Abstract
Biomedical implants play an important role in today’s clinical practice. Unfortunately, biomedical implant-mediated host responses may lead to implant failure. Thus, all implants are tested for tissue compatibility prior to clinical trials. For that, after implantation in animals for different periods of time, the implants and surrounding tissues are isolated for histological analyses. Unfortunately, histological evaluation methods are labor intensive, time consuming, expensive and do not produce quantitative outcomes. With the advent of in vivo imaging technology, many imaging methods have been developed for evaluating biomedical implant-associated immune responses. In this review, we summarize the recent progress in the use of in vivo real-time imaging techniques for assessing acute phase foreign body reactions, including fibrin deposition, inflammatory cell recruitment and responses surrounding biomaterial implants. These new technologies may serve as powerful tools to characterize tissue compatibility of medical implants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medical implant devices have been used widely for more than 40 years. Recent estimates based on NIH Consensus Development Program report suggest that 20–25 million American currently have at least one medical implant. Despite of the health benefits from implants, almost all of them prompt various types and extents of foreign body reactions, including inflammation, infection, fibrosis and coagulation.91,93 In fact, shortly after implantation, many medical implants are surrounded by substantial numbers of inflammatory cells, including polymorphonuclear leukocytes [PMN] and macrophages/monocytes [MΦ].1,5,93,97,111 Subsequently, inflammatory cells release products which may cause the degradation and failure of many implantable devices and the dissolution of surrounding tissue, including surgical mesh,78 degradable implants/scaffold, soft tissue filler,75 encapsulated cell implants,72,79 implantable sensor,61 temporomandibular,12 and other joint implants.48 To reduce biomaterial-mediated inflammatory responses, intense research efforts have been placed on the development and modification of biomaterials with improved biocompatibility and safety by changing the material’s physical and chemical properties.4,10 As a result, a wide variety of materials with different material characteristics have been generated. Many in vitro methods have been established to assess the cytotoxicity, genotoxicity, mutagenicity, and hemocompatibility of biomaterials in large quantities. Yet, none of these in vitro models can be used to simulate the complex immune reactions in vivo. Therefore, almost all materials passing the in vitro tests have to be further tested using in vivo implantation models. However, many technical challenges associated with animal studies substantially delay the search for better materials and medical implants.
Histological evaluations are the gold standard for assessment of tissue responses to biomaterial implants. Typically, hematoxylin and eosin (H&E), Masson trichrome and immunohistochemical staining techniques help assess the extent of accumulation of cells and their products in response to biomaterial implants. Nevertheless, histological evaluations have many limitations. First, tissue processing, sectioning and staining are labor-intensive and expensive. Quantitative assessment of overall tissue responses necessitates analyses of many sections throughout the samples.5,16,33,77,105,115 Even with automation in sample preparation and basic staining, it would easily take a few weeks to process all samples from a study. Second, histological analyses require isolation of tissues from sacrificed animals. To provide quantitative and statistically significant assessment of the extent of foreign body reactions, large number of animals (total animal number = ~5 animals/sample group X test group number X time points) are needed. There is a great interest among biomedical scientists to reduce the numbers of animals needed for research. Third, to assess the extent of cellular responses to biomaterial implants in situ, most of the current methods, such as microarray and RT-PCR array on tissue sections, can only be carried out with skin biopsies from animals at the end point.63,64,98 It should be noted that microdialysis apparatus and various catheters have been employed to collect body fluids from implantation sites.28,62,103 Unfortunately, these techniques can only be used on rats or larger animals. Due to the limited availability of specific antibodies to these species of animals, this system is rarely used to characterize tissue responses to biomaterial implants. In addition, microdialysis implants take time to heal and are not suitable for measuring acute inflammatory responses (from hours to 3 days) to small size implants (<5 mm diameter). Finally, due to the high costs, lengthy procedures, and methodology limitations, most animal studies have to be carried out with limited time points and measurements. As a consequence of these limitations, most published works have had to deal with inconsistent time points and different measurements that prohibited direct comparison of the results obtained from different studies. In conclusion, high animal numbers and multiple-time replicates for each test at every time point make histological analyses costly, time-consuming and tedious in spite of all the advancements in automation of tissue sectioning and staining processes. Therefore, a better method needs to be developed for rapid, low-cost, non-invasive, and real-time analysis of biomaterial-mediated tissue reactions.
Several non-invasive imaging techniques, including computed tomography, magnetic resonance imaging, positron emission tomography and ultrasonography,49,116 have been developed to visualize and quantify the inflammatory responses in vivo. These techniques rely solely on structural changes and, therefore, are not able to identify non-traumatic, acute and localized inflammatory reactions. Several fluorescence-labeled cells and particle-based sensors have also been developed for a wide range of applications in biological research and clinical diagnosis.13,22,29,41,60,89,104,108 Of late, near Infrared (NIR) fluorescence has gained popularity for in vivo imaging because of reduced absorption and scattering of photons by tissues, and also reduced interference from auto-fluorescence signal.31 NIR technique has been applied in several clinical studies in recent years. For example, NIR fluorophore was used to image lymph flow in breast cancer patients.82 Indocyanine green (ICG) has also been used clinically for hepatic clearance measurement, cardiovascular function testing, and retinal angiography for over 50 years.56 Additionally, NIR dyes have been used for Intraoperative imaging-guided cancer resection surgery.21,65
In this paper, we briefly review the emerging imaging techniques which have been or can be used to assess foreign body reactions (edema formation, inflammatory cell recruitment, inflammatory product release and cell/tissue death, as depicted in Fig. 1) and to characterize tissue compatibility of biomaterial implants.
Schematic illustration of the key cells and cellular products that influence cell and tissue compatibility of biomaterial implants. Shortly after implantation, the biomaterial implant will trigger mast cell activation which lead to fibrin deposition and edema formation. Subsequently, inflammatory cells such as neutrophils and macrophages are recruited to the implant site. The activated inflammatory cells then release a variety of inflammatory products which include free radicals, reactive oxygen species, and many inflammatory cytokines. The release of these products lead to tissue damage/acidosis and cell death.
Imaging Inflammatory Cell Accumulation
The accumulation of inflammatory cell, especially PMN and MΦ, at the implant sites is the hallmark of inflammatory responses (Fig. 1). It is well established that the numbers of accumulated inflammatory cells reflect the extent of biomaterial-mediated inflammatory responses and/or tissue incompatibility of the biomaterial implants. Several imaging methods have been developed to evaluate the extent of implant-mediated inflammatory cell accumulation.
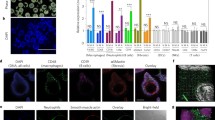
PMNs are the first-responders of inflammatory cells to the inflammatory or injured sites. Shortly after biomaterial implantation, a larger amount of PMNs migrate to the implant sites through the blood stream. These activated PMNs then release many acute inflammatory products (such as granular enzymes and reactive oxygen species), which can lead to degradation of polymeric implants and even tissue damage.32,40,87,110 Therefore, implant-associated PMNs are quantified to indicate tissue compatibility of biomaterial implants.91–93 Although the numbers of recruited PMNs can be estimated via PMN-specific enzyme (such as myeloperoxidase) measurement or histological staining,42,47,90,94 these methods cannot be used as a tool to monitor PMN responses to biomaterial implants in real time. Several imaging techniques have been developed so far to monitor the migration of PMNs during inflammatory processes. For instance, by labeling autologous leukocytes labeled with either 111In or 99mTc, the role of PMNs in myocardial infarction and inflammatory diseases was investigated using scintigraphic imaging system.51,96 To avoid cell isolation and labeling procedures, the extent of PMNs recruitment can be quantified using PMN-targeting optic imaging probes. For example, formyl-methionyl-leucylphenylalanine (fMLP)-based optic probes have been used to bind PMN via formyl peptide receptor and visualize PMNs’ migration in vivo. Unfortunately, fMLP, the N-fomylated oligopeptide family, is a potent PMN chemotactic activator and can induce cell chemotaxis, adhesion and degranulation.7,30 Recently, identification of a new formyl peptide receptor-specific ligand—cinnamoyl-Phe-(d)Leu-Phe-(d)Leu-Phe (cFLFLF) peptide—with no or minimal influence on PMN activity has led to fabrication of PMN-targeting NIR imaging probe.107,114 Using eight-arm PEG as nanocarrier, the PMN-targeting imaging probe was prepared by conjugated the peptide cFLFLF and NIR dye Oyster®-800 onto the nanocarrier. In vitro tests were carried out using isolated mice inflammatory cells which contain MΦ and PMN. Our results demonstrated that the probe is able to specifically identify activated PMN in vitro. Furthermore, the cFLFLF-based PMN-targeting probes are found to be able to assess the extent of PMN recruitment in responding to different material implants.114 In addition, PMN-targeting probes were found to be able to detect infected catheter, since device-centered infection is well established to attract large number of PMNs (Figs. 2a and 2b).114
Characterization of biomaterial-mediated inflammatory cell recruitment and polarization using optical imaging probes. (a) PMN probes were used to assess the extent of inflammatory responses associated with poly-l-lactic acid (PLA) and polyethylene glycol (PEG) particle implants. Both groups of particles were implanted subcutaneously in the back of animals for 24 h prior to the administration of PMN-targeting probes. Images of the animals were taken 3 h after probe administration. PLA implants prompted more PMN accumulation than PEG implants. (b) In an infected catheter inflammation model, PU catheters were colonized with Staphylococcus aureus and then transplanted subcutaneously on the back of animals for 24 h. The animals were then administered with PMN-targeting probes for 3 h prior to image analyses. Infected PU catheters recruited more neutrophils than sterile ones. Adapted from Zhou et al.114 (c) MΦ-targeting probes were used to assess the degree of foreign body reactions. The fluorescent signal was merged with a white-light image captured at different time points following MΦ probes injection (top), with fluorescence intensities in different implantation sites (PLA and PEG) at different time points (shown at the bottom). Significantly more MΦ accumulated around PLA implants than around PEG implants over time. Adapted from Zhou et al.112 (d) MΦ polarization twin probes—M1 and M2 probes—could assess the behavior of implant-associated MΦ. Merged M1/M2 images showed that either LPS-treated PLA (+LPS) or bacteria-infected PLA (+Bacteria) particles triggered much more M1 accumulation around the implant sites while sterile PLA particles induced more M2 recruitment. Adapted from Baker et al.8
MΦ plays a vital role in chronic inflammatory responses and foreign body reactions. Their encounter with “foreign” implants leads to their activation and release of a wide variety of inflammatory cytokines that promote chronic fibrotic tissue formation.93 Thus, it is well established that the extent of MΦ recruitment can be used to assess tissue compatibility of medical implants.92,93 Once again, histological staining is a traditional method to determine the extent of MΦ recruitment surrounding biomaterial implants. As mentioned earlier, traditional histological measurements cannot be used to analyze foreign body reactions—complex tissue reactions. To overcome such drawbacks, several optical imaging strategies have been employed to monitor MΦ recruitment around biomaterial implants in vivo. For instance, fluorescein isothiocyanate (FITC) tagged MΦ-specific antibodies has been used to image MΦ recruitment at biomaterial implants in vivo.14 In spite of the interesting results, this approach has several limitations. First, antibody-based imaging probes are costly, primarily due to high manufacturing cost. Second, antibodies are sensitive to changes in environment (such as temperature and pH), and typically have short shelf life. Finally, both human and animal skins have autofluorescence which emits light in the visible range. This property prevents the use of fluorescent dyes at the visible ranges, including FITC, for in vivo imaging applications.
Previous studies have shown that activated MΦ highly express folate receptor (FR) on the cell membrane and FR-conjugated imaging probes can be used to detect activated MΦ in the inflamed joints of animals with inflammatory arthritis.14,19,26,39,53,54,70,73,101,102,107,114 Based on these early observations, using PNIPAM-co-polystyrene nanoparticle as vehicle, the FR-targeting imaging probe was fabricated by physically loading NIR dye into the nanoparticles and then covalently conjugating folic acid onto the surface of the dye-loading nanoparticle. In vitro studies have shown that FR-targeting probes have no/minimal toxicity to cells and there was very good relationship between the MΦ numbers and fluorescent intensities. The FR-targeting imaging probes were then used to non-invasively assess the extent of MΦ accumulation in foreign body reactions in vivo.112 Following intravenous injection, FR-targeting imaging probes were found to accumulate at the biomaterial implant sites over time.112 Interestingly, the accumulation of imaging probes at the implant sites varied among test materials and there was a very good linear relationship between probe-associated fluorescent intensities (based on whole body animal imaging) and inflammatory cell numbers (quantified by immunohistochemistry analyses) (Fig. 2c).112 This study demonstrated that FR-targeting imaging probes can be used as a powerful tool to quantify the extent of biomaterial-mediated MΦ recruitment and inflammatory responses in vivo.
Imaging MΦ Polarization
In recent years, growing knowledge has revealed the important role of MΦ polarization on affecting the fate of biomaterial implants between chronic foreign body reactions and tissue regeneration.57,85 Typically, based on their functions and activities, polarized MΦ can be categorized into two groups—classically activated M1 and alternatively activated M2. M1 cells are pro-inflammatory in nature and promote tissue destruction. On the other hand, M2 cells are regulatory in nature and trigger tissue regeneration.57,85 Thus the relative degree of MΦ polarization is believed to affect the delicate balance between destructive and regenerative tissue responses. With increasing interest in developing medical implants to enhance tissue regeneration, it is desirable to select biomaterials with the ability to enhance M2 differentiation. In support of this need, a recent study to examine the role of MΦ in the remodeling response of surgical meshes found that an increased M2/M1 ratio was strongly associated with more positive remodeling outcomes.15
A twin imaging probe modality was established to monitor MΦ polarization in vivo.8 In this investigation, folate- and mannose-conjugated, PEG-based NIR probes with different excitation/emission wavelengths were prepared to target M1 and M2 cells, respectively.8 As described earlier, inflammatory MΦ had highly upregulated folate receptor.112 On the other hand, M2 cells expressed high levels of the mannose receptor which was up-regulated by regulatory cytokines such as interleukin-4 (IL-4), IL-10, and IL-13.34,38,58 The ability of the NIR imaging probes to detect M1/M2 cells were then investigated both in vitro and in vivo. As expected, with in vitro competition binding tests, these two probes showed good cellular specificity to M1 or M2 cells. The folate-conjugated probe was observed to bind to M1 cells while the mannose-conjugated probe was observed to bind to M2 cells. Animal studies revealed a good relationship between fluorescent ratios (M2 probe/M1 probe) and histological analyses (CD80 + M1 cells/CD206 + M2 cells). In agreement with previous observations and assumptions, M2 probe/M1 probe fluorescent ratios reflected inflammatory environments, such as the poly-l lactic acid implant sites and bacterial infected tissue (Fig. 2d). This investigation showed that an in vivo optical imaging modality can be used to determine the subtle changes of MΦ polarized phenotypes. The non-invasive characterization of such processes may offer a critical method to non-invasively and in real-time, determine whether the test materials are pro-inflammatory or pro-regenerative in vivo.
Imaging the Release of Inflammatory Products
As discussed above, the accumulation of inflammatory cells (PMN, MΦ, mast cells, dendritic cells, and others) in tissue is a critical indicator of all inflammatory diseases such as foreign body reactions, device-centered infection, atherosclerosis, arthritis, and many others. It is well documented that upon arrival at injured or inflamed sites, inflammatory cells (especially PMNs) activate the respiratory burst, release a number of reactive oxygen species (ROS), including superoxide, hydrogen peroxide, and hyperchlorous acid.35 These released ROS play a key role in eradicating invading foreign bodies, such as foreign microorganisms and biomaterial implants.35,37 Therefore, the extent of ROS production has been used to characterize the cell compatibility and toxicity of biomaterials.68,69 Many methods, including spectrophotometric measurements, electron spin resonance spectroscopy, ELISA, chemiluminescence etc., have been used to assess the release of ROS by inflammatory cells.37 Unfortunately, these established methods cannot be used to assess the extent of ROS in vivo in real time. Some progress has been made in recent years to develop fluorescent probes to detect ROS responses. Specifically, ROS-sensitive NIR dye (Hydro-indocyanine green) had been synthesized and used to monitor biomaterial-associated infections in vivo.27 Later on, ROS-sensitive NIR probe (hydro-sulfo-Cy5) and nitric oxide-sensitive NIR probe (DAC-S) were also investigated for in vivo fluorescence imaging of PET disk-associated inflammation and infection.86
Chemiluminescent imaging is an emerging method to detect ROS activity in vivo. ROS chemiluminescent imaging has many advantages including high sensitivity and specificity, easy quantitative analysis of the light emission at the single-photon level with no excitation light requirement and minimal background noise as fluorescence.44,76 Several chemiluminescent probes have been developed for non-invasive real time ROS imaging in vivo. For instance, peroxalate nanoparticles were fabricated and used as a chemiluminescence probe to detect H2O2 activity in inflammation and infection model in vivo.18,45,46 Oxazine conjugated nanoparticles have been synthesized and used for ex vivo detection of hypochlorous acid and peroxynitrite generation in mouse hearts after myocardial infarction.67 A chemiluminescence probe, luminol, has been used in studies to detect myeloperoxidase activity, the pathogenesis of arthritis, and biomaterial-induced ROS activities in vivo.20,36 Recently, luminol has been used to qualitatively detect implant-associated foreign body reactions in vivo.50 In another investigation, L-012, a luminol-derivative chemiluminescence probe with higher sensitivity toward ROS, has been employed to image ROS activity in vivo using various inflammation and inflection models including allergy- and biomaterial-mediated inflammatory responses.113 Most recently, L-012 was used to determine the extent of biomaterial-mediated ROS production in vivo. Interestingly, it was found that chemiluminescence signal was strongly dependent on type of biomaterial. ROS activities around PNIPAM-NH2, PLA, and PEG implant sites were ~52, 16, and 4 times higher than that around saline sites. In addition, there was a linear relationship between ROS activities and PMNs density in tissue surrounding biomaterial implants (Figs. 3a and 3b).113 These studies reveal that chemiluminescence of ROS generation is a promising modality for characterizing the biocompatibility and pro-inflammatory property of biomaterials and medical implants.
Implant-mediated cellular responses and microenvironment changes. (a) L-012 probe was used to assess the degree of ROS generation by various implants; (b) A good linear relationship was seen between number of recruited PMNs and ROS-associated chemiluminescence intensity at various implant sites. Adapted from Zhou et al.113 (c) Ratiometric pH probes were used to measure the extent of tissue acidosis triggered by various implant biomaterials; (d) The extent of tissue acidosis (pH change) and inflammatory cell accumulation at the implant sites showed a good relationship. Abbreviations for particles made of different materials-PEG (polyethylene glycol); PLA (poly-l-lactic acid); PNIPAM-NH2 (amine-rich poly-N-isopropylacrylamide-co-N-(3-aminopropyl) methacryl-amide); PS (polystyrene); SiO2 (silicone dioxide). Adapted from Tsai et al.99
Tissue acidosis is another well characterized phenomenon which usually occurs in and around inflamed tissue.25,74 Inflammatory cellular responses and associated products will lead to tissue acidosis and cell death in the surrounding tissue in most inflammatory diseases. High hydrogen ion concentrations found in inflamed tissues result in pH as low as 5.7 in cardiac ischemia, and sometimes even down to 4.7 in hematomas.74,84 Previous studies suggest that the acidification of diseased tissues is strongly associated with cell death and inflammatory products such as ROS (hypochloric acid and H2O2).23,55,106 Therefore, monitoring pH change of implants and surrounding tissues in vivo could be a feasible approach for determining the microenvironment inside and surrounding the implants. Although several pH sensitive probes have been developed,6,9,17 these probes have limited capabilities for use in vivo due to diffusion of the dyes in and out of cells and tissues at different rates. To overcome these limitations, an pH ratiometric imaging probe has recently been developed by chemically conjugating a pH-sensitive dye (CypHer5E dye) and a pH-insensitive dye (Oyster®-800) onto a biocompatible PNIPAM nanoparticle which is used as a carrier.99 The ability of ratiometric probes to quantitate pH changes was evaluated both in vitro and in vivo. Using in vitro system, we first determined that the ratio of the average fluorescence intensities between CypHer5E and Oyster®-800 had a strong linear correlation with pH values from 5.53 to 7.55. In addition, the ratiometric pH probe could accurately measure pH without interference from probe concentrations and tissue thickness. The ability of the pH ratiometric probes for in vivo determination of degree of acidosis was then tested in ischemic kidney injury, tumorigenesis and foreign body reaction models.99 Indeed, we found that there was a very good relationship between tissue acidosis (pH values 7.4) and inflammatory cell accumulation (based on histological analyses) (Figs. 3c and 3d).99 These results support that pH imaging probes can be used to assess the potential impact (inflammatory responses and toxicity) of biomaterial implants on the surrounding tissues. Furthermore, pH ratiometric imaging probes can also be used to assess the microenvironment and health of transplanted cells in scaffolds for tissue engineering application.
It should be noted that several commercially available probes have been developed to assess the production of different inflammatory and wound healing products. For example, MMPSense®680 and IntegriSense®750 have been used to assess the activities of two inflammatory products, matrix metalloproteinases- and integrin-expression, respectively.24
Imaging Implant-Associated Fibrin Deposition
Previous studies have shown that fibrinogen/fibrin accumulation is critical in triggering foreign body reactions, including coagulation, inflammation (such as heart attack, ischemic stroke, and pulmonary embolism) and infection.43,83,90,92 Localized fibrin deposition has been demonstrated to be the main driving force for localized immune cell recruitment.3,43,52 Meanwhile, the interaction of Mac-1 (CD11b/CD18) and fibrin is able to trigger the production and release of inflammatory chemokines, such as tumor necrosis factor alpha (TNF-α) and IL-1β.71,88 Furthermore, many studies have shown that fibrin depletion substantially alleviates many inflammatory diseases including glomerulonephritis, lung ischemia, and rheumatoid arthritis. Based on these studies, it is generally agreed that fibrin-mediated immune responses are essential to the pathogenesis of many inflammatory diseases.2 The extent of fibrin deposition in tissue is commonly determined using histological methods.109 However, fibrin accumulation in tissue is a dynamic process with fibrin deposition and fibrinolysis that cannot be quantified based on histological analyses.
Recently, in vivo imaging methods have been developed to detect fibrin deposition in inflammation diseases. For example, ruptured atherosclerotic plaques in a rabbit model can be observed using a short fibrin-affinity peptide conjugated with Gd-DTPA groups and later with a more stable Gd-DOTA chelator for MR signal enhancement.11,66 Later on, fluorescent dye-labeled cross-linked iron oxide (CLIO) nanoparticles functionalized with FXIII-specific peptide (GNQEQVSPLTLLKC) and fibrin(ogen)-affinity peptide (GPRPPGGSKGC) was prepared for detection of clots by both MR and optical imaging modalities.59 Encouraged by these promising outcomes, a NIR fibrin-affinity probe was developed by directly conjugating peptide (GPRPPGGSKGC) with NIR dye (Oyster®-800) to in vivo monitor implant-associated fibrin deposition.100 In vitro tests revealed that the fibrin-affinity probes could preferentially bind to immobilized fibrin. In addition, there was a good relationship between fibrin quantity and fluorescent intensity. Animal studies showed that the fibrin-affinity probe could assess degree of implant-associated fibrin deposition as early as 15 min following biomaterial implantation.100 Furthermore, by combining the technique with histology measurements, this study discovered a linear relationship between implant-associated fibrin-affinity probe accumulation and localized fibrin deposition (Fig. 4).100 Interestingly, the study also found a good relationship between the extent of early fibrin deposition and subsequent inflammatory cell accumulation at the implant sites.100 It is believed that the fibrin-affinity probe can be used as a tool for the early detection of foreign body response to biomaterial implant.
Fibrin-affinity probes were used to assess acute implant-mediated edema formation and fibrin deposition. (a) After particle implantation for 10 min prior to fibrin-affinity probe injection, different implants triggered different degree of fibrin deposition within 30 min. The animal images were taken 1 h after probe administration. (b) There was very good agreement between probe accumulation and fibrin deposition in tissue. Abbreviations for particles made of different materials—SiO2 (silicone dioxide); PLA (poly-l-lactic acid particle); TiO2 (titanium dioxide). Adapted from Tsai et al.100
Conclusions
Biomaterial characterization is essential to the development of medical implants with optimal tissue compatibility. In spite of intensive efforts in the development of in vitro models to predict foreign body reactions, animal models and histological analyses remain the gold standards for assessing tissue compatibility of biomedical implants. This method allows us to examine the numbers and types of cells at the implant sites. However, this method only allows us to examine a slice of the tissue responses at one time point per animal. For histological measurements, many animals are needed to study the kinetics of cellular responses to biomaterial implants. To reduce the number of animals needed for testing and also the costs/time associated with histological evaluations, several in vivo optical imaging probes/methods have been established to evaluate the cellular and tissue compatibility of biomaterial implants. Compared to histological methods, these techniques are non-invasive, more rapid and cost-effective. Most importantly, they can be used to continuously monitor the dynamics of inflammatory processes and the microenvironment surrounding biomedical implants in real time at cellular- and molecular-level. Recently, some breakthroughs have been made on translating inflammation monitoring into clinical diagnosis. Specifically, NIR probes have been fabricated to detect bacterial colonization and device-centered infection.95 In addition, several optical imaging probes have been developed to monitor wound healing status and outcomes after treatments.80,81 Although promising, some shortcomings associated with optical imaging methods have to be overcome for routine use in research and clinical setting. For instance, limited penetration depth of light, light scattering as well as attenuation, prevent these optical imaging probes from detecting inflammatory response to deeply-implanted medical devices. To overcome this limitation, other imaging modalities such as PET and MRI could be included. We believe that with continued development of in vivo imaging techniques, in vivo evaluation and analysis of medical implant-associated immune responses will become realistic in clinical applications in near future.
References
Adams, Jr., W. P., M. S. Haydon, J. Raniere, Jr., S. Trott, M. Marques, M. Feliciano, et al. A rabbit model for capsular contracture: development and clinical implications. Plast. Reconstr. Surg. 117:1214–1219, 2006.
Akassoglou, K., R. A. Adams, J. Bauer, P. Mercado, V. Tseveleki, H. Lassmann, et al. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 101:6698–6703, 2004.
Altieri, D. C., R. Bader, P. M. Mannucci, and T. S. Edgington. Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J. Cell Biol. 107:1893–1900, 1988.
Alves, N. M., I. Pashkuleva, R. L. Reis, and J. F. Mano. Controlling cell behavior through the design of polymer surfaces. Small 6:2208–2220, 2010.
Anderson, J. M., A. Rodriguez, and D. T. Chang. Foreign body reaction to biomaterials. Semin. Immunol. 20:86–100, 2008.
Andreev, O. A., A. D. Dupuy, M. Segala, S. Sandugu, D. A. Serra, C. O. Chichester, et al. Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc. Natl. Acad. Sci. U.S.A. 104:7893–7898, 2007.
Babich, J. W., R. G. Tompkins, W. Graham, S. A. Barrow, and A. J. Fischman. Localization of radiolabeled chemotactic peptide at focal sites of Escherichia coli infection in rabbits: evidence for a receptor-specific mechanism. J. Nucl. Med. 38:1316–1322, 1997.
Baker, D. W., J. Zhou, Y. T. Tsai, K. M. Patty, H. Weng, E. N. Tang, et al. Development of optical probes for in vivo imaging of polarized macrophages during foreign body reactions. Acta Biomater. 10:2945–2955, 2014.
Bartsch, I., E. Willbold, B. Rosenhahn, and F. Witte. Non-invasive pH determination adjacent to degradable biomaterials in vivo. Acta Biomater. 10:34–39, 2014.
Biggs, M. J., R. G. Richards, and M. J. Dalby. Nanotopographical modification: a regulator of cellular function through focal adhesions. Nanomedicine 6:619–633, 2010.
Botnar, R. M., A. S. Perez, S. Witte, A. J. Wiethoff, J. Laredo, J. Hamilton, et al. In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent. Circulation 109:2023–2029, 2004.
Bouloux, G. F. Temporomandibular joint pain and synovial fluid analysis: a review of the literature. J. Oral Maxillofac. Surg. 67:2497–2504, 2009.
Brasuel, M., R. Kopelman, T. J. Miller, R. Tjalkens, and M. A. Philbert. Fluorescent nanosensors for intracellular chemical analysis: decyl methacrylate liquid polymer matrix and ion-exchange-based potassium PEBBLE sensors with real-time application to viable rat C6 glioma cells. Anal. Chem. 73:2221–2228, 2001.
Bratlie, K. M., T. T. Dang, S. Lyle, M. Nahrendorf, R. Weissleder, R. Langer, et al. Rapid biocompatibility analysis of materials via in vivo fluorescence imaging of mouse models. PLoS ONE 5:e10032, 2010.
Brown, B. N., R. Londono, S. Tottey, L. Zhang, K. A. Kukla, M. T. Wolf, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 8:2871, 2012.
Cai, T., P. D. Hu, M. Sun, J. Zhou, Y. T. Tsai, D. Baker, et al. Novel thermogelling dispersions of polymer nanoparticles for controlled protein release. Nanomedicine 8:1301–1308, 2012.
Carmo, V. A., C. S. Ferrari, E. C. Reis, G. A. Ramaldes, M. A. Pereira, M. C. De Oliveira, et al. Biodistribution study and identification of inflammation sites using 99mTc-labelled stealth pH-sensitive liposomes. Nucl. Med. Commun. 29:33–38, 2008.
Chen, Z., Z. Liu, Z. Li, E. Ju, N. Gao, L. Zhou, et al. Upconversion nanoprobes for efficiently in vitro imaging reactive oxygen species and in vivo diagnosing rheumatoid arthritis. Biomaterials 39:15–22, 2015.
Chen, W. T., U. Mahmood, R. Weissleder, and C. H. Tung. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res. Ther. 7:R310–R317, 2005.
Chen, W. T., C. H. Tung, and R. Weissleder. Imaging reactive oxygen species in arthritis. Mol. Imaging 3:159–162, 2004.
Chi, C., Y. Du, J. Ye, D. Kou, J. Qiu, J. Wang, et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 4:1072–1084, 2014.
Clark, H. A., R. Kopelman, R. Tjalkens, and M. A. Philbert. Optical nanosensors for chemical analysis inside single living cells. 2. Sensors for pH and calcium and the intracellular application of PEBBLE sensors. Anal. Chem. 71:4837–4843, 1999.
Conus, S., and H. U. Simon. Cathepsins: key modulators of cell death and inflammatory responses. Biochem. Pharmacol. 76:1374–1382, 2008.
Daghighi, S., J. Sjollema, D. J. Dijkstra, V. Jaspers, S. A. Zaat, H. C. van der Mei, et al. Real-time quantification of matrix metalloproteinase and integrin αvβ3 expression during biomaterial-associated infection in a murine model. Eur. Cell Mater. 27:26–37, 2014.
De Backer, D. Lactic acidosis. Minerva Anestesiol. 69:281–284, 2003.
Derian, C. K., H. F. Solomon, J. D. Higgins, 3rd, M. J. Beblavy, R. J. Santulli, G. J. Bridger, et al. Selective inhibition of N-formylpeptide-induced neutrophil activation by carbamate-modified peptide analogues. Biochemistry 35:1265–1269, 1996.
Dinjaski, N., S. Suri, J. Valle, S. M. Lehman, I. Lasa, M. A. Prieto, et al. Near-infrared fluorescence imaging as an alternative to bioluminescent bacteria to monitor biomaterial-associated infections. Acta Biomater. 10:2935–2944, 2014.
Duo, J., and J. A. Stenken. In vitro and in vivo affinity microdialysis sampling of cytokines using heparin-immobilized microspheres. Anal. Bioanal. Chem. 399:783–793, 2011.
Fehr, M., W. B. Frommer, and S. Lalonde. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc. Natl. Acad. Sci. U.S.A. 99:9846–9851, 2002.
Fischman, A. J., M. C. Pike, D. Kroon, A. J. Fucello, D. Rexinger, C. ten Kate, et al. Imaging focal sites of bacterial infection in rats with indium-111-labeled chemotactic peptide analogs. J. Nucl. Med. 32:483–491, 1991.
Frangioni, J. V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 7:626–634, 2003.
Freeman, T. A., J. Parvizi, C. J. Dellavalle, and M. J. Steinbeck. Reactive oxygen and nitrogen species induce protein and DNA modifications driving arthrofibrosis following total knee arthroplasty. Fibrogenesis Tissue Repair 2:5, 2009.
Gerstner, A. O., C. Trumpfheller, P. Racz, P. Osmancik, K. Tenner-Racz, and A. Tarnok. Quantitative histology by multicolor slide-based cytometry. Cytometry A 59:210–219, 2004.
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35, 2003.
Greenhalgh, D. G. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 30:1019–1030, 1998.
Gross, S., S. T. Gammon, B. L. Moss, D. Rauch, J. Harding, J. W. Heinecke, et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 15:455–461, 2009.
Halliwell, B., and M. Whiteman. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 142:231–255, 2004.
Hayes, E. M., A. Tsaousi, K. Di Gregoli, S. R. Jenkinson, A. R. Bond, J. L. Johnson, et al. Classical and alternative activation and metalloproteinase expression occurs in foam cell macrophages in male and female ApoE null mice in the absence of T and B lymphocytes. Front. Immunol. 5:537, 2014.
Hilgenbrink, A. R., and P. S. Low. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J. Pharm. Sci. 94:2135–2146, 2005.
Hoemann, C. D., G. P. Chen, C. Marchand, N. Tran-Khanh, M. Thibault, A. Chevrier, et al. Scaffold-guided subchondral bone repair implication of neutrophils and alternatively activated arginase-1+ macrophages. Am. J. Sports Med. 38:1845–1856, 2010.
Ji, J., N. Rosenzweig, C. Griffin, and Z. Rosenzweig. Synthesis and application of submicrometer fluorescence sensing particles for lysosomal pH measurements in murine macrophages. Anal. Chem. 72:3497–3503, 2000.
Josefsson, E., A. Tarkowski, and H. Carlsten. Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell. Immunol. 142:67–78, 1992.
Languino, L. R., J. Plescia, A. Duperray, A. A. Brian, E. F. Plow, J. E. Geltosky, et al. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an Icam-1-dependent pathway. Cell 73:1423–1434, 1993.
le Masne de Chermont, Q., C. Chaneac, J. Seguin, F. Pelle, S. Maitrejean, J. P. Jolivet, et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. U.S.A. 104:9266–9271, 2007.
Lee, D., V. R. Erigala, M. Dasari, J. Yu, R. M. Dickson, and N. Murthy. Detection of hydrogen peroxide with chemiluminescent micelles. Int. J. Nanomed. 3:471–476, 2008.
Lee, D., S. Khaja, J. C. Velasquez-Castano, M. Dasari, C. Sun, J. Petros, et al. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 6:765–769, 2007.
Lefer, A. M., B. Campbell, R. Scalia, and D. J. Lefer. Synergism between platelets and neutrophils in provoking cardiac dysfunction after ischemia and reperfusion—role of selectins. Circulation 98:1322–1328, 1998.
Lidgren, L. Chronic inflammation, joint replacement and malignant lymphoma. J. Bone Joint Surg. Br. 90:7–10, 2008.
Lindner, J. R., J. Song, F. Xu, A. L. Klibanov, K. Singbartl, K. Ley, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation 102:2745–2750, 2000.
Liu, W. F., M. Ma, K. M. Bratlie, T. T. Dang, R. Langer, and D. G. Anderson. Real-time in vivo detection of biomaterial-induced reactive oxygen species. Biomaterials 32:1796–1801, 2011.
Liu, Z., L. Wyffels, C. Barber, M. M. Hui, and J. M. Woolfenden. A (99m)Tc-labeled dual-domain cytokine ligand for imaging of inflammation. Nucl. Med. Biol. 38:795–805, 2011.
Loike, J. D., B. Sodeik, L. Cao, S. Leucona, J. I. Weitz, P. A. Detmers, et al. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc. Natl. Acad. Sci. U.S.A. 88:1044–1048, 1991.
Low, P. S., W. A. Henne, and D. D. Doorneweerd. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 41:120–129, 2008.
Lu, Y., T. W. Stinnette, E. Westrick, P. J. Klein, M. A. Gehrke, V. A. Cross, et al. Treatment of experimental adjuvant arthritis with a novel folate receptor-targeted folic acid-aminopterin conjugate. Arthritis Res. Ther. 13:R56, 2011.
Mainnemare, A., B. Megarbane, A. Soueidan, A. Daniel, and I. L. Chapple. Hypochlorous acid and taurine-N-monochloramine in periodontal diseases. J. Dent. Res. 83:823–831, 2004.
Marshall, M. V., J. C. Rasmussen, I. C. Tan, M. B. Aldrich, K. E. Adams, X. Wang, et al. Near-infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surg. Oncol. J. 2:12–25, 2010.
Martinez, F. O., A. Sica, A. Mantovani, and M. Locati. Macrophage activation and polarization. Front. Biosci. 13:453–461, 2008.
Martinez-Pomares, L., D. M. Reid, G. D. Brown, P. R. Taylor, R. J. Stillion, S. A. Linehan, et al. Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J. Leukoc. Biol. 73:604–613, 2003.
McCarthy, J. R., P. Patel, I. Botnaru, P. Haghayeghi, R. Weissleder, and F. A. Jaffer. Multimodal nanoagents for the detection of intravascular thrombi. Bioconjug. Chem. 20:1251–1255, 2009.
McNamara, K. P., T. Nguyen, G. Dumitrascu, J. Ji, N. Rosenzweig, and Z. Rosenzweig. Synthesis, characterization, and application of fluorescence sensing lipobeads for intracellular pH measurements. Anal. Chem. 73:3240–3246, 2001.
Morais, J. M., F. Papadimitrakopoulos, and D. J. Burgess. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 12:188–196, 2010.
Mou, X., M. R. Lennartz, D. J. Loegering, and J. A. Stenken. Long-term calibration considerations during subcutaneous microdialysis sampling in mobile rats. Biomaterials 31:4530–4539, 2010.
Nguyen, K. T., N. Shaikh, K. P. Shukla, S. H. Su, R. C. Eberhart, and L. Tang. Molecular responses of vascular smooth muscle cells and phagocytes to curcumin-eluting bioresorbable stent materials. Biomaterials 25:5333–5346, 2004.
Nguyen, K. T., N. Shaikh, D. Wawro, S. Zhang, N. D. Schwade, R. C. Eberhart, et al. Molecular responses of vascular smooth muscle cells to paclitaxel-eluting bioresorbable stent materials. J. Biomed. Mater. Res. A 69:513–524, 2004.
Okusanya, O. T., E. M. DeJesus, J. X. Jiang, R. P. Judy, O. G. Venegas, C. G. Deshpande, et al. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J. Thorac. Cardiovasc. Surg. 150:28.e1–35.e1, 2015.
Overoye-Chan, K., S. Koerner, R. J. Looby, A. F. Kolodziej, S. G. Zech, Q. Deng, et al. EP-2104R: a fibrin-specific gadolinium-based MRI contrast agent for detection of thrombus. J. Am. Chem. Soc. 130:6025–6039, 2008.
Panizzi, P., M. Nahrendorf, M. Wildgruber, P. Waterman, J. L. Figueiredo, E. Aikawa, et al. Oxazine conjugated nanoparticle detects in vivo hypochlorous acid and peroxynitrite generation. J. Am. Chem. Soc. 131:15739–15744, 2009.
Park, M. V., A. M. Neigh, J. P. Vermeulen, L. J. de la Fonteyne, H. W. Verharen, J. J. Briede, et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32:9810–9817, 2011.
Patel, J. D., T. Krupka, and J. M. Anderson. iNOS-mediated generation of reactive oxygen and nitrogen species by biomaterial-adherent neutrophils. J. Biomed. Mater. Res. A 80:381–390, 2007.
Paulos, C. M., M. J. Turk, G. J. Breur, and P. S. Low. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv. Drug Deliv. Rev. 56:1205–1217, 2004.
Perez, R. L., and J. Roman. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J. Immunol. 154:1879–1887, 1995.
Piemonti, L., L. G. Guidotti, and M. Battaglia. Modulation of early inflammatory reactions to promote engraftment and function of transplanted pancreatic islets in autoimmune diabetes. Adv. Exp. Med. Biol. 654:725–747, 2010.
Puig-Kroger, A., E. Sierra-Filardi, A. Dominguez-Soto, R. Samaniego, M. T. Corcuera, F. Gomez-Aguado, et al. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 69:9395–9403, 2009.
Reeh, P. W., and K. H. Steen. Tissue acidosis in nociception and pain. Prog. Brain Res. 113:143–151, 1996.
Requena, L., C. Requena, L. Christensen, U. S. Zimmermann, H. Kutzner, and L. Cerroni. Adverse reactions to injectable soft tissue fillers. J. Am. Acad. Dermatol. 64:1–34, 2011.
Roda, A., M. Guardigli, P. Pasini, M. Mirasoli, E. Michelini, and M. Musiani. Bio- and chemiluminescence imaging in analytical chemistry. Anal. Chim. Acta 541:25–36, 2005.
Sabaliauskas, N. A., C. A. Foutz, J. R. Mest, L. R. Budgeon, A. T. Sidor, J. A. Gershenson, et al. High-throughput zebrafish histology. Methods 39:246–254, 2006.
Sailes, F. C., J. Walls, D. Guelig, M. Mirzabeigi, W. D. Long, A. Crawford, et al. Synthetic and biological mesh in component separation: a 10-year single institution review. Ann. Plast. Surg. 64:696–698, 2010.
Santos, E., J. Zarate, G. Orive, R. M. Hernandez, and J. L. Pedraz. Biomaterials in cell microencapsulation. Adv. Exp. Med. Biol. 670:5–21, 2010.
Schreml, S., R. J. Meier, K. T. Weiss, J. Cattani, D. Flittner, S. Gehmert, et al. A sprayable luminescent pH sensor and its use for wound imaging in vivo. Exp. Dermatol. 21:951–953, 2012.
Schreml, S., R. J. Meier, O. S. Wolfbeis, M. Landthaler, R. M. Szeimies, and P. Babilas. 2D luminescence imaging of pH in vivo. Proc. Natl. Acad. Sci. USA 108:2432–2437, 2011.
Sevick-Muraca, E. M., R. Sharma, J. C. Rasmussen, M. V. Marshall, J. A. Wendt, H. Q. Pham, et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology 246:734–741, 2008.
Smiley, S. T., J. A. King, and W. W. Hancock. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol. 167:2887–2894, 2001.
Steen, K. H., A. E. Steen, and P. W. Reeh. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J. Neurosci. 15:3982–3989, 1995.
Stout, R. D., and J. Suttles. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 76:509–513, 2004.
Suri, S., S. M. Lehman, S. Selvam, K. Reddie, S. Maity, N. Murthy, et al. In vivo fluorescence imaging of biomaterial-associated inflammation and infection in a minimally invasive manner. J. Biomed. Mater. Res. A. 103:76–83, 2015.
Sutherland, K., J. R. Mahoney, A. J. Coury, and J. W. Eaton. Degradation of biomaterials by phagocyte-derived oxidants. J. Clin. Invest. 92:2360–2367, 1993.
Szaba, F. M., and S. T. Smiley. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood 99:1053–1059, 2002.
Tan, M., G. Wang, X. Hai, Z. Ye, and J. Yuan. Development of functionalized fluorescent europium nanoparticles for biolabeling and time-resolved fluorometric applications. J. Mater. Chem. 14:2896–2901, 2004.
Tang, L., and J. W. Eaton. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J. Exp. Med. 178:2147–2156, 1993.
Tang, L., and J. W. Eaton. Inflammatory responses to biomaterials. Am. J. Clin. Pathol. 103:466–471, 1995.
Tang, L., and J. W. Eaton. Natural responses to unnatural materials: a molecular mechanism for foreign body reactions. Mol. Med. 5:351–358, 1999.
Tang, L., and W. Hu. Molecular determinants of biocompatibility. Expert Rev. Med. Devices 2:493–500, 2005.
Tang, L., A. H. Lucas, and J. W. Eaton. Inflammatory responses to implanted polymeric biomaterials: role of surface-adsorbed immunoglobulin G. J. Lab. Clin. Med. 122:292–300, 1993.
Tang, E. N., A. Nair, D. W. Baker, W. Hu, and J. Zhou. In vivo imaging of infection using a bacteria-targeting optical nanoprobe. J. Biomed. Nanotechnol. 10:856–863, 2014.
Thakur, M. L., A. Gottschalk, and B. L. Zaret. Imaging experimental myocardial infarction with indium-111-labeled autologous leukocytes: effects of infarct age and residual regional myocardial blood flow. Circulation 60:297–305, 1979.
Thevenot, P., W. Hu, and L. Tang. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 8:270–280, 2008.
Thevenot, P. T., A. M. Nair, J. Shen, P. Lotfi, C. Y. Ko, and L. Tang. The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 31:3997–4008, 2010.
Tsai, Y. T., J. Zhou, H. Weng, J. Shen, L. Tang, and W. J. Hu. Real-time noninvasive monitoring of in vivo inflammatory responses using a pH ratiometric fluorescence imaging probe. Adv. Healthc. Mater. 3:221–229, 2014.
Tsai, Y. T., J. Zhou, H. Weng, E. N. Tang, D. W. Baker, and L. Tang. Optical imaging of fibrin deposition to elucidate participation of mast cells in foreign body responses. Biomaterials 35:2089–2096, 2014.
Turk, M. J., G. J. Breur, W. R. Widmer, C. M. Paulos, L. C. Xu, L. A. Grote, et al. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 46:1947–1955, 2002.
Turk, M. J., D. J. Waters, and P. S. Low. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 213:165–172, 2004.
von Grote, E. C., V. Venkatakrishnan, J. Duo, and J. A. Stenken. Long-term subcutaneous microdialysis sampling and qRT-PCR of MCP-1, IL-6 and IL-10 in freely-moving rats. Mol. BioSyst. 7:150–161, 2011.
Wang, L., C. Yang, and W. Tan. Dual-luminophore-doped silica nanoparticles for multiplexed signaling. Nano Lett. 5:37–43, 2005.
Weng, H., J. Zhou, L. Tang, and Z. Hu. Tissue responses to thermally-responsive hydrogel nanoparticles. J. Biomater. Sci. Polym. Ed. 15:1167–1180, 2004.
Whiteman, M., and J. P. Spencer. Loss of 3-chlorotyrosine by inflammatory oxidants: implications for the use of 3-chlorotyrosine as a bio-marker in vivo. Biochem. Biophys. Res. Commun. 371:50–53, 2008.
Xiao, L., Y. Zhang, Z. Liu, M. Yang, L. Pu, and D. Pan. Synthesis of the cyanine 7 labeled neutrophil-specific agents for noninvasive near infrared fluorescence imaging. Bioorg. Med. Chem. Lett. 20:3515–3517, 2010.
Xu, H., J. W. Aylott, R. Kopelman, T. J. Miller, and M. A. Philbert. A real-time ratiometric method for the determination of molecular oxygen inside living cells using sol-gel-based spherical optical nanosensors with applications to rat C6 glioma. Anal. Chem. 73:4124–4133, 2001.
Yamamoto, K., and D. J. Loskutoff. Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J. Clin. Invest. 97:2440–2451, 1996.
Ye, Q. S., M. C. Harmsen, M. J. A. van Luyn, and R. A. Bank. The relationship between collagen scaffold cross-linking agents and neutrophils in the foreign body reaction. Biomaterials 31:9192–9201, 2010.
Zdolsek, J., J. W. Eaton, and L. Tang. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J. Transl. Med. 5:31, 2007.
Zhou, J., Y. T. Tsai, H. Weng, D. W. Baker, and L. Tang. Real time monitoring of biomaterial-mediated inflammatory responses via macrophage-targeting NIR nanoprobes. Biomaterials 32:9383–9390, 2011.
Zhou, J., Y. T. Tsai, H. Weng, and L. Tang. Noninvasive assessment of localized inflammatory responses. Free Radic. Biol. Med. 52:218–226, 2012.
Zhou, J., Y. T. Tsai, H. Weng, E. N. Tang, A. Nair, D. P. Dave, et al. Real-time detection of implant-associated neutrophil responses using a formyl peptide receptor-targeting NIR nanoprobe. Int. J. Nanomed. 7:2057–2068, 2012.
Zhou, J., G. Wang, L. Zou, L. Tang, M. Marquez, and Z. Hu. Viscoelastic behavior and in vivo release study of microgel dispersions with inverse thermoreversible gelation. Biomacromolecules 9:142–148, 2008.
Zhuang, H., and A. Alavi. 18-Fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin. Nucl. Med. 32:47–59, 2002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Agata Exner oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Zhou, J., Hu, W. & Tang, L. Non-invasive Characterization of Immune Responses to Biomedical Implants. Ann Biomed Eng 44, 693–704 (2016). https://doi.org/10.1007/s10439-015-1470-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1470-9