtar, S., K. M. Meek, and V. James. Immunolocalization of elastin, collagen type i and type iii, fibronectin, and vitronectin in extracellular matrix components of normal and myxomatous mitral heart valve chordae tendineae. Cardiovasc. Pathol. 8(4):203–211, 1999.
Alvarez-Llamas, G., et al. Modification of the secretion pattern of proteases, inflammatory mediators, and extracellular matrix proteins by human aortic valve is key in severe aortic stenosis. Mol. Cell. Proteomics 12(9):2426–2439, 2013.
Angel, P. M., et al. Networked-based characterization of extracellular matrix proteins from adult mouse pulmonary and aortic valves. J. Proteome Res. 10(2):812–823, 2011.
Aronow, W. S. Valvular aortic stenosis in the elderly. Cardiol. Rev. 15(5):217–225, 2007.
Balasubramanian, S., et al. mTOR in growth and protection of hypertrophying myocardium. Cardiovasc. Hematol. Agents Med. Chem. 7(1):52–63, 2009.
Barrick, C. J., et al. Reduced egfr causes abnormal valvular differentiation leading to calcific aortic stenosis and left ventricular hypertrophy in c57bl/6j but not 129s1/svimj mice. Am. J. Physiol. Heart Circ. Physiol. 297(1):H65–H75, 2009.
Benjamini, Y., and Y. Hochberg. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57(1):289–300, 1995.
Chu, Y., et al. Pioglitazone attenuates valvular calcification induced by hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 33(3):523–532, 2013.
Dabovic, B., et al. Bone abnormalities in latent tgf-β binding protein (ltbp)-3–null mice indicate a role for ltbp-3 in modulating tgf-β bioavailability. J. Cell Biol. 156(2):227–232, 2002.
Duncan, D., N. Prodduturi, and B. Zhang. Webgestalt2: an updated and expanded version of the web-based gene set analysis toolkit. BMC Bioinform. 11(Suppl 4):P10, 2010.
Guilak, F., L. G. Alexopoulos, M. A. Haider, H. P. Ting-Beall, and L. A. Setton. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Ann. Biomed. Eng. 33(10):1312–1318, 2005.
Harikrishnan, K., et al. Fibulin-1 suppresses endothelial to mesenchymal transition in the proximal outflow tract. Mech. Dev. 136:123–132, 2015.
Hinton, Jr, R. B., et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ. Res. 98(11):1431–1438, 2006.
Hwang, S.-K., and H.-H. Kim. The functions of mtor in ischemic diseases. BMB Rep. 44(8):506–511, 2011.
Iwamoto, R., and E. Mekada. Erbb and hb-egf signaling in heart development and function. Cell Struct. Funct. 31(1):1–14, 2006.
Jiang, X., et al. Modularity in the genetic disease-phenotype network. FEBS Lett. 582(17):2549–2554, 2008.
Johnson, K., D. Farley, S. I. Hu, and R. Terkeltaub. One of two chondrocyte-expressed isoforms of cartilage intermediate-layer protein functions as an insulin-like growth factor 1 antagonist. Arthritis Rheum. 48(5):1302–1314, 2003.
Jones, W. R., et al. Alterations in the young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech. 32(2):119–127, 1999.
Koli, K., M. J. Ryynänen, and J. Keski-Oja. Latent tgf-β binding proteins (ltbps)-1 and-3 coordinate proliferation and osteogenic differentiation of human mesenchymal stem cells. Bone 43(4):679–688, 2008.
Krishnamurthy, V. K., F. Guilak, D. A. Narmoneva, and R. B. Hinton. Regional structure-function relationships in mouse aortic valve tissue. J. Biomech. 44(1):77–83, 2011.
Krishnamurthy, V. K., et al. Maladaptive matrix remodeling and regional biomechanical dysfunction in a mouse model of aortic valve disease. Matrix Biol. 31(3):197–205, 2012.
Li, F., et al. Pioglitazone attenuates progression of aortic valve calcification via down-regulating receptor for advanced glycation end products. Basic Res. Cardiol. 107(6):1–14, 2012.
Liu, H., R. G. Sadygov, and J. R. Yates. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76(14):4193–4201, 2004.
Loeys, B., et al. Homozygosity for a missense mutation in fibulin-5 (fbln5) results in a severe form of cutis laxa. Hum. Mol. Genet. 11(18):2113–2118, 2002.
Makki, N., K. W. Thiel, and F. J. Miller. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int. J. Mol. Sci. 14(10):20597–20613, 2013.
Martín-Rojas, T., et al. Proteomic profile of human aortic stenosis: insights into the degenerative process. J. Proteome Res. 11(3):1537–1550, 2012.
Merryman, W. D., and F. J. Schoen. Mechanisms of calcification in aortic valve disease: role of mechanokinetics and mechanodynamics. Curr. Cardiol. Rep. 15(5):1–7, 2013.
Miller, J. D., R. M. Weiss, and D. D. Heistad. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ. Res. 108(11):1392–1412, 2011.
Moremen, K. W., M. Tiemeyer, and A. V. Nairn. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 13(7):448–462, 2012.
Munjal, C., et al. Tgf-β mediates early angiogenesis and latent fibrosis in an emilin1-deficient mouse model of aortic valve disease. Dis. Models Mech. 7(8):987–996, 2014.
Munjal, C., et al. Inhibition of mapk-erk pathway in vivo attenuates aortic valve disease processes in emilin1-deficient mouse model. Physiol. Rep. 5(5):e13152, 2017.
Nkomo, V. T., et al. Burden of valvular heart diseases: a population-based study. Lancet 368(9540):1005–1011, 2006.
Pezet, M., et al. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res. 11(1):97–112, 2008.
Rajamannan, N. M., et al. Calcific aortic valve disease: not simply a degenerative process a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group executive summary: calcific aortic valve disease: 2011 update. Circulation 124(16):1783–1791, 2011.
Robertson, I. B., et al. Latent tgf-β-binding proteins. Matrix Biol. 47:44–53, 2015.
Saeed, A. I., et al. Tm4 microarray software suite. Methods Enzymol. 411:134–193, 2006.
Seki, S., et al. Cartilage intermediate layer protein promotes lumbar disc degeneration. Biochem. Biophys. Res. Commun. 446(4):876–881, 2014.
Sewell-Loftin, M. K., C. B. Brown, H. S. Baldwin, and W. D. Merryman. A novel technique for quantifying mouse heart valve leaflet stiffness with atomic force microscopy. J. Heart Valve Dis. 21:513–520, 2012.
Sharan, R., I. Ulitsky, and R. Shamir. Network-based prediction of protein function. Mol. Syst. Biol. 3:1–13, 2007.
Sibilia, M., et al. Mice humanised for the egf receptor display hypomorphic phenotypes in skin, bone and heart. Development 130(19):4515–4525, 2003.
Steitz, S. A., et al. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am. J. Pathol. 161(6):2035–2046, 2002.
Sullivan, K. M., R. Bissonnette, H. Yanagisawa, S. N. Hussain, and E. C. Davis. Fibulin-5 functions as an endogenous angiogenesis inhibitor. Lab. Invest. 87(8):818–827, 2007.
Theret, D. P., M. J. Levesque, M. Sato, R. M. Nerem, and L. T. Wheeler. The application of a homogeneous half-space model in the analysis of endothelial cell micropipette measurements. J. Biomech. Eng. 110(3):190–199, 1988.
Varki, A. Biological roles of glycans. Glycobiology 27(1):3–49, 2017.
Vehviläinen, P., et al. Latent tgf-β binding proteins (ltbps) 1 and 3 differentially regulate transforming growth factor-β activity in malignant mesothelioma. Hum. Pathol. 42(2):269–278, 2011.
Weichhart, T., M. Hengstschläger, and M. Linke. Regulation of innate immune cell function by mtor. Nat. Rev. Immunol. 15(10):599–614, 2015.
Wiltz, D., et al. Extracellular matrix organization, structure, and function. In: Calcific Aortic Valve Disease, edited by E. Aikawa. Hicksville: InTech, 2013.
Wirrig, E. E., R. B. Hinton, and K. E. Yutzey. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J. Mol. Cell. Cardiol. 50(3):561–569, 2011.
Xu, L., and M. Brink. mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochim. Biophys. Acta Mol. Cell Res. 1863:1894–1903, 1863.
Yoshioka, M., et al. Chondromodulin-i maintains cardiac valvular function by preventing angiogenesis. Nat. Med. 12(10):1151–1159, 2006.
Zacchigna, L., et al. Emilin1 links tgfbeta maturation to blood pressure homeostasis. Cell 124(5):929–942, 2006.
Zanetti, M., et al. Emilin-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell. Biol. 24(2):638–650, 2004.
Zhang, B., S. Kirov, and J. Snoddy. Webgestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 33(Web Server Issue):W741, 2005.
Acknowledgments
We thank Aaron Reed for help in microscopy work and Susana Comte-Walters for help in proteomics data analysis. This study was supported by the National Center for Advancing Translational Sciences of the NIH (P.M.A., UL1 TR000445), National Institute of General Medical Sciences (P.M.A., P20 GM103542-06) the National Heart Lung and Blood Institute of the NIH (R.B.H., HL117851) an Institutional Clinical and Translational Science Award (R.B.H., NIH/NCRR 8UL1TR000077), and the Cincinnati Children’s Research Foundation (R.B.H.).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Scott I Simon oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Angel, P.M., Narmoneva, D.A., Sewell-Loftin, M.K. et al. Proteomic Alterations Associated with Biomechanical Dysfunction are Early Processes in the Emilin1 Deficient Mouse Model of Aortic Valve Disease. Ann Biomed Eng 45, 2548–2562 (2017). https://doi.org/10.1007/s10439-017-1899-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1899-0