Abstract
The field of regenerative medicine has progressed tremendously over the past few decades in its ability to fabricate functional tissue substitutes. Conventional approaches based on scaffolding and microengineering are limited in their capacity of producing tissue constructs with precise biomimetic properties. Three-dimensional (3D) bioprinting technology, on the other hand, promises to bridge the divergence between artificially engineered tissue constructs and native tissues. In a sense, 3D bioprinting offers unprecedented versatility to co-deliver cells and biomaterials with precise control over their compositions, spatial distributions, and architectural accuracy, therefore achieving detailed or even personalized recapitulation of the fine shape, structure, and architecture of target tissues and organs. Here we briefly describe recent progresses of 3D bioprinting technology and associated bioinks suitable for the printing process. We then focus on the applications of this technology in fabrication of biomimetic constructs of several representative tissues and organs, including blood vessel, heart, liver, and cartilage. We finally conclude with future challenges in 3D bioprinting as well as potential solutions for further development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue engineering has emerged as a promising solution to the unmet demand of tissues and organs for regenerative medicine and pharmaceutical research. Tissue engineering uses a combination of cells, biomaterials, and engineering technologies to fabricate biological constructs that mimic and improve the functions of their counterparts in human body.7,52,59 – 61,72,101,125 The concept and scope have significantly expanded during the past decades, leading to widespread applications such as regeneration of damaged tissues in vivo that are beyond the ability of self-repairing in the conventional sense, as well as construction of in vitro models for understanding cellular behaviors and performing drug screening using microfluidic organs-on-a-chip platforms, among many others. While several poorly vascularized tissues such as cornea90 are less complicated to engineer, fabrication of most other tissues relies on high density of multiple cell types to achieve full recapitulation of tissue/organ-level functions (Fig. 1a).
Approaches for tissue/organ fabrication. (a) Scale of cell numbers encountered in tissue engineering spans at least eight orders of magnitude. The minimum therapeutic threshold for recapitulating solid organ functions in humans is estimated at the level of 1–10 billion functioning cells. Reproduced with permission from Ref. 76, copyright 2014 public library of science. (b) Schematic illustrations of common approaches for tissue engineering. In the scaffold-based approach, cells are seeded into a porous scaffold to populate the matrix and deposit their own ECM. The modular approach, on the other hand, building blocks are utilized to build up large tissue constructs via multiple assembling techniques. Reproduced with permission from Ref. 68, copyright 2013 Dove Medical Press.
A variety of tissue engineering strategies have been developed to tackle the challenges for regenerating or modeling highly complex and functional tissues.1,72,99,101,125 The conventional methodology makes use of scaffolds as matrices to load cells (Fig. 1b).67 These scaffolds can be fabricated from either naturally derived polymers such as gelatin,24,46,88 collagen,14,39,46 hyaluronic acid,12,46 and alginate,2,24,46 or synthetic polymers such as poly(ɛ-caprolactone) (PCL), poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lactic-co-glycolic acid) (PLGA).47,49,69,108,123 The scaffolds serve as three-dimensional (3D) templates that support cells to attach, proliferate, and expand throughout the entire structure before they develop their own extracellular matrix (ECM), which eventually leads to the generation of mature cell-laden grafts with comparable properties to their native counterparts. Studies have shown that the phenotypes of seeded cells can be regulated in the scaffolds by applying a combination of different biological and physical stimuli, including growth factors,99,114 shear stress,89,100 as well as electrical93,112,122 and mechanical cues.31,48,53,119 However, there are limitations for these conventional scaffold-based approaches, including the intrinsic inability to mimic the complex microstructures of biological tissues.67 Particularly, it is widely acknowledged that physiologically relevant activities and functions of organs critically rely on their microarchitectures, such as the capillaries of the nephron system in kidneys,104 the hepatic lobules of livers,44 and the aligned cardiac fibers of the myocardium.16,122
Alternatively, the modular tissue engineering methodology aims to mimic the microstructural features of native tissues and organs.25,29,67 In this approach, the complex architecture of a tissue construct is divided into basic functional building blocks, which can be further assembled unit by unit into larger biomimetic structures. One distinctive advantage of the modular approach lies in its ability to precisely produce microscopic structural features, allowing for subsequent assembly in a controlled manner (Fig. 1b).29,67
Among different approaches, the recently developed 3D bioprinting technology promises to bridge the divergence between artificially engineered tissue constructs and native tissues. It is believed that 3D bioprinting offers unprecedented versatility and capability to deliver cells and biomaterials with precise control over spatial distributions. As a result, it is possible to recreate engineered constructs with accurate, detailed, or even personalized features that mimic the fine shape, structure, architecture, and therefore, function of targeting tissues and organs.70,83 In general, current 3D bioprinting technologies can be divided into indirect and direct fabrications. Indirect 3D bioprinting first creates negative sacrificial molds, followed by casting with desired positive biomaterial and then selective removal of the molds.6,57,63,77 Direct 3D bioprinting techniques, on the other hand, generate 3D structures in a point-by-point and/or layer-by-layer manner, which offer feasibility in depositing multiple cell types and/or biomaterials to achieve tissue constructs with improved reproducibility and heterogeneity to mimic in vivo systems.83
In this review we briefly describe recent progresses of 3D bioprinting technology and associated bioinks suitable for the printing process. We then focus on the applications of this versatile technology, in fabrication of biomimetic constructs of several representative tissues and organs that have been widely explored for live cell deposition, including blood vessel, heart, liver, and cartilage, largely due to the unique challenges associated with construction of these highly complex biological structures using conventional tissue engineering approaches. We finally conclude with future challenges in 3D bioprinting and perspectives.
From Blueprints to Grafts
Typically, 3D bioprinting starts with a computer-assisted process for depositing biomaterials and living cells in a determinate configuration in order to produce a defined 3D biological structure.80,83 The general process contains three steps: (i) pre-processing for acquisition of 3D computer-aided design (CAD) model of the tissue to be engineered; (ii) processing by automated deposition of cells and/or biomaterials of interest; and (iii) post-processing involving maturation of cell-laden constructs to reinforce the development of desired tissue constructs.78,79,83
Many current imaging and diagnostic technologies, such as magnetic resonance imaging (MRI) and computer tomography (CT), have been explored to acquire information about the targeting tissues and achieve the CAD “blueprints” of the grafts.83 The 3D CAD models can be subsequently segregated into 2D horizontal slices to provide instructions to the bioprinter and direct the layer-by-layer depositions of the biological elements.79,83 In addition to an appropriate software that coordinates the deposition, the other key component of a bioprinting system include the bioink, which refers to the (cell-laden) biomaterials used as the ink for the bioprinters.79,83
Selection of Bioinks
Selection of proper biomaterials as the bioink is a key step towards successful bioprinting. Bioinks based on both naturally derived and synthetic biomaterials have been developed to afford a spectrum of properties, such as biocompatibility and appropriate physical assets, to ensure printability and long-term functionality following deposition.18,83,84,109 For example, viscosity of the bioink is an important rheological parameter to determine flexibility in deposition of free-standing structures and maintenance of architectural integrity immediately after bioprinting.18,57 Shear-thinning biomaterials such as those based on Pluronic, gelatin, polyethylene glycol (PEG), or their combinations with other hydrogels, are often utilized as bioinks, which possess a liquid-like behavior under high shear stress during the extrusion process, but can quickly recover their gel state once bioprinted and thus prevent the structure from collapsing.8,42,43,57 Long-term stability of the bioprinted tissue constructs, however, typically depends on a secondary crosslinking mechanism to further stabilize the bioprinted structures.19 There are two general crosslinking mechanisms: (i) physical crosslinking through non-covalent interactions such as thermally induced sol–gel transitions or ionic interactions, and (ii) chemical crosslinking through the formation of new covalent bonds.70 For example, it is well-known that alginate solutions can be quickly crosslinked in the presence of Ca2+ ions to form a solid physical hydrogel.2,17 Other systems such as gelatin methacryloyl (GelMA) hydrogels can be photocrosslinked to form permanent 3D polymeric networks in the presence of a photoinitiator upon light exposure.88,121 Since physically crosslinked gels are typically unstable over an extended period of time and are subject to dissolution, they can function effectively as fugitive templates where only temporal stability is required, such as in cases of fabricating sacrificial bioprinted constructs like the vasculature systems.6,57,63,77 In contrast, chemically crosslinked gels possess better long-term stability and are suitable for constructive bioprinting to function as the biomimetic ECM.
To date, hydrogels based on natural biopolymers, such as alginate,17,19 gelatin,8,19,63 collagen,64,65,110 fibrin,110 hyaluronic acid,42 chitosan,82 and agarose,6 as well as many synthetic polymers like PEG15,21 and Pluronic,57 have been demonstrated to fulfill some essential requirements for use as bioinks. These bioinks may not only provide the basis as sacrificial/constructive scaffolds, but can also maintain the viability and promote the activity of bioprinted living cells. Recently, decellularized extracellular matrix (dECM), a class of naturally derived composite biomaterials, has attracted increasing attention for their use as bioinks (Fig. 2a).83,95 One unique advantage of the dECM bioinks lies in the ability to apply materials from the same tissue of interest in the bioprinting process, which promises to present well-matched compositional complexity in addition to architectural fidelity between the printed biological structures and the target tissues (Fig. 2b).83
3D Bioprinting with dECM bioinks of different tissue constructs. (a) dECM materials are obtained from various tissues via a multi-step decellularization process that combines physical, chemical and enzymatic treatments. The collected soluble dECM materials are mixed with stem cells and used as bioinks in a layer-by-layer bioprinting approach to fabricate tissue analogues. (b) Native tissues and bioprinted constructs from dECM of the corresponding tissues show similar morphological or histological appearance. Reproduced with permission from Ref. 95, copyright 2014 Nature Publishing Group.
Besides biomaterials, encapsulated cells comprise another critical component of bioinks. The cells need to be widely available due to the fact that bioprinting generally requires large densities of cells to maintain post-printing functionality.76 In addition, the cells should be able to survive under the high shear stress caused by the viscous bioink during the bioprinting process, and resist the relatively harsh crosslinking steps (e.g., in the presence of chemical reagents or UV light) associated with the design of bioinks.83 Indeed, it has been shown that short-time exposure to high levels of shear stress during the bioprinting procedure could both affect immediately cell viability as well as induce long-term alterations in the proliferation and potentially functionality of those that have survived the bioprinting process; for a certain cell type a specific threshold of shear stress may exist without noticeable side effects.11 It is further expected that mature somatic cell types in general are more resistant to these harsh environments than stem cells, which tend to respond to externally applied physical stimuli such as the mechanics.31,48 To this end, an optimized shear rate for dispensing of each cell type may be obtained by carefully tuning the extrusion rate of the bioink at a balanced bioprinting speed to ensure high cell viability and unaltered cell functions to support tissue formation.11 Shear stress that the cells experience during the bioprinting process may also be adjusted by changing the viscosity of the bioink.11,19 While most current technologies focus on single-cell-type bioprinting, the need for simultaneous deposition of multiple cells types to mimic the in vivo scenario has been increasingly acknowledged.19,40 Additionally, the cells can be encapsulated as either individually dispersed cells or as aggregates of cells (e.g., spheroids).9,22,81 While single-cell bioprinting allows for better flexibility in fabricating tissues on smaller scales and requires less efforts in the preparation of bioinks, the advantages related to bioprinting spheroids include reduction of time to produce larger tissues and much higher cell viability due to the protection of cells in the interiors of spheroids from the shear stress.9
Bioprinting the Vasculature: From Expressways to Alleys in the Body
Cells embedded in any tissue construct require optimal nutrition and oxygen delivery, as well as removal of produced wastes, to maintain viability and functionality.3,91,92 Diffusion of growth factors and other signaling biomolecules is also of critical importance to direct cellular behaviors. Large tissues and organs are integrated with complex vasculature in vivo that provides blood flow to sustain all the necessary supplies and functionalities. Therefore, introduction of vessel-like structures is a prerequisite for successful engineering of functional tissues suitable for regeneration as well as construction of in vitro models to understand underlying disease causes and screen pharmaceutical compounds.124
Native arteries and veins present a multi-layered structure where blood flow in the lumen is surrounded by three layers of distinct components and cell types. The innermost layer is called ‘tunica intima’, which is formed by endothelial cells; the middle layer ‘tunica media’ and the outmost ‘tunica externa’ layer are composed of smooth muscle cells [SMCs] supported by connective tissues of elastic and collagenous fibers, respectively (Fig. 3a).20,105 From a functional point of view, however, in vitro vessels should possess at least hollow lumens ideally covered by one or more layers of undamaged endothelium and pericytes.92 Recently, numerous approaches have been developed to recreate vasculature in vitro. Although major efforts have been devoted to understanding factors that promote vascularization (i.e., angiogenic growth factors),98,99,102 it remains highly challenging to induce the formation of vessels with desired organization. A promising solution is to create tissue constructs with pre-defined microarchitecture (such as interconnected microchannels) that mimic the vasculature and support surrounding stromal cells to survive and function. To realize this aim, 3D bioprinting techniques have been explored, which ensure precise control over the spatial arrangements of the vascular cells in the matrix.
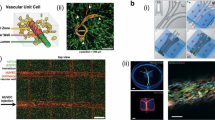
Bioprinting of vascular structures. (a) Physiology of arteries and veins. Arteries and veins sharing certain features in the multi-layered structures but differ in many other ways. Reproduced with permission from Ref. 117, copyright 2011 John Wiley & Sons. (b) Construction of macroscale vessels: (i) 3D bioprinted hydrogel; (ii) cross-sectional view; (iii) perspective view of the aorta model. Reproduced with permission from Ref. 58, copyright 2013 Elsevier. (c) Templated bioprinting based on sacrificial agarose fibers: (i) graphic mode of the agarose template fibers for micromolding; schematic representation of bioprinting of agarose template fibers and subsequent formation of microchannels via template micromolding; (ii) bifurcating bioprinted microchannel network in a GelMA hydrogel; and (iii) confocal image of HUVEC-lined microchannel generated by template micromolding. The inset shows a cross-sectional view of the channel. Scale bars: 250 μm. Reproduced with permission from Ref. 6, copyright 2014 Royal Society of Chemistry. (d) Schematic views of a heterogeneous bioprinting based on fugitive Pluronics inks: (i) blue filament corresponds to 10T1/2 fibroblast-laden GelMA, red fugitive filament, and green HNDF-laden GelMA ink; (ii) bright-field image of the 3D printed tissue construct, which is overlayed with the green fluorescent channel; (iii) stacked composition of tissue construct. Reproduced with permission from Ref. 57, copyright 2014 Wiley–VCH. (e) Sacrificial bioprinting based on sugar struts: (i) schematic overview of an bioprinted interconnected, self-supporting carbohydrate-glass lattice; (ii) stacked composition of 10T1/2 uniformly distributed in the fibrin gel and HUVECs in the vascular space; scale bar: 1 mm; (iii) cross-section image of a representative channel; scale bar: 200 μm. Reproduced with permission from Ref. 77, copyright 2012 Nature Publishing Group.
Scaffold-free vessel-like tubular structures have been reported as potential vessel substitutes by direct 3D bioprinting techniques. For example, Ozbolat et al. used alginate solutions as the bioink, which could be physically crosslinked by CaCl2 solutions. The two solutions were delivered using a customized coaxial needle to achieve in situ crosslinking upon deposition of the bioinks to form lumen-like structures.126 In a successive study, they further demonstrated the ability of the bioprinted network to provide nutrients to encapsulated cells in the surrounding matrix.120 Significantly, patient-inspired bioprinting of scaffold-free macrovascular structures has been demonstrated by Koc et al. MRI/CT data of the human aorta were segmented and converted into a CAD model for the bioprinter.58 Layer-by-layer printing of cylindrical aggregates of cell-laden hydrogels in a supporting structure consisted of crossing vertical and horizontal rods, as illustrated in Fig. 3b.
While direct bioprinting of macrovessels represents a breakthrough in generating blood vessels at larger scales, fabrication of hollow vessels within cell-laden tissue constructs is typically more complex and requires entirely different methodologies based on sacrificial bioprinting. A common strategy to sacrificially bioprint a vascularized tissue generally involves three steps: (i) bioprinting of a network of solid fibers embedded in a hydrogel matrix encapsulating stromal cells; (ii) selective removal of the fibers to form perfusable channels; and (iii) seeding of endothelial cells in the interiors of the channels to build functional vessels.45 Such techniques to fabricate perfusable matrices are also referred as indirect bioprinting, since they require the printing of sacrificial templates in the matrices that are subsequently removed to reveal the hollow channel structures.
Khademhosseini et al. applied agarose, a naturally derived polysaccharide, as the sacrificial template to bioprint hollow vessels within hydrogel constructs.6 Agarose solutions (>2 wt%) formed a solid gel below 32 °C to function as a fugitive bioink that could be removed later on. A cell-laden hydrogel precursor (SMCs and fibroblasts in 5–20 wt% GelMA or polyethylene glycol diacrylate [PEGDA] solution) was then poured around the patterned agarose fibers and photocrosslinked to form the matrix. After stabilizing the construct, the agarose fibers could be removed under mild vacuum to obtain hollow channels with diameters down to 100 µm (Fig. 3c). The presence of these channels significantly improved the viability of the surrounding stromal cells in the construct due to enhanced nutrient and oxygen delivery. Significantly, the bioprinted microchannels might be further coated with a layer of endothelium to recapitulate the biological function of the microvasculature (Fig. 3ciii).
Direct retraction of the sacrificial templates, however, may compromise the integrity of the channels in tissue constructs. To this end, an alternative bioink was introduced by Lewis et al. that could be liquefied by simply tuning the temperature.57 Specifically, it was found that Pluronic F127 solutions formed a shear-thinning hydrogel at room temperature, but returned to its solution state below 4 °C. Therefore, Pluronic F127 solution was used as the fugitive bioink to bioprint microfibers at a higher temperature, while hollow channels in the crosslinked GelMA hydrogel matrix could be easily generated by subsequently decreasing the temperature to remove the Pluronic F127 microfibers (Fig. 3d). Although providing a simple strategy for the construction of hollow vessels, minor cytotoxic effects associated with the use of high-concentration Pluronic-F127 solutions were observed, which might partially limit its applications in the fabrication of cell-laden constructs.
It has been long known that gelatin solutions solidify at lower temperatures but liquefy at around 37 °C, which allows for easy removal of gelatin-based sacrificial templates. Taking advantage of this unique property of gelatin, 3D vascular channels were created within a collagen I matrix by Dai et al.63 Interestingly, due to the excellent biocompatibility of gelatin, endothelial cells could be directly encapsulated within the gelatin bioink during the bioprinting process. The gelatin bioink diffused into the surround medium from the channels over the course of culture at 37 °C in an incubator. After liquefying the gelatin template, the endothelial cells were released from the gelatin fibers and could adhere to the interface between the liquefied bioink and the surface of the channels, where they would eventually form a confluent layer of endothelium.
Another explored template material is carbohydrates, which could be fabricated into self-supporting templates and subsequently removed by dissolving in aqueous solutions. Carbohydrate glass lattices were printed by Chen et al. as the sacrificial template inside a 3D hydrogel pre-polymer with encapsulated cells.77 After crosslinking the matrix, the carbohydrate lattice simply dissolved using the culture medium (Figs. 3ei and 3ii). The glass fibers comprising of the lattice were covered by a thin layer of PLGA to prevent during the gel casting process. This carbohydrate sacrificial material was used in combination with a large variety of synthetic ECM materials without showing any negative effects on encapsulated cells, such as HUVECs, 10T1/2 mouse embryo cells, human fibroblasts, and human embryonic kidney (HEK) cells. The strong mechanical properties of the carbohydrate glass rendered it possible to fabricate constructs at larger scales containing multiple layers of interconnected vasculature. It was further observed that, the endothelialized microchannels were highly biomimetic, where the coated endothelial cells could sprout into the surrounding matrix to form neovessels with lumen structures (Fig. 3eiii).
Forging the Heart
Heart is the first functional organ formed during embryonic development, when cells are confined to different layers due to differential affinities. Embryonic mesoderm germ-layer cells then form the blood vessels, the blood cells, as well as the heart (Fig. 4a).116 After gastrulation, the embryonic mesoderm cell layer further develops into mesothelium, endothelium, and myocardium. Mesothelial pericardium derives into the outer lining of the heart, while endothelium matures into the inner lining of the heart, the lymphatic vessels and the blood vessels.116 The main cellular components that make up the heart include cardiomyocytes, cardiac fibroblasts, and endothelial cells.4,13,122 Previous studies suggested that in a normal adult heart, cardiomyocytes take up to 30–40% of the entire population of the heart and the rest are non-myocytes with the majority being fibroblasts.85,122 At the tissue level, heart is composed of three different types of cardiac tissues: myocardium, endocardium, and pericardium.116,122 The myocardium is the thick muscular layer of the heart wall consisting of cardiomyocytes. The sinoatrial node (SAN), a group of specialized pacemaker cells located in the right atrium, can generate electrical impulses that set off contractions of myocytes without any stimulation from the nerves.116,122 Despite the intrinsic automaticity, this pacemaker activity is normally controlled by opposing input from the parasympathetic and sympathetic nervous systems. The myocytes align themselves in an anisotropic manner that promotes the electrical activation of the cardiac muscles.116,122 The endocardium is the innermost layer of the heart chambers and heart valves. It is primarily made up of endothelial cells that form overlapping regions to seal the heart and connect the surrounding blood vessels.116 Apart from preventing the leakage, it also has the functions as blood-heart barrier to filter certain types of molecules to enter or exit the tissue. The pericardium is a double-wall fibroserous sac that encloses the heart and the root of the blood vessels.116 The pericardial cavity, the space between the two membranes of the pericardium, contains pericardial fluid that acts as lubricant to allow membranes to slide over each other. Besides the three major cardiac tissues, ECM also plays an important role in shaping the fate of cells, regulating protein expression and differentiation.23,96,97 In normal myocardium, the elasticity of the collagen-based ECM and cardiomyocytes must be matched to generate actomyosin forces and pump the heart.30
(a) Schematics showing the geographical anatomy of a mature human heart. Reproduced with permission from Ref. 117, copyright 2011 John Wiley & Sons. (b) Bioprinting of heart valves: (i) heart valve model designed by Solidworks; as-printed valve conduit; Safranin-O staining to stain the glycosaminoglycans red which also stained the MeHA within the hydrogel red. Reproduced with permission from Ref. 27, copyright 2014 Elsevier. (ii) Heterogeneous aortic valve e-printing software sliced the geometries into layers and generated extrusion paths for each layer along with viable HAVIC-seeded valve scaffolds containing cells across the entire surface of the conduits. Reproduced with permission from Ref. 26, copyright 2013 Wiley–VCH. (c) Cardiac cells bioprinted in decellularized cardiac extracellular matrix. Scale bars: 5 mm and 400 µm. Reproduced with permission from Ref. 95, copyright 2014 Nature Publishing Group. (d) Bioprinting of the whole-heart structure: (i) A darkfield image of an explanted embryonic chick heart. (ii) A confocal fluorescence micrograph of the heart stained for fibronectin (green), nuclei (blue), and F-actin (red). (iii) The 3D CAD model of the heart with complex internal architecture based on the confocal data. (iv) A cross section of the 3D bioprinted heart showing recreation of the internal trabecular structure from the CAD model. (v) A dark-field image of the 3D-printed heart with internal structure visible through the translucent heart wall. Scale bars: 1 mm in (i) and (ii) and 1 cm in (iii) and (iv). Reproduced with permission from Ref. 43, copyright 2015 American Association for the Advancement of Science.
A number of techniques have thus been developed to improve the functionality of engineered cardiac tissues. Besides conventional tissue engineering approaches, 3D bioprinting has recently shown to be a promising alternative to produce functional cardiac tissues, and particularly, the heart valves. The aortic valve has a semilunar valves conformation with three main components.116 The relatively stiff heart valve root populated by contractile SMCs. Three thin flexible leaflets contain fibroblastic interstitial cells and three sinuses. Along with the pulmonary valve, it allows blood to be forced into the arteries and prevent the backflows. It is crucial that the valves open and close properly to keep the heart perform efficiently. However, conventional options to treat dysfunctional valves caused by stenosis or regurgitation, such as medication, surgical repair, and percutaneous balloon valvotomy, have shown limited effectiveness. Heart valve replacement is still one important procedure to correct the symptoms.
Butcher et al. designed bioprinted trileaflet valve hydrogels that regulate behaviors of encapsulated human aortic vascular interstitial cells (HAVICs).27 In this study, the geometries of the trileaflet valve were designed by Solidworks (Fig. 4bi). Hybrid hydrogel properties were varied by changing concentrations of the two compositions: methacrylated hyaluronic acid (MeHA) and GelMA. The optimized hydrogel formulation was mixed with HAVICs and used as bioink to print the heart valve conduit. After 7 days in static culture, the bioprinted valve conduit showed well maintained structure, high viability of the encapsulated cells (>90%), as well as promising remodeling potentials. This study expanded the range of biomaterials that could be used for bioprinting heart tissues and provided an important understanding about the bioprintable microenvironment architecture for controlling HAVIC behaviors. Another study from the same group successfully bioprinted an aortic valve conduit with direct encapsulation of sinus SMCs in the valve root and HAVIC in the leaflet (Fig. 4bii).26 The 3D model of the aortic valve was obtained by micro-CT scan on the freshly harvest porcine aortic valves. Live/Dead assay after 7 days of the encapsulated cells in alginate/gelatin hydrogels showed 83.2 and 81.4% viability for HAVICs and SMCs, respectively. Moreover, decreased cell circularity suggested high cell spreading in both types of cells. This study proved that 3D bioprinting is capable of constructing a complex heterogeneous aortic valve conduit.
Until now, however, the bioprinted aortic valves have not been tested in a human body. Many studies are being conducted toward implementation of clinical trials. The bioprinted heart valves cannot open and close by itself without the presence of the rest of the heart. Hoerstrup et al. developed an in vitro cell culture system that stimulated the heart valve with the physiological pressure and flow.28 Other than testing the bioprinted heart valve, this stimulation also improved the strength of the heart valve before a possible implantation. Bioreactor systems have been used to mature decellularized heart valves, which could be beneficial to bioprinted heart valves for in vitro testing and maturation.
Myocardial infarction, another major cause of heart failure, leads to congestive heart failure, derived by irreversible necrosis of the heart muscle resulted from prolonged ischemia to the myocardium.116 It was commonly believed that cardiac muscle cells were terminally differentiated cells and therefore did not have the ability to regenerate.56 By using the C14 isotope labeling technique, Bergmann et al. recently showed that the cardiomyocytes could indeed renew, with an annual turnover rate ranging from 1% at the age of 25 to 0.45% at the age of 75.5 The low renew rate, however, is insufficient for repairing extensive myocardial injuries that occur in human heart diseases and fully regaining the functions of the heart.50 Currently, there is no practical therapy to cure and recover injured cardiomyocytes.
To this end, the capability to fabricate functional myocardium for regeneration becomes crucial. Sluijter et al. demonstrated that human cardiomyocyte progenitor cells (hCMPCs) are capable of being bioprinted and cultivated in alginate scaffolds for the generation of myocardium constructs.36 Moreover, cultured hCMPCs showed an increase of cardiac commitment while at the same time maintaining viability and proliferation (Fig. 4c). In another study, the same group applied the laser-induced-forward-transfer (LIFT) cell printing technique to fabricate a cardiac patch made from polyesterurethane urea (PEUU) with defined patterns and seeded with co-cultured HUVECs and human mesenchymal stem cells (hMSCs).35 The bioprinted patches were transplanted to the infarction area of rat heart and showed increased vessel formation as well as significant functional improvement of the infarcted regions. More recently, Feinberg et al. developed a 3D bioprinting technology termed freeform reversible embeddeing of suspended hydrogels, for fabrication of complex biological structures.43 This method relied on direct bioprinting of the bioinks into a support bath of gelatin microparticles and took advantage of the physical support by the supporting hydrogel under room temperature to construct volumetric objects at large scales that were impossible to achieve before. The support bath could then be liquefied at elevated temperature to release the bioprinted structures. Using this novel 3D bioprinting approach, the authors demonstrated the capability to recapitulate the complex trabecular structures of a whole heart through CAD modeling (Fig. 4d).
Building the Liver
Liver has the extensive capacity to regenerate even with vast damages.75,113 The functional unit of liver is the hepatic lobule, a hexagonally structured unit with a side-to-side length of approximately 1 mm and a thickness of around 2 mm (Fig. 5a).44,105 The lobules carry on the crucial functions of complex exocrine and endocrine metabolism and detoxification. Millions of lobules together constitute each of the Couinaud segments that make up the liver.44,105 The parenchymal hepatocytes have an endodermic origin and constitute the major part of liver.33 Other cells that compose the liver include portal fibroblasts, sinusoidal endothelial cells (SECs), and biliary epithelial cells. In addition, there are mesoderm derived cells such as hepatic stellate cells (HSCs), stromal cells, and Kupffer cells.33,86 These non-parenchymal cells play significant roles in certain liver functions. For example, HSCs are heavily involved in the synthesis of growth factors and regeneration of ECM proteins, both of which possess pivotal roles in hemostasis and cell signaling.107 Collagen and glycosaminoglycan compose a considerable portion of the ECMs that ensure the mechanical integrity of hepatocytes and are responsible for providing bioactive molecular signals to cells.62
Bioprinting of liver tissues. (a) Layout of typical structural units of the hepatic lobule. In cross-sectional views, the microstructures appear as a hexagonal lattice, with the hepatic artery, bile duct, and portal vein triads placed at the hexagon vertices. Reproduced with permission from Ref. 117, copyright 2011 John Wiley & Sons. (b–d) Bioprinted liver tissue constructs with similar arrangement of the hepatic lobules to native liver tissues and tissue-like cellular density and tight intercellular junctions, using human primary hepatocytes, endothelial cells, and hepatic stellate cells. (b, c) Photographs showing the liver organoids immediately after bioprinting. (d) Fluorescence micrograph of the planar cross-section after tissue maturation, highlighting the compartmentalization of the non-parenchymal cells relative to the hepatocytes. The hepatic stellate cells and endothelial cells were pre-labeled in green and red, respectively, while the nuclei of all cells were stained in blue. Adapted with permission from Ref. 103, copyright 2015 Organovo™.
Various techniques have been used over the past few years to fabricate biomimetic liver tissues, starting from 2D culture of parenchymal cells that showed successful differentiation.62 With these simple techniques, however, it was not possible to achieve the microenvironment sufficient for interactions between the cells and the ECMs, and the cell survival rate was thus limited. Further investigations in the field suggested that the intercellular adhesion is important, leading to the development of several techniques to construct volumetric liver tissues.54,55 Recently, 3D bioprinting techniques have also been adopted to fabricate liver-like microstructures. For example, studies have exploited the possibility for 3D bioprinting of hepatoma cells9,111 and hepatocytes118 using a variety of hydrogels such as MeHA, PEG, gelatin, and alginate in different combinations. Particularly, Organovo™, one of the first bioprinting companies, has successfully achieved 3D vascularized liver constructs with high cell viability and reliable zonation through bioprinting of high-density hepatocytes, endothelial cells, and hepatic stellate cells in an architecture that mimicked the native hepatic lobules (Figs. 5b–5d).87,103 Alternatively, liver spheroids were used in bioprinting to replace single hepatocytes. Using liver spheroids can protect the cells from the negative effects exerted by the shear stress during the printing process and recapitulate the volumetric cell–cell interactions.9 The bioprinted liver spheroids embedded in GelMA hydrogel exhibited long-term functionality for up to 30 days as revealed by their stable secretion of hepatic biomarkers including albumin, ceruloplasmin, alpha-1 antitrypsin (A1AT), and transferrin. The liver spheroids, when combined with a microfluidic bioreactors, successfully functioned as a viable platform to evaluate hepatotoxic drugs, which induced dose- and time-dependent responses of biomarker secretion by the organoids.
Constructing the Cartilage
Cartilages or cartilaginous tissues refer to the connective tissues widely existing in vivo, constituting the major components of joints between bones, ears, and nose. In contrast to many other tissues, cartilaginous tissues are featured by the avascular and aneural structures containing a relatively low density of cells, which limits the ability for the cartilages to spontaneously repair defects. Cartilage tissue engineering aims to enhance regeneration by fabricating cell-laden cartilage constructs for implantation. To this end, 3D bioprinting offers unparalleled ability to deposit bioinks and cells with precise spatial control, which mimic the structural and compositional heterogeneity of native cartilage tissues.
Since chondrocytes are the major cell type found in cartilages, efforts towards bioprinting 3D cartilage tissues generally apply various designs of bioinks to encapsulate chondrocytes and recreate the desired shapes, from simple grid-like shapes to complex cartilaginous tissues such as ears and noses. D’Lima and Cui et al. applied photocrosslinkable PEG dimethacrylate (PEGDMA) or GelMA as the bioinks and demonstrated direct printing of human chondrocytes or hMSCs-laden constructs to repair cartilage defects. The layer-by-layer bioprinting followed by simultaneous crosslinking ensured homogeneous cell distributions within the hydrogel matrix. High cell viability and good integration of the bioprinted constructs with the defect smoothly interfaced the osteochondral plug model.21,37
Similar with other organ bioprinting, one major challenge in bioprinting 3D cartilage tissues lies in finding proper bioink formulations with high biocompatibility and printability. For example, while GelMA hydrogels are known to support chondrocyte encapsulation, the prepolymer solutions typically possess a low viscosity that impedes the fidelity of printed structures. To solve this issue, Malda et al. reported the use of GelMA/hyaluronic acid (HA) composites as bioinks to print cartilage constructs. The addition of HA into GelMA prepolymer significantly increased viscosity of the resulting mixture, and thus allowed direct extrusion of continuous hydrogel strands that can further fuse into grid-like structures. Histological and immunohistochemical staining of the cell-laden constructs confirmed glycosaminoglycan formation and cartilaginous matrix production after 4 weeks in vitro culture.106 To increase printability and structural fidelity, Gatenholm et al. developed a composite of cellulose nanofibrils and alginate as a shear-thinning bioink suitable for extrusion-based 3D bioprinting. After printing, the constructs could be further crosslinked in the presence of calcium. Human chondrocytes were encapsulated in this bioink to demonstrate the printing of simple grid-like structures as well as complex 3D anatomically shaped ear-like structures.73
Recently, Zenobi-Wong et al. reported successful 3D bioprinting of complex cartilaginous structures using bioinks based on FDA-compliant materials: gellan, alginate, and a commercial product Biocartilage made of cartilage ECM particles.51 The composite bioink showed excellent biocompatibility, and optimized rheological properties including shear-thinning and shear recovery. Various anatomically relevant structures possessing auricular, nasal, and meniscal shapes were demonstrated. The introduction of Biocartilage promoted chondrocyte proliferation during in vitro culturing, which also suggested versatility of this method to print tissue-specific constructs using different ECM components.51
McAlpine et al. demonstrated that interweaving of the 3D bioprinted cell-laden constructs with electronic devices allowed fabrication of bionic artificial ears that not only anatomically mimicked the ears, but also were able to capture auditory signals. Cell-laden hydrogels were bioprinted to form the structural part of the bionic ear, which were integrated with a cochlea-shaped electrode and a readout wire composed of silver nanoparticle-infused silicone polymers. This study suggested possible strategies to merge biological and electronic functionalities via sophisticated 3D bioprinting in conjunction with fabrication technology.71
Conclusions
Over the past few years, researchers not only have demonstrated proof-of-concept examples of different bioprinting technologies, but also have shown possibilities how 3D bioprinting may change the future of tissue engineering, ranging from fabrication of organ and tissue constructs for functional regeneration to relevant models for pharmacological investigations.9,41 The 3D cell-embedding volumes of biomaterials generated by bioprinting could serve as biomimetic constructs with desired composition, structure, and architecture to ensure better cell viability and more importantly support the functionality of the tissues, as demonstrated by numerous studies where tissues such as vasculature, heart, liver, cartilage, bladder,34 and skin10,66,110 have been bioprinted. Each of these tissues/organs is highly complex and may require a combination of several bioprinting techniques along with specifically designed bioinks to introduce structural heterogeneity and functionality. For example the sacrificial bioprinting strategy may be integrated into other deposition methods to produce hierarchically vascularized tissues; and bioinks derived from tissue-specific dECM may be fitted on a multi-material bioprinter to enable spatially defined deposition of bioinks that matches the architecture of the target organs to be printed. Although challenges still present, with new niches for technological developments on the instrumentation with improved spatial and temporal resolutions as well as optimized bioinks and cell sources for specific organs, it is expected that 3D bioprinting will eventually become one of the most efficient, reliable, and convenient methods to biofabricate tissue constructs in the near future. Combination with the stem cell technologies32,74,94 and advanced materials engineering approaches featuring stimuli-responsiveness38,115 will further allow temporal evolution of bioprinted tissue constructs that potentially meet the requirements of dynamic tissue remodeling during developmental processes.
References
Atala, A., F. K. Kasper, and A. G. Mikos. Engineering complex tissues. Sci. Transl. Med. 4:160rv12, 2012.
Augst, A. D., H. J. Kong, and D. J. Mooney. Alginate hydrogels as biomaterials. Macromol. Biosci. 6:623–633, 2006.
Bae, H., A. S. Puranik, R. Gauvin, F. Edalat, B. Carrillo-Conde, N. A. Peppas, and A. Khademhosseini. Building vascular networks. Sci. Transl. Med. 4:160ps23, 2012. doi:10.1002/smll.201501798
Baudino, T. A., W. Carver, W. Giles, and T. K. Borg. Cardiac fibroblasts: friend or foe? Am. J. Physiol. Heart Circ. Physiol. 291:H1015–H1026, 2006.
Bergmann, O., R. D. Bhardwaj, S. Bernard, S. Zdunek, F. Barnabe-Heider, S. Walsh, J. Zupicich, K. Alkass, B. A. Buchholz, H. Druid, S. Jovinge, and J. Frisen. Evidence for cardiomyocyte renewal in humans. Science 324:98–102, 2009.
Bertassoni, L. E., M. Cecconi, V. Manoharan, M. Nikkhah, J. Hjortnaes, A. L. Cristino, G. Barabaschi, D. Demarchi, M. R. Dokmeci, Y. Yang, and A. Khademhosseini. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab. Chip 14:2202–2211, 2014.
Berthiaume, F., T. J. Maguire, and M. L. Yarmush. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2:403–430, 2011.
Bhattacharjee, T., S. M. Zehnder, K. G. Rowe, S. Jain, R. M. Nixon, W. G. Sawyer, and T. E. Angelini. Writing in the granular gel medium. Sci. Adv. 1:e1500655, 2015.
Bhise, N. S., V. Manoharan, S. Massa, A. Tamayol, M. Ghaderi, M. Miscuglio, Q. Lang, Y. S. Zhang, S. R. Shin, G. Calzone, N. Annabi, T. Shupe, C. Bishop, A. Atala, M. R. Dokmeci, and A. Khademhosseini. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 8:014101, 2016.
Binder, K. W., W. Zhao, T. Aboushwareb, D. Dice, A. Atala, and J. J. Yoo. In situ bioprinting of the skin for burns. J. Am. Coll. Surg. 211:S76, 2010.
Blaeser, A., D. F. D. Campos, U. Puster, W. Richtering, M. M. Stevens, and H. Fischer. Controlling shear stress in 3d bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 5:326–333, 2016.
Burdick, J. A., and G. D. Prestwich. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 23:H41–H56, 2011.
Camelliti, P., T. K. Borg, and P. Kohl. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 65:40–51, 2005.
Cen, L., W. Liu, L. Cui, W. Zhang, and Y. Cao. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr. Res. 63:492–496, 2008.
Censi, R., W. Schuurman, J. Malda, G. Di Dato, P. E. Burgisser, W. J. A. Dhert, C. F. Van Nostrum, P. Di Martino, T. Vermonden, and W. E. Hennink. A printable photopolymerizable thermosensitive p (hpmam-lactate)-peg hydrogel for tissue engineering. Adv. Funct. Mater. 21:1833–1842, 2011.
Chiu, L. L., and M. Radisic. Cardiac tissue engineering. Curr. Opin. Chem. Eng. 2:41–52, 2013.
Christensen, K., C. Xu, W. Chai, Z. Zhang, J. Fu, and Y. Huang. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 112:1047–1055, 2015.
Chung, J. H. Y., S. Naficy, Z. Yue, R. Kapsa, A. Quigley, S. E. Moulton, and G. G. Wallace. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 1:763–773, 2013.
Colosi, C., S. R. Shin, V. Manoharan, S. Massa, M. Constantini, A. Barbetta, M. R. Dokmeci, M. Dentini, and A. Khademhosseini. Microfluidic bioprinting of heterogeneous 3d tissue constructs using low viscosity bioink. Adv. Mater. 28:677–684, 2015.
Comparative structure of blood vessels [Online]. Wiley, New York, 2011. http://higheredbcs.wiley.com/legacy/college/tortora/0470565101/hearthis_ill/pap13e_ch21_illustr_audio_mp3_am/simulations/hear/blood_vessels.html. Accessed 29 Nov 2015.
Cui, X., K. Breitenkamp, M. G. Finn, M. Lotz, and D. D. D’lima. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. A 18:1304–1312, 2012.
Dababneh, A. B., and I. T. Ozbolat. Bioprinting technology: a current state-of-the-art review. J. Manuf. Sci. Eng. 136:061016, 2014.
Discher, D. E., P. Janmey, and Y. L. Wang. Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143, 2005.
Drury, J. L., and D. J. Mooney. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24:4337–4351, 2003.
Du, Y., E. Lo, S. Ali, and A. Khademhosseini. Directed assembly of cell-laden microgels for fabrication of 3d tissue constructs. Proc. Natl. Acad. Sci. USA 105:9522–9527, 2008.
Duan, B., L. A. Hockaday, K. H. Kang, and J. T. Butcher. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 101:1255–1264, 2013.
Duan, B., E. Kapetanovic, L. A. Hockaday, and J. T. Butcher. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 10:1836–1846, 2014.
Dumont, K., J. Yperman, E. Verbeken, P. Segers, B. Meuris, S. Vandenberghe, W. Flameng, and P. R. Verdonck. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif. Organs 26:710–714, 2002.
Elbert, D. L. Bottom-up tissue engineering. Curr. Opin. Biotechnol. 22:674–680, 2011.
Engler, A. J., C. Carag-Krieger, C. P. Johnson, M. Raab, H. Y. Tang, D. W. Speicher, J. W. Sanger, J. M. Sanger, and D. E. Discher. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 121:3794–3802, 2008.
Engler, A. J., S. Sen, H. L. Sweeney, and D. E. Discher. Matrix elasticity directs stem cell lineage specification. Cell 126:677–689, 2006.
Faulkner-Jones, A., C. Fyfe, D.-J. Cornelissen, J. Gardner, J. King, A. Courtney, and W. Shu. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3d. Biofabrication 7:044102, 2015.
Fukumitsu, K., H. Yagi, and A. Soto-Gutierrez. Bioengineering in organ transplantation: targeting the liver. Transpl. Proc. 43:2137–2138, 2011.
Fullhase, C., R. Soler, A. Atala, K.-E. Andersson, and J. J. Yoo. A novel hybrid printing system for the generation of organized bladder tissue. J. Urol. 181:282–283, 2009.
Gaebel, R., N. Ma, J. Liu, J. Guan, L. Koch, C. Klopsch, M. Gruene, A. Toelk, W. Wang, P. Mark, F. Wang, B. Chichkov, W. Li, and G. Steinhoff. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 32:9218–9230, 2011.
Gaetani, R., P. A. Doevendans, C. H. G. Metz, J. Alblas, E. Messina, A. Giacomello, and J. P. G. Sluijter. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 33:1782–1790, 2012.
Gao, G., A. F. Schilling, K. Hubbell, T. Yonezawa, D. Truong, Y. Hong, G. Dai, and X. Cui. Improved properties of bone and cartilage tissue from 3d inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in peg-gelma. Biotechnol. Lett. 37:2349–2355, 2015.
Gladman, A. S., E. A. Matsumoto, R. G. Nuzzo, L. Mahadevan, and J. A. Lewis. Biomimetic 4d printing. Nat. Mater. 15:413–418, 2016.
Glowacki, J., and S. Mizuno. Collagen scaffolds for tissue engineering. Biopolymers 89:338–344, 2008.
Hardin, J. O., T. J. Ober, A. D. Valentine, and J. A. Lewis. Microfluidic printheads for multimaterial 3d printing of viscoelastic inks. Adv. Mater. 27:3279–3284, 2015.
Henmi, C., M. Nakamura, Y. Nishiyama, K. Yamaguchi, S. Mochizuki, K. Takiura, and H. Nakagawa. Development of an effective three dimensional fabrication technique using inkjet technology for tissue model samples. AATEX 14:689–692, 2007.
Highley, C. B., C. B. Rodell, and J. A. Burdick. Direct 3d printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 27:5075–5079, 2015.
Hinton, T. J., Q. Jallerat, R. N. Palchesko, J. H. Park, M. S. Grodzicki, H. J. Shue, M. H. Ramadan, A. R. Hudson, and A. W. Feinberg. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 1:e1500758, 2015.
Ho, C. T., R. Z. Lin, R. J. Chen, C. K. Chin, S. E. Gong, H. Y. Chang, H. L. Peng, L. Hsu, T. R. Yew, S. F. Chang, and C. H. Liu. Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue. Lab. Chip 13:3578–3587, 2013.
Hoch, E., G. E. Tovar, and K. Borchers. Bioprinting of artificial blood vessels: current approaches towards a demanding goal. Eur. J. Cardiothorac. Surg. 46:767–778, 2014.
Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug Del. Rev. 64:18–23, 2012.
Hubbell, J. A. Biomaterials in tissue engineering. Biotechnology 13:565–576, 1995.
Huebsch, N., P. R. Arany, A. S. Mao, D. Shvartsman, O. A. Ali, S. A. Bencherif, J. Rivera-Feliciano, and D. J. Mooney. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9:518–526, 2010.
Hutmacher, D. W. Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 12:107–124, 2001.
Kajstura, J., N. Gurusamy, B. Ogorek, P. Goichberg, C. Clavo-Rondon, T. Hosoda, D. D’amario, S. Bardelli, A. P. Beltrami, D. Cesselli, R. Bussani, F. Del Monte, F. Quaini, M. Rota, C. A. Beltrami, B. A. Buchholz, A. Leri, and p Anversa. Myocyte turnover in the aging human heart. Circ. Res. 107:1374–1386, 2010.
Kesti, M., C. Eberhardt, G. Pagliccia, D. Kenkel, D. Grande, A. Boss, and M. Zenobi-Wong. Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv. Funct. Mater. 25:7406–7417, 2015.
Khademhosseini, A., J. P. Vacanti, and R. Langer. Progress in tissue engineering. Sci. Am. 300:64–71, 2009.
Khetan, S., M. Guvendiren, W. R. Legant, D. M. Cohen, C. S. Chen, and J. A. Burdick. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12:458–465, 2013.
Khetani, S. R., and S. N. Bhatia. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 26:120–126, 2008.
Kim, M., J. Y. Lee, C. N. Jones, A. Revzin, and G. Tae. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials 31:3596–3603, 2010.
Knezevic, I., A. Patel, N. R. Sundaresan, M. P. Gupta, R. J. Solaro, R. S. Nagalingam, and M. Gupta. A novel cardiomyocyte-enriched microRNA, miR-378, targets insulin-like growth factor 1 receptor: implications in postnatal cardiac remodeling and cell survival. J. Biol. Chem. 287:12913–12926, 2012.
Kolesky, D. B., R. L. Truby, A. S. Gladman, T. A. Busbee, K. A. Homan, and J. A. Lewis. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26:3124–3130, 2014.
Kucukgul, C., B. Ozler, H. E. Karakas, D. Gozuacik, and B. Koc. 3D hybrid bioprinting of macrovascular structures. Procedia Eng. 59:183–192, 2013.
Langer, R. Tissue engineering: status and challenges. e-Biomed. J. Regen. Med. 1:5–6, 2000.
Langer, R., and J. P. Vacanti. Tissue engineering. Science 260:920–926, 1993.
Langer, R., J. P. Vacanti, C. A. Vacanti, A. Atala, L. E. Freed, and G. Vunjak-Novakovic. Tissue engineering: biomedical applications. Tissue Eng. 1:151–161, 1995.
Lee, J. S., and S.-W. Cho. Liver tissue engineering: recent advances in the development of a bio-artificial liver. Biotechnol. Bioprocess Eng. 17:427–438, 2012.
Lee, V. K., D. Y. Kim, H. Ngo, Y. Lee, L. Seo, S.-S. Yoo, P. A. Vincent, and G. Dai. Creating perfused functional vascular channels using 3d bio-printing technology. Biomaterials 35:8092–8102, 2014.
Lee, W., J. Pinckney, V. Lee, J. H. Lee, K. Fischer, S. Polio, J. K. Park, and S. S. Yoo. Three-dimensional bioprinting of rat embryonic neural cells. NeuroReport 20:798–803, 2009.
Lee, Y. B., S. Polio, W. Lee, G. Dai, L. Menon, R. S. Carroll, and S. S. Yoo. Bio-printing of collagen and vegf-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 223:645–652, 2010.
Lee, V., G. Singh, J. P. Trasatti, C. Bjornsson, X. Xu, T. N. Tran, S.-S. Yoo, G. Dai, and P. Karande. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 20:473–484, 2014.
Leijten, J., J. Rouwkema, Y. S. Zhang, A. Nasajpour, M. R. Dokmeci, and A. Khademhosseini. Advancing tissue engineering: a tale of nano-, micro-, and macroscale integration. Small 12:2130–2145, 2016. doi:10.1002/smll.201501798.
Lu, T., Y. Li, and T. Chen. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 8:337–350, 2013.
Ma, P. X. Scaffolds for tissue fabrication. Mater. Today 7:30–40, 2004.
Malda, J., J. Visser, F. P. Melchels, T. Jungst, W. E. Hennink, W. J. Dhert, J. Groll, and D. W. Hutmacher. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 25:5011–5028, 2013.
Mannoor, M. S., Z. Jiang, T. James, Y. L. Kong, K. A. Malatesta, W. O. Soboyejo, N. Verma, D. H. Gracias, and M. C. Mcalpine. 3D printed bionic ears. Nano Lett. 13:2634–2639, 2013.
Mao, A. S., and D. J. Mooney. Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci. USA 112:14452–14459, 2015.
Markstedt, K., A. Mantas, I. Tournier, H. M. Avila, D. Hagg, and P. Gatenholm. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 16:1489–1496, 2015.
Mehrban, N., G. Z. Teoh, and M. A. Birchall. 3D bioprinting for tissue engineering: stem cells in hydrogels. Int. J. Bioprint. 2:6–19, 2016. doi:10.18063/IJB.2016.01.006.
Michalopoulos, G. K., and M. C. Defrances. Liver regeneration. Science 276:60–66, 1997.
Miller, J. S. The billion cell construct: will three-dimensional printing get us there? PLoS Biol. 12:e1001882, 2014.
Miller, J. S., K. R. Stevens, M. T. Yang, B. M. Baker, D. H. Nguyen, D. M. Cohen, E. Toro, A. A. Chen, P. A. Galie, X. Yu, R. Chaturvedi, S. N. Bhatia, and C. S. Chen. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11:768–774, 2012.
Mironov, V., T. Boland, T. Trusk, G. Forgacs, and R. R. Markwald. Organ printing: computer-aided jet-based 3d tissue engineering. Trends Biotechnol. 21:157–161, 2003.
Mironov, V., V. Kasyanov, C. Drake, and R. R. Markwald. Organ printing: promises and challenges. Regen. Med. 3:93–103, 2008.
Mironov, V., N. Reis, and B. Derby. Review: bioprinting: a beginning. Tissue Eng. 12:631–634, 2006.
Mironov, V., R. P. Visconti, V. Kasyanov, G. Forgacs, C. J. Drake, and R. R. Markwald. Organ printing: tissue spheroids as building blocks. Biomaterials 30:2164–2174, 2009.
Müller, W. E. G., E. Tolba, H. C. Schröder, M. Neufurth, S. Wang, T. Link, B. Al-Nawas, and X. Wang. A new printable and durable n, o-carboxymethyl chitosan–ca2+–polyphosphate complex with morphogenetic activity. J. Mater. Chem. B 3:1722–1730, 2015.
Murphy, S. V., and A. Atala. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32:773–785, 2014.
Murphy, S. V., A. Skardal, and A. Atala. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A 101:272–284, 2013.
Nag, A. C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28:41–61, 1979.
Nahmias, Y., F. Berthiaume, and M. L. Yarmush. Integration of technologies for hepatic tissue engineering. Tissue engineering II. New York: Springer, pp. 309–329, 2007.
Nguyen, D., J. Robbins, C. Crogan-Grundy, V. Gorgen, P. Bangalore, D. Perusse, O. Creasey, S. King, S. Lin, and C. Khatiwala. Functional characterization of three-dimensional (3d) human liver tissues generated by an automated bioprinting platform. FASEB J. 29:LB424, 2015.
Nichol, J. W., S. T. Koshy, H. Bae, C. M. Hwang, S. Yamanlar, and A. Khademhosseini. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31:5536–5544, 2010.
Niklason, L. E., J. Gao, W. M. Abbott, K. K. Hirschi, S. Houser, R. Marini, and R. Langer. Functional arteries grown in vitro. Science 284:489–493, 1999.
Nishida, K., M. Yamato, Y. Hayashida, K. Watanabe, K. Yamamoto, E. Adachi, S. Nagai, A. Kikuchi, N. Maeda, H. Watanabe, T. Okano, and Y. Tano. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351:1187–1196, 2004.
Nomi, M., A. Atala, P. D. Coppi, and S. Soker. Principals of neovascularization for tissue engineering. Mol. Asp. Med. 23:463–483, 2002.
Novosel, E. C., C. Kleinhans, and P. J. Kluger. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 63:300–311, 2011.
Nunes, S. S., J. W. Miklas, J. Liu, R. Aschar-Sobbi, Y. Xiao, B. Zhang, J. Jiang, S. Masse, M. Gagliardi, A. Hsieh, N. Thavandiran, M. A. Laflamme, K. Nanthakumar, G. J. Gross, P. H. Backx, G. Keller, and M. Radisic. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 10:781–787, 2013.
Ouyang, L., R. Yao, S. Mao, X. Chen, J. Na, and W. Sun. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication 7:044101, 2015.
Pati, F., J. Jang, D. H. Ha, S. W. Kim, J. W. Rhie, J. H. Shim, D. H. Kim, and D. W. Cho. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5:3935, 2014.
Pelham, Jr., R. J., and Y. Wang. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94:13661–13665, 1997.
Peyton, S. R., and A. J. Putnam. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell. Physiol. 204:198–209, 2005.
Phelps, E. A., N. Landazuri, P. M. Thule, W. R. Taylor, and A. J. Garcia. Bioartificial matrices for therapeutic vascularization. Proc. Natl. Acad. Sci USA 107:3323–3328, 2010.
Place, E. S., N. D. Evans, and M. M. Stevens. Complexity in biomaterials for tissue engineering. Nat. Mater. 8:457–470, 2009.
Ratcliffe, A. Tissue engineering of vascular grafts. Matrix Biol. 19:353–357, 2000.
Rice, J. J., M. M. Martino, L. De Laporte, F. Tortelli, P. S. Briquez, and J. A. Hubbell. Engineering the regenerative microenvironment with biomaterials. Adv. Healthc. Mater. 2:57–71, 2013.
Richardson, T. P., M. C. Peters, A. B. Ennett, and D. J. Mooney. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19:1029–1034, 2001.
Robbins, J. B., V. Gorgen, P. Min, B. R. Shepherd, and S. C. Presnell. A novel in vitro three-dimensional bioprinted liver tissue system for drug development. FASEB J. 27:872.12, 2013.
Rosines, E., K. Johkura, X. Zhang, H. J. Schmidt, M. Decambre, K. T. Bush, and S. K. Nigam. Constructing kidney-like tissues from cells based on programs for organ development: toward a method of in vitro tissue engineering of the kidney. Tissue Eng. Part A 16:2441–2455, 2010.
Saladin, K. S., and L. Miller. Anatomy & physiology. New York: McGraw-Hill, 1998.
Schuurman, W., P. A. Levett, M. W. Pot, P. R. Van Weeren, W. J. Dhert, D. W. Hutmacher, F. P. Melchels, T. J. Klein, and J. Malda. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 13:551–561, 2013.
Senoo, H. Structure and function of hepatic stellate cells. Med. Electron Microsc. 37:3–15, 2004.
Shin, H., S. Jo, and A. G. Mikos. Biomimetic materials for tissue engineering. Biomaterials 24:4353–4364, 2003.
Skardal, A., and A. Atala. Biomaterials for integration with 3-d bioprinting. Ann. Biomed. Eng. 43:730–746, 2015.
Skardal, A., D. Mack, E. Kapetanovic, A. Atala, J. D. Jackson, J. Yoo, and S. Soker. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 1:792–802, 2012.
Skardal, A., J. Zhang, L. Mccoard, X. Xu, S. Oottamasathien, and G. D. Prestwich. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 16:2675–2685, 2010.
Tandon, N., C. Cannizzaro, P. H. Chao, R. Maidhof, A. Marsano, H. T. Au, M. Radisic, and G. Vunjak-Novakovic. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc. 4:155–173, 2009.
Taub, R. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 5:836–847, 2004.
Tayalia, P., and D. J. Mooney. Controlled growth factor delivery for tissue engineering. Adv. Mater. 21:3269–3285, 2009.
Tibbits, S. 4d printing: multi-material shape change. Archit. Design 84:116–121, 2014.
Tomanek, R. J., and R. B. Runyan. Formation of the Heart and Its Regulation. Boston: Birkhäuser, 2012.
Tortora, G. J., and B. H. Derrickson. Principles of Anatomy and Physiology. New York: Wiley, 2011.
Wang, X., Y. Yan, Y. Pan, Z. Xiong, H. Liu, J. Cheng, F. Liu, F. Lin, R. Wu, R. Zhang, and Q. Lu. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 12:83–90, 2006.
Wen, J. H., L. G. Vincent, A. Fuhrmann, Y. S. Choi, K. C. Hribar, H. Taylor-Weiner, S. Chen, and A. J. Engler. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 13:979–987, 2014.
Yu, Y., Y. Zhang, J. A. Martin, and I. T. Ozbolat. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 135:91011, 2013.
Yue, K., G. Trujillo-De Santiago, M. M. Alvarez, A. Tamayol, N. Annabi, and A. Khademhosseini. Synthesis, properties, and biomedical applications of gelatin methacryloyl (gelma) hydrogels. Biomaterials 73:254–271, 2015.
Zhang, Y. S., J. Aleman, A. Arneri, S. Bersini, F. Piraino, S. R. Shin, M. R. Dokmeci, and A. Khademhosseini. From cardiac tissue engineering to heart-on-a-chip: beating challenges. Biomed. Mater. 10:034006, 2015.
Zhang, Y. S., S.-W. Choi, and Y. Xia. Inverse opal scaffolds for applications in regenerative medicine. Soft Matter 9:9747–9754, 2013.
Zhang, Y. S., and A. Khademhosseini. Seeking the right context for evaluating nanomedicine: from tissue models in petri dishes to microfluidic organs-on-a-chip. Nanomedicine 10:685–688, 2015.
Zhang, Y. S., and Y. Xia. Multiple facets for extracellular matrix mimicking in regenerative medicine. Nanomedicine 10:689–692, 2015.
Zhang, Y., Y. Yu, H. Chen, and I. T. Ozbolat. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 5:025004, 2013.
Acknowledgments
The authors gratefully acknowledge funding from the Office of Naval Research Young National Investigator Award, the National Institutes of Health (EB012597, AR057837, DE021468, HL099073, R56AI105024), and the Presidential Early Career Award for Scientists and Engineers (PECASE).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jos Malda oversaw the review of this article.
Kan Yue, Julio Aleman, Kamyar Mollazadeh-Moghaddam, Syeda Mahwish Bakht authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Y.S., Yue, K., Aleman, J. et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng 45, 148–163 (2017). https://doi.org/10.1007/s10439-016-1612-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1612-8