Abstract
A majority of traumatic brain injuries (TBI) in motor vehicle crashes and sporting environments are mild and caused by high-rate acceleration of the head. For injuries caused by rotational acceleration, both magnitude and duration of the acceleration pulse were shown to influence injury outcomes. This study incorporated a unique rodent model of rotational acceleration-induced mild TBI (mTBI) to quantify independent effects of magnitude and duration on behavioral and neuroimaging outcomes. Ninety-two Sprague–Dawley rats were exposed to head rotational acceleration at peak magnitudes of 214 or 350 krad/s2 and acceleration pulse durations of 1.6 or 3.4 ms in a full factorial design. Rats underwent a series of behavioral tests including the Composite Neuroscore (CN), Elevated Plus Maze (EPM), and Morris Water Maze (MWM). Ex vivo diffusion tensor imaging (DTI) of the fixed brains was conducted to assess the effects of rotational injury on brain microstructure as revealed by the parameter fractional anisotropy (FA). While the injury did not cause significant locomotor or cognitive deficits measured with the CN and MWM, respectively, a main effect of duration was consistently observed for the EPM. Increased duration caused significantly greater activity and exploratory behaviors measured as open arm time and number of arm changes. DTI demonstrated significant effects of both magnitude and duration, with the FA of the amygdala related to both the magnitude and duration. Increased duration also caused FA changes at the interface of gray and white matter. Collectively, the findings demonstrate that the consequences of rotational acceleration mTBI were more closely associated with duration of the rotational acceleration impulse, which is often neglected as an independent factor, and highlight the need for animal models of TBI with strong biomechanical foundations to associate behavioral outcomes with brain microstructure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) results from a variety of mechanisms including impact, inertia, penetration, and blast overpressure. In civilian sporting and motor vehicle crash environments, injuries commonly result from head impact leading to high rate head acceleration. In general, head impacts with a vector directed away from the center of gravity will lead to rotational accelerations. Diffuse injuries occur within the tissues of the brain as shear strains develop due to inertia, centrifugal force, and coriolis forces during head rotational acceleration. This results in pathologies distributed more diffusely than for other mechanisms such as skull deflection due to impact or penetration resulting in focal injury. The pattern of diffuse injury is dependent upon a number of factors including anatomy/geometry63 and plane of rotational acceleration.33 The importance of head acceleration in the mechanism of concussive brain injury has been understood for decades.20 It was generally acknowledged that acceleration characteristics modulate outcomes, with higher accelerations resulting in more severe injuries.35 Whereas the historical focus of brain injury tolerance research was on linear acceleration, with the Head Injury Criterion (HIC)96 correlating the risk of injury with the magnitude of linear acceleration over a specific time duration, other research has demonstrated a higher risk of injury during rotational acceleration.34,46
Experimental and computational research has attempted to outline the role of rotational acceleration in the mechanism of TBI. A recent computational modeling study revealed that 90% of the total strain within the tissues of the brain during head acceleration due to blunt impact results from the rotational component, with only 10% attributed to the translational component.101 Additionally, different characteristics of the rotational acceleration vs. time pulse were correlated to injury risk or severity. The peak magnitude of head rotational acceleration was identified as a determinant of injury or injury severity. Specifically, multiple studies identified a correlation between increasing magnitude of rotational acceleration and increasing injury severity.2,45,62,80 In 2003, Gennarelli et al., reported a theoretical relationship between increasing rotational acceleration magnitude and injury severity from mild concussion through severe diffuse axonal injury.32 Acknowledging the confounding influence of acceleration duration, other studies correlated injury tolerance with rotational velocity, computed as the integral of the acceleration vs. time pulse.62,94 Those studies identified lower injury tolerance at higher velocities. However, given its inherent limitations in the prediction of injury, i.e., high velocities resulting from very low acceleration with long duration, the focus of injury tolerance research may be better applied to the investigation of magnitude and duration as separate characteristics. To that end, Ommaya and colleagues identified a complex relationship between rotational acceleration magnitude and duration.79 However, that relationship was applicable to more severe injuries and neurobehavioral assessments were not performed. Because of the contemporary importance of neurocognitive outcomes in the determination of brain injury severity,53,68,74,86 there is particular need for studies relating injury tolerance to neurobehavioral changes associated with mild TBI. The current study employed a rodent model for TBI that is capable of inducing brain injury in rats through pure coronal plane rotational acceleration of the head. The model is capable of controlling rotational acceleration and duration independently to assess the role of those factors on injury tolerance. Well-characterized behavioral assessments were used to quantify motor, cognitive, and emotional outcomes and diffusion tensor imaging (DTI) was used to evaluate brain microstructural changes.
Methods
Animals
Adult female Sprague–Dawley rats (weight: 289 ± 21 g) were used. Rats were housed individually in standard cages, maintained on a 12-h light/dark cycle, and provided free access to food and water throughout the experimental period. All injury and behavioral testing was conducted with approval from the Institutional Animal Care and Use Committee at our Institution.
Injury Procedure
Rats were exposed to high rate head rotational acceleration using the MCW Rotational Injury Device (Fig. 1a).26 The model consisted of a rat helmet with laterally extended moment arm and an impactor rod. The impactor rod was accelerated by gravity down a drop tower to impact the moment arm and generate sufficient force to rotate the helmet. A rotational bearing was used to constrain helmet motion to pure coronal plane rotation, without linear displacement. Helmet rotation was centered about an axis in the mid-sagittal plane located in the lower half of the brain (Fig. 1b). Characteristics of the rotational acceleration vs. time pulse were modulated by the mass of the impactor, initial drop height, and characteristics of the elastomer interface material between the impactor and the moment arm.24 An accelerometer attached to the distal end of the moment arm measured tangential linear acceleration, which was converted to rotational acceleration vs. time of the helmet. Our previous studies and extensive tests ensured that magnitude and duration of the rotational acceleration vs. time pulse were independently modulated and could be accurately measured for each injury.24–27
(a) MCW rotational injury device that was used to produce mild TBI in rats through coronal plane rotational acceleration of the head. The three images demonstrate helmet positioning just prior to impact from the rod (left), and during impact causing the helmet to rotate 40° (middle) and 80° (right). Total rotation of the helmet was limited to 90°. Motion was constrained to pure coronal plane rotation, without linear displacement, using a fixed bearing located anterior to the helmet. (b) The axis of helmet rotation was located in the mid-sagittal plane in the lower half of the brain
On the day of the injury procedure, rats were individually transported to the laboratory and anesthetized using 4.0% Isoflurane. When rats were no longer responsive to the toe pinch reflex, they were given a single dose of Carprofen (5.0 mg/kg), placed within the device, and exposed to a single head rotational acceleration of a predetermined magnitude and duration. Following exposure to head rotational acceleration, rats were removed from the device and placed on a warming blanket until reappearance of the righting reflex, returned to their cages, and supervised for at least 15 min.
Experimental Groups
A full 2 × 2 factorial design was used to assess the independent effects of rotational acceleration magnitude and duration, with targeted magnitudes of 200 and 350 krad/s2 and durations of 1.6 and 3.5 ms. Each of the four injury groups consisted of fourteen to eighteen rats (Table 1). Magnitude and duration were calculated for each animal based on the acquired rotational acceleration profile using custom Matlab routines (MathWorks, Natick, MA). A group of sham animals (n = 28) was subjected to the entire experimental protocol, including anesthesia and placement in the helmet, without exposure to head rotational acceleration.
Composite Neuroscore Testing
The Composite Neuroscore Motor Assessment Task was used to grade post-traumatic gross neurological dysfunction. The task assessed six neurologically related reflexes with a score between 0 (severely impaired) and 2 (normal). The composite score from all tests was used to grade overall neuro-motor function. The six tasks included: (1) left and (2) right forelimb flexion during suspension by the tail; (3) left and (4) right hind limb flexion with forelimbs remaining on a flat surface as hind limbs are lifted up and down by the tail; and (5) ability to resist lateral pulsion to the left and (6) right. Composite Neuroscore was quantified on post-injury day 4. These metrics were scored by an observer blinded to the injury status of the rat using videos obtained during the test. Lower Composite Neuroscores were associated with higher levels of neuromotor dysfunction.
Morris Water Maze (MWM) Testing
The MWM Visuo-Spatial Learning Paradigm was used to grade post-traumatic anterograde amnesia and spatial learning following TBI in rats.15,90 The paradigm consisted of three days of testing on post-injury days 1, 2, and 3. Each day of testing included one set of four trials for a total of 12 trials across three days. The four trials per set consisted of initially placing the rats at the four cardinal locations within the 183-cm diameter maze (N, E, S, W), facing the outer wall. During each trial, rats were allowed to swim in the 25-cm deep water until finding and mounting a 10-cm diameter hidden platform submerged 1 cm below the water surface, or until 60 s had passed. Trials were considered to be ‘unsuccessful’ if the rat was unable to find the hidden platform in less than 60 s. In the event of an unsuccessful trial, rats were guided to the platform by the technician and allowed to remain on the platform for 30 s. Rats that successfully found the platform in less than 60 s were also allowed to remain on the platform for 30 s. The platform was located between the cardinal axes (e.g., SE) halfway between the center and outer wall. Location remained constant for the four trials of a set, but was changed to a randomized location from set-to-set. Water temperature remained within one degree of 24 °C for all trials and the maze was located in a room with numerous visual cues external to the maze and oriented identically for each session. Visual cues were also placed inside the maze. A computerized tracking system and software (Ethovision V8.0, Noldus Information Technology, Wageningen, The Netherlands) recorded several metrics during each MWM trial. Latency to find the hidden platform (s) and distance traveled (cm) were measured for each trial and compared between injury groups and between successive trials/sets. The number of unsuccessful trials for the second, third, and fourth trials of each set was counted for each rat over the entire three set assessment. The cumulative number of unsuccessful trials from only the second, third, and fourth trials were used, for a total of nine trials across the three sets. The first trial for each set was not included in the analysis of unsuccessful trials because the hidden platform was moved to a new location for each set and the ability to find the platform on the first trial of each set did not involve spatial learning. Cognitive deficits were associated with greater latency and swim distance, and a greater number of unsuccessful trials.
Elevated Plus Maze (EPM) Testing
The EPM assessment was used to quantify activity and emotional-type behaviors following TBI in rats. Rats underwent the EPM assessment on day 2 post-injury. The maze consisted of four perpendicular 10 × 90-cm arms suspended 50 cm above the floor. A 10 × 10-cm central platform connected the arms. One pair of opposing arms was enclosed by 40-cm-high walls, while the other two arms and the center platform were uncovered. Rats were initially placed on the central platform facing one of the two open arms. The animals were allowed to explore the maze for 5 min and tracked using a digital video camera mounted above the maze. Metrics quantified during the test include: total distance traveled, total number of arm changes, the number of entries into and time spent in open areas (center platform and uncovered arms), the number of entries into and time spent in the closed arms, and the number of rearing events and head dips. These metrics were automatically quantified using Ethovision computer tracking system. Behaviors associated with post-injury activity included total number of arm changes and total distance traveled. Behaviors associated with changes in emotionality included the percentage of time spent in open arms and number of rearing events.
Statistical Analysis for Behavioral Testing
The four injury groups were paired to independently assess effects of rotational acceleration magnitude and duration on behavioral outcomes. Therefore, the assessment of increasing rotational acceleration magnitude included three factor levels: sham, low magnitude (M1D1 and M1D2), and high magnitude (M2D1 and M2D2). Likewise, the assessment of increasing rotational acceleration duration on behavioral outcomes included three factor levels: sham, short duration (M1D1 and M2D1), and long duration (M1D2 and M2D2). Unconsciousness Time A two factor ANOVA was used to test for significant effects of magnitude or duration. If significant main effects were observed, Bonferroni post hoc analysis was used to test for significant factor-level differences. Morris Water Maze A two-factor mixed effects ANOVA was used to test for significant differences in latency to find the hidden platform and total distance traveled, with trial number as a repeated factor and magnitude or duration as group factors. Group differences in the total number of unsuccessful trials were assessed using two factor ANOVA. Elevated Plus Maze A two factor ANOVA was used to test for significant effects of magnitude or duration in total EPM metrics (across the 5-min duration). If significant main effects were observed, Bonferroni post hoc analyses were used to determine significant differences between factor levels. Additionally, if significant main effects were observed for a specific EPM metric, separate single-factor ANOVA analyses were used to assess group effects on a minute-by-minute basis. Those data are presented in the EPM figures. All results are expressed as mean ± standard error (SE) unless otherwise noted. A p value of <0.05 was considered statistically significant for all tests.
Magnetic Resonance Imaging
At 4 days post-injury, following the completion of behavioral studies, animals were euthanized with an overdose of sodium pentobarbital and perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS through the left cardiac ventricle. Brains were immediately excised from the skull and placed in fixative for 24 h and transferred to PBS for long term storage at 4 °C. A 9.4T Bruker Biospec (Bruker Corporation, Billerica, MA) was employed for MRI procedures using a custom 20-mm diameter loop-gap coil. Brains were submerged in room-temperature susceptibility-matching fluid (Fomblin) during imaging. A multiple-echo spin-echo sequence was used to acquire diffusion-weighted images (TR = 2000 ms, TE = 21 ms, echo separation = 6 ms, number of echoes = 3). Twenty axial slices were obtained with a slice thickness of 0.5 mm and an in-plane resolution of 0.2 mm2. Diffusion weighting was realized with a pair of Stejskal-Tanner trapezoidal gradients with durations (δ) of 6 ms and a separation (Δ) of 10 ms performed along the 12 icosahedral directions42 at a b-value of 1200 s/mm2, along with two non-diffusion weighted images (b = 0).
MRI Data Analysis
The multiple-echo images were first averaged to increase the signal-to-noise ratio, and the diffusion weighted images were smoothed with a non-local means method for improved DTI analysis.98 The diffusion tensor was calculated using a linear least-squares procedure implemented in fMRI Software Library (FSL).49 A tensor-based spatial registration procedure was employed using an iterative process to perform rigid, affine, and diffeomorphic warping using the DTI-TK102 software package, using the average of all brains as the initial template and a final resolution of 0.2 mm3. Maps of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), and mode of the tensor (MO) were derived on a voxel-by-voxel basis and were smoothed using a 0.2 mm3 Gaussian filter to reduce the potential effects of misregistration in the subsequent voxelwise statistical analysis. A two-factor ANOVA was used to identify main effects of magnitude and duration on a voxel-by-voxel basis, using only the injured animals. An F-test was performed to test for main effects for each of the DTI parameters. In the voxel that reached statistical significance, subsequent post hoc t-tests were performed to examine the direction of the relationship between the DTI parameters and either magnitude or duration. A second analysis used a multiple linear regression to test for significant linear relationships between each of the DTI metrics and either magnitude or duration, while controlling for the other parameter. The regression analysis included 12 sham animals for a total of 54 animals. Finally, a conjunction analysis was performed to identify the brain regions that exhibited both a main effect and a significant linear relationship for either magnitude or duration. All statistical procedures used permutation-based inference testing implemented in the software package FSL.43 For all tests, voxels with p < 0.05 (corrected for multiple comparisons) and clusters of greater than 200 contiguous voxels were considered statistically significant.
Results
Injury Biomechanics
All rats survived the experimental protocol without skull fracture. The rotational injury device had highly accurate and reproducible injury biomechanics as measured from the rotational acceleration profiles. Magnitudes of 214 ± 25 and 350 ± 34 krad/s2 (mean ± SD) were obtained for the low (M1) and high (M2) magnitude conditions, respectively, reflecting less than a 7% deviation from the targeted values (Fig. 2). Durations of 1.6 ± 0.4 and 3.4 ± 0.4 ms (mean ± SD) were obtained for the short (D1) and long (D2) duration conditions, respectively, reflecting less than 1% deviation from the targeted values. Moreover, the within-group coefficient of variation for magnitude and duration were 10.5 and 16.5%, respectively, reflecting good reproducibility. There were no significant differences in magnitude or duration between any of the groups within the same level.
Mean and standard deviation values for the four experimental groups focused on two levels of rotational acceleration magnitude (M1 and M2) and two levels of rotational acceleration duration (D1 and D2). Biomechanics for each group were distinct and demonstrated reasonable repeatability, with coefficient of variation of 10.5 and 16.5% for magnitude and duration, respectively
Unconscious Time
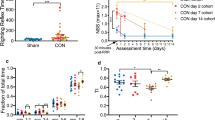
Acute injury severity was assessed as the time duration until return of the righting reflex following the removal of anesthesia (which occurred immediately prior to the rotational acceleration injury). Unconscious time had a significant main effect of head rotational acceleration magnitude (p < 0.0001) but not duration (p = 0.1087). Post-hoc analysis demonstrated that unconscious time in the high magnitude groups (M2) was significantly longer than the low magnitude groups (M1) and shams (Fig. 3), but the low magnitude groups were not significantly different from the sham group. No signs of injury such as pain or discomfort were observed throughout the experimental period.
Duration of unconsciousness following exposure to head rotational acceleration was significantly dependent (p < 0.0001) upon acceleration magnitude but not duration, wherein rats exposed to high magnitude accelerations remained unconscious for a significantly longer time than shams or rats exposed to low magnitude accelerations
Motor Assessment (CN)
The Composite Neuroscore test for motor dysfunction did not reveal a significant effect of magnitude (p = 0.06) or duration (p = 0.17). Composite Neuroscores for the five groups were as follows (mean ± SD): shams 8.9 ± 1.7; M1D1 8.6 ± 1.2; M1D2 8.4 ± 1.7; M2D1 9.9 ± 1.3; M2D2 8.9 ± 1.2.
Spatial Learning (MWM)
Spatial learning was assessed using the Morris Water Maze Visuo-Spatial Learning Paradigm. All groups of rats exposed to the MWM demonstrated the ability to acquire spatial memory as evidenced by a significant decrease (p < 0.05) in the latency and distance travelled with successive trials of each set (Fig. 4). However, there were no significant main effects for magnitude (p = 0.2322) or duration (p = 0.8816) in latency to find the hidden platform. Likewise, there were no significant main effects for magnitude (p = 0.2819) or duration (p = 0.7941) in swim distance to find the hidden platform. Additionally, the total number of unsuccessful trials did not have a significant effect of magnitude (p = 0.9581) or duration (p = 0.9598) (Fig. 5). The mean number of unsuccessful trials across the entire sample for the nine included trials was 2.9 ± 0.2 (mean ± standard error).
Rats were subjected to the Morris Water Maze Visuo-Spatial Learning Paradigm on the first, second, and third days post injury. One set per day consisted of four trials with the platform located in the same location, but moved between days. No statistically significant differences in latency as an effect of increasing magnitude or duration were evident
Total number of unsuccessful trials across all three sets of the Morris Water Maze assessment. Unsuccessful trials that occurred in the first trial of each set were not included because the platform was moved to a new location for each set. The number of unsuccessful trials was not different between shams and any combination of injured groups, indicating a lack of any significant spatial learning deficits
Activity and Emotionality (EPM)
Activity and emotionality were assessed using the Elevated Plus Maze. Both magnitude and duration had significant effects on EPM behaviors. Specifically, significant main effects of duration were evident for a majority of analyzed EPM metrics including total number of arm changes (p = 0.0103), open area entries (p = 0.0098), open area time (p = 0.0097), closed arm entries (p = 0.0275), and closed arm time (p = 0.0091). In general, post hoc tests demonstrated rats exposed to rotational acceleration with longer durations (D2) had abnormal behaviors compared to both the short duration (D1) and sham animals. Specifically, the D2 animals had significantly increased number of arm changes (p < 0.05) (Fig. 6) and open entries (p < 0.05) (Fig. 7) compared to the D1 and sham animals. The D2 animals also had significantly greater open area time (p < 0.05) than the D1 animals (Fig. 8) and significantly greater closed arm entries (p < 0.05) than sham animals (Fig. 9). The D2 animals also had significantly reduced closed arm time (p < 0.05) than the D1 animals (Fig. 10). Additionally, the D1 animals were not significantly different (p = 1.000) from the sham group for any of the measures.
Rats were exposed to the Elevated Plus Maze for 5 min on the second day post injury. Total number of arm changes between closed arms, the center platform, and open arms were counted and used as an assessment of post-injury activity. Results for magnitude/duration are presented as high magnitude/long duration (red), low magnitude/short duration (green), and shams (blue). The number of arm changes was significantly dependent upon duration (p < 0.05) but not magnitude of rotational acceleration. Independent minute-by-minute analyses revealed significantly increased activity (p < 0.05) in the long duration group during the third and fifth minutes of the assessment
Rats were exposed to the Elevated Plus Maze for 5 min on the second day post injury. Total number of open area entries (center platform and open arms) were counted and used as an assessment of post-injury activity and inhibition. Results for magnitude/duration are presented as high magnitude/long duration (red), low magnitude/short duration (green), and shams (blue). The number of open area entries was significantly dependent upon duration (p < 0.05) but not magnitude of rotational acceleration. Independent minute-by-minute analyses revealed significantly increased entries (p < 0.05) in the long duration group during the third and fifth minutes of the assessment
Rats were exposed to the Elevated Plus Maze for 5 min on the second day post injury. Total amount of time spent in the open areas (center platform and open arms) was used as an assessment of post-injury inhibition. Results for magnitude/duration are presented as high magnitude/long duration (red), low magnitude/short duration (green), and shams (blue). The total amount of time spent in open areas was significantly dependent upon duration (p < 0.05) but not magnitude of rotational acceleration. Independent minute-by-minute analyses revealed significantly increased open area time (p < 0.05) in the long duration group during the third and fifth minutes of the assessment
Rats were exposed to the Elevated Plus Maze for 5 min on the second day post injury. Total number of closed arm entries were counted and used as an assessment of post-injury activity and inhibition. Results for magnitude/duration are presented as high magnitude/long duration (red), low magnitude/short duration (green), and shams (blue). The number of closed arm entries was significantly dependent upon duration (p < 0.05) and magnitude (p < 0.05) of rotational acceleration. Independent minute-by-minute analyses revealed significantly increased entries (p < 0.05) in the long duration group during the third and fifth minutes of the assessment
Rats were exposed to the Elevated Plus Maze for 5 min on the second day post injury. Total amount of time spent in the closed arms was used as an assessment of post-injury inhibition. Results for magnitude/duration are presented as high magnitude/long duration (red), low magnitude/short duration (green), and shams (blue). The total amount of time spent in the closed arms was significantly dependent upon duration (p < 0.05) but not magnitude of rotational acceleration. Independent minute-by-minute analyses revealed significantly decreased closed arm time (p < 0.05) in the long duration group during the third and fifth minutes of the assessment
Rats exposed to higher magnitude rotational accelerations demonstrated significantly increased EPM activity including a greater total number of arm changes (p = 0.0103), open arm entries (p = 0.0267), and closed arm entries (p = 0.0026). Specifically, the high magnitude group had a greater total number of arm changes and closed arm entries compared to the low magnitude group and shams. Both low magnitude and high magnitude groups had greater open arm entries than shams. Neither total open area duration or closed arm duration were significantly dependent on magnitude of rotational acceleration. None of the other investigated metrics, including total number of rearings and head dips, were significantly different between groups based on either rotational acceleration magnitude or duration.
Diffusion Tensor Imaging
The effects of rotational injury on the brain microstructure were examined using ex vivo DTI to independently identify effects of magnitude or duration on brain microstructure on a voxel-by-voxel basis. A two-way ANOVA of injured animals revealed several regions with a significant main effect of acceleration magnitude. Specifically, greater rotational acceleration magnitude was associated with a reduction in FA in the right internal capsule and corticospinal tract (Fig. 11). Regions of increased FA were evident in the right amygdala, thalamus, and a portion of the medial cerebral cortex. Greater rotational acceleration magnitude was also associated with a diffuse reduction of MD in the right cortex, white matter, and ventral basal ganglia. In contrast, an increase in the duration of the rotational acceleration was associated with a diffuse reduction in FA throughout much of the brain, but principally located at the interface of the gray and white matter. Increasing rotational acceleration duration did not cause a significant change in MD.
DTI groupwise factorial analysis. Voxels with a significant main effect of either magnitude or duration solely within the animals exposed to injury. Regions of decreased FA in the high magnitude group were evident in portions of the corticospinal tracts and internal capsule, whereas FA was increased in the high magnitude group in the amygdala. MD was increased in the high magnitude group predominantly in portions of the right ventral cortex and white matter. Regions of decreased FA were evident in the high duration groups, predominantly located bilaterally at the interface of gray and white matter in ventral regions. MD did not exhibit any effects of duration. Only voxels with a significant main effect (p < 0.05 and cluster size >200 voxels) for either magnitude or duration in an F-test are shown, with the colors reflecting the p-value and sign of the post hoc T-statistic
A multiple linear regression was subsequently performed to identify brain regions that exhibited a significant relationship between DTI parameters and either magnitude or duration of rotational acceleration, while controlling for the other parameter (Fig. 12). With the inclusion of sham animals, the results were similar to the group-wise analysis, with FA of the amygdala having a significant positive relationship with magnitude, and a large portion of the brain having a significant negative relationship between FA and duration of the rotational acceleration. Only small, sparely located clusters of voxels had a significant association between MD and either magnitude or duration.
DTI regression analysis. Voxels with a significant linear regression with the magnitude or duration measured from each injury are shown for FA and MD. Increasing magnitude was associated with increased FA in the right amygdala and ventral white matter, whereas decreased FA was evident in the right dorsolateral cortex. Increasing duration was significantly associated with a much larger portion of the brain, localized bilaterally in the ventral cortex and white matter and large portions of the basal ganglia. Only few small regions were significant in the regression analysis between MD and either magnitude or duration. The colored regions depict the p value of the T-statistic for either magnitude or duration, while controlling for the other parameter, with a p value threshold of 0.05 (two-tailed) and cluster size threshold of 200 voxels
Finally, a conjunction analysis was performed to identify the regions of the brain that were independently significant in both the group-wise and regression analysis (Fig. 13). The right amygdala was the primary region that exhibited a large cluster with significance in both analyses. The corresponding region of interest analysis (ROI) showed a clear association between FA and the magnitude of rotational acceleration (partial regression R 2 = 0.33). Although the voxelwise analysis did not exhibit a significant association between duration and FA in the amygdala, the ROI analysis did show a strong association with duration that was slightly weaker than magnitude (partial regression R 2 = 0.31) and of the opposite direction. The voxelwise conjunction analysis revealed a strong association between FA and duration of the acceleration impulse as evidenced by the large region of brain tissue having significance in both the group-wise and regression analysis. The region was primarily located at the interface between white and gray matter near the ventral external capsule. Using the FA map to identify white matter (an FA greater than 0.3), 82% of the significant voxels were located within the gray matter. Overall, duration had a more pronounced effect on FA than magnitude, with 6.68 and 0.610% of the total brain voxels significant in the conjunction analysis, respectively.
DTI conjunction analysis. Regions having both a significant main effect and a significant correlation with either magnitude or duration are shown. The amygdala is the most pronounced region exhibiting a strong effect of magnitude. The average FA from the significant voxels was derived from each animal, and the partial regression plots are shown in (a). For duration, the ventral portion of the gray/white matter interface was highly significant, and the mean FA from this region is shown in the partial regression plots in (b). The conjunction analysis revealed most of the FA changes were associated with changes in AD. Other DTI parameters (MD & RD) did not have any significant voxels in the conjunction analysis
To provide further insight into the relationship between DTI and rotational acceleration injury, maps of AD and RD were similarly examined using the conjunction analysis. The amygdala and a small portion of the medial cortex had a significant association with AD and magnitude, and sparse regions of the ventral brain had an association between AD and duration. The diffusion tensors in the amygdala and ventral cortex were composed of linear tensors having a mode (MO) of 0.32 and 0.25, respectively. Neither MD nor RD had any voxels reaching significance in the conjunction analysis.
Discussion
The rotational injury model has previously been reported to be a preclinical model of brain injury that more closely approximates the vast majority of human TBI events, which are characterized as mild.14 Importantly, the rotational acceleration model produces injuries though diffuse mechanisms related to brain shear, which again replicates the majority of mild human injury events involving no skull fracture or blunt trauma.63 The model was shown to be reproducible and accurate,26 with measurable biomechanical data available for the understanding of injury mechanisms and development of preliminary tolerance information.28 Injury biomechanics reported in this study were accurate and reproducible with respect to both magnitude and duration of the rotational acceleration impulse. To place the findings in the context of human mild TBI and concussion, injury biomechanics of the model need to be related to human exposures, wherein scaling from the rat to the human is often based on brain mass, although other proposed relationships account for brain mass, material properties and mechanical tolerance of injured tissues.48 This association was first outlined by Holbourn, and referenced by Ommaya et al.79 and Ommaya and Hirsch,76 which states that the ratio of head rotational acceleration between species is equivalent to the inverse ratio of brain mass to the two-thirds power. Accordingly, head rotational accelerations used in the current study reflect human-equivalent accelerations of 2729 and 4464 rad/s2, which are associated with mild and classical concussion, respectively, according to a clinical concussion scale correlating head rotational acceleration with injury outcomes in humans,32 or associated with less than 10% risk of injury in a more recent study.89 Mild and classical concussion rank as the lowest severity classifications on a six-point scale from mild concussion through severe diffuse axonal injury (DAI). The level of injury commonly results in either very short periods of unconsciousness or no loss of consciousness. However, building on the findings of Gennarelli et al.,32 severity of outcomes were based not only on the magnitude of rotational acceleration, but also the duration. Thus, the current study utilized a factorial design to differentiate effects of magnitude and duration of the rotational acceleration impulse. The measurable biomechanical data facilitates the interpretation of the behavioral findings in the context of human injuries, and also allows per-subject evaluation of the relationship between behavioral outcomes, brain microstructure, and injury biomechanics.
Incorporation of advanced measurement and analysis techniques during sporting events encourages the correlation of the present findings with human exposures. Scaled higher magnitude accelerations from the present study were within one standard deviation of the mean for concussion-producing head impacts during National Football League (NFL) games.82 Scaled acceleration durations from the current study (14-30 ms) were consistent with another study from the same group (approximately 20 ms).97 However, present head accelerations were associated with a low risk (<10%) of concussion according to data derived from college football players using the HITS system,89 but higher than 7 of 13 players that sustained concussion according to another study using the same system.39 Although clinical classification of concussion varied between the studies, each incorporated symptoms that were somewhat more severe than outcomes from the current study. For example, Pellman et al. incorporated cognitive dysfunction, loss of consciousness, and vertigo whereas Rowson et al. included vomiting and balance disturbances. Additionally, scaled rotational accelerations from this study were higher than the mean level for non-concussed high school football players.8 The present study produced significant emotional changes in the absence of cognitive deficit for lower magnitude accelerations, which highlights the complex clinical scenario associated with concussion and the difficulty in determination of biomechanically based injury thresholds and return-to-play guidelines. Nonetheless, this study has provided valuable data highlighting significant changes in activity and emotionality, in the absence of cognitive deficits, and for relatively low magnitude exposures. Given the significant clinical implications associated with changes in emotionality following concussion,37 continued investigations along this line and with similar rotational-based preclinical models are warranted.
It should be noted that human concussive events most often involve head impact resulting in complex head kinematics involving multi-axial linear and rotational accelerations. As such, the present experimental injury model is a necessary simplification of that mechanism, focusing only on pure coronal plane rotational acceleration in the absence of linear acceleration. Nonetheless, the present model remains more biofidelic than other rodent models for TBI that incorporate cortical impact or transient intracranial pressure gradients and allows for in vivo study of rotational biomechanics and tolerance. The focus on rotational acceleration builds on prior experiments incorporating primates33 and computer modeling investigations that demonstrated brain tissue strains primarily attributable (>90%) to the rotational component and not the translational component during more “realistic” head accelerations.101 Given this knowledge, the present model was intentionally limited to coronal plane rotational accelerations to limit the number of experimental variables. As such, the model provides a great deal of control over the rotational acceleration vs. time pulse, which has enabled this study. However, the authors acknowledge possibly important contributions of coupled linear accelerations and more complex multi-axial rotational accelerations. Investigation of these factors remains a focus of continued experimental and computational efforts of this and other laboratories.
In the acute phase, animals exposed to higher magnitude rotational accelerations (M2) took longer to recover from anesthesia than animals exposed to lower magnitude rotational accelerations (M1) and shams. However, changing acceleration duration did not have a similar effect. This finding mirrors the human environment, wherein increasing acceleration magnitude is biomechanically associated with greater acute injury severities,32,82,89 clinically assessed using a number of grading protocols including the Glasgow Coma Score (GCS). Indeed, the GCS is focused on determining the depth of unconsciousness and longer duration unconsciousness is clinically correlated with more severe injury.92 Neither injury magnitude nor duration produced deficits in locomotor function as assessed by the Composite Neuroscore. Although human studies have demonstrated balance impairments following mild injury or concussion, it is likely that the rat is less susceptible to gross motor impairment. For example, even removal of large portions of the motor cortex in the rat produces only deficits in skilled motor movements.73 The current study did not explicitly test highly skilled motor behaviors.
Further dissociation between magnitude and duration was evident in the behavioral testing paradigm conducted during the first week post-injury. Many human studies have demonstrated strong changes in cognitive performance following concussion or mild TBI,16,22,61 although the current study did not reveal deficits in spatial learning following injury as assessed by the Morris Water Maze. Interestingly, a similar rotational acceleration model reported increased MWM escape latencies following exposure to lower magnitude head accelerations with similar acceleration durations.31,100 As discussed previously, scaled biomechanics from the present study were in the range of mild concussion. Therefore, injury severity might have been sufficiently low to avoid producing significant cognitive deficits. Continued research using the model and focusing on higher magnitude/longer duration accelerations will explore this area. Alternatively, the injury distribution may be another important factor in the lack of spatial learning impairments in the present study. Specifically, the dorsal hippocampus was shown to have a more significant role in spatial learning than the ventral hippocampus.71,72 Rodent TBI models such as the controlled cortical impact (CCI) and lateral fluid perfusion (LFP) that cause considerable damage to the hippocampus (more often the dorsal hippocampus), often reveal pronounced deficits in spatial memory and learning. However, our DTI results did not reveal substantial microstructural abnormalities in the hippocampus, which is consistent with the lack of behavioral effects.
The primary behavioral deficits observed in this study were abnormal behaviors in the Elevated Plus Maze, which were strongly associated with duration of rotational acceleration. Specifically, increasing rotational acceleration duration affected EPM measures of activity (i.e., arm changes) and emotionality (i.e., open arm duration), whereas increasing magnitude affected only activity-related metrics. The EPM behaviors reflected increasing activity and exploratory behavior for injuries with longer durations (D2). This is seemingly in contrast to the majority of TBI studies that report decreased activity in the EPM1,4,5,30,51,56,57 compared to uninjured animals. In these studies, which most often employ the CCI or LFP models, decreased activity is commonly attributed to elevated anxiety.13 However, different outcomes in our model may be reflective of different injury mechanisms or severities. Previous studies employing the impact acceleration81 or rotational acceleration87 (sagittal vs. coronal in the current study) injury models reported increased activity in the EPM, similar to this study. Likewise, post-injury time may also play a role in the observed behaviors, since fluid percussion models demonstrate acute increases in open arm time3,91 that resolved91 or reversed3 at chronic time points. Increased exploratory behaviors in the current study may reflect increased impulsivity or disinhibition6,59,69 or decreased anxiety.23,47 The behaviors may also be consistent with depression-related changes,81,83,84 which could be significant given the importance of post-injury depression as a co-morbidity to TBI in the human condition.37,41,54,85
The DTI findings, coupled with reproducible and measureable biomechanics, provide further insight into the relationship between biomechanics and brain injury. Rotational acceleration of the brain is largely believed to result in shearing forces in the brain tissues. The magnitude of shearing is dependent on, among other factors, characteristics of the insult, mass/inertia/geometry of the brain, and material properties of the tissues. Indeed, finite element models of rotational brain injury in the rat29,58 predict greater strains and stress/strain time-based metrics with increasing rotational acceleration and duration. In our model, with the axis of rotation located directly ventral to the base of the brain, we consistently observed DTI abnormalities in right ventral brain regions. This pattern of injury is largely consistent with a finite element model of rotational acceleration of the rat brain,29 in which the ventral brain regions exhibited high strains. The unilateral nature of the changes is likely related to the direction of the rotation (counter-clockwise), but remains to be demonstrated. We also observed considerable microstructural changes at the interface between the gray and white matter.40 In patients with TBI, injury is often observed in this region, and although the reasons for its vulnerability are not entirely known, the microstructural anatomy and orientation of neuronal fibers9,10 may be critical to understand the effects of shearing forces and the resulting injury.
Interestingly, the change in FA with greater injury severity had opposing effects for magnitude and duration. A decrease in FA is often associated with injury and damage, particularly in white matter fibers. However, increased FA is often observed following TBI. It may be related to the temporal effects of injury60 and neuronal swelling.99 However, changes in FA are somewhat ambiguous with respect to the underlying pathology,52 since axonal injury, myelin integrity, and gliosis11 can change FA in complicated ways. It should also be noted that while significant DTI changes were observed in this study, the strength of the relationship (slope of the regression), at this time precludes individual-subject analysis or prediction, which will be important for clinical translation. Moreover, advanced diffusion measures of tissue microstructure50 may also improve pathologic specificity and patient-specific diagnosis. While DTI of fixed tissues allows improvements in image quality and resolution, and anisotropy is preserved through perfusion fixation,93 longitudinal in vivo studies will allow better translation to findings in human studies. The ability to acquire biomechanical data in large populations of athletes12,88 coupled with follow-up MRI has yielded considerable insight into the relationship between injury biomechanics and microstructural brain injury for both concussive65 and subconcussive-level impacts.17 The rotational injury model reported here is likely to have an important role in establishing a causal link between the biomechanics and brain injury in an experimental setting.
The DTI results also shed light on the link between structural brain injury and behavioral outcomes. As evidenced by the multiple regression ROI analysis, the FA of the right amygdala demonstrated a clear association with both magnitude and duration (Fig. 12), although the effect was slightly greater for magnitude in the conjunction analysis (Fig. 13). The prominence of the DTI changes in the amygdala is particularly intriguing given its role in anxiety, which is consistent with the behaviors observed in the EPM. Lesions to the central amygdala increase EPM open arm time in experimental models of stress and anxiety.70,95 Furthermore, even extensive amygdala lesions have been found to be without effect on the MWM or on open field locomotor activity.19 The fact that only the EPM revealed an injury-related difference is likely related to the complex interplay between injury biomechanics and functional brain anatomy, with DTI suggesting a link between the two. On the other hand, an interconnected network of brain regions is involved in controlling anxiety, and the more widespread changes in the brain associated with duration may also contribute to the observed behaviors. Given that the amygdala may be prone to the effects of subconcussive head trauma64 and is intimately involved in post-traumatic stress disorder (PTSD)55 and chronic traumatic encephalopathy (CTE), the current findings are likely to have important translational relevance to these and other disorders related to mild TBI.
Investigation of the opposing effects of rotational acceleration magnitude and duration is not unique to the current study. The most comprehensive series of studies to focus on rotational acceleration as the mechanism of concussion was conducted by Ommaya and collaborators in the 1960s using a primate injury model.77 Those studies recognized that peak rotational acceleration of the head significantly correlated with the production of concussion and also underlined the importance that rotational accelerations occur over a specific duration window. This eventually led to the formation of concussive injury tolerance curves that included head rotational acceleration and duration.75,76,79 However, the diagnosis of concussion in those studies was likely of higher severity than injuries produced in the current study and included the loss of response to a piece of apple, loss of response to ear pinching, apnea greater than 3 s followed by irregular slow respirations, and bradycardia.78 Some of those symptoms (e.g., apnea and bradycardia) are more commonly associated with severe head injury for contemporary diagnoses. Macroscopic pathological outcomes from those specimens included skull fractures, massive epidural and subdural hematomas, subarachnoid hemorrhage, intraventricular hemorrhage, and multiple brain contusions.75 Other studies investigating head injury tolerance due to rotational acceleration have also focused on higher severities including diffuse axonal injury.44,62
Head injury tolerance to rotational acceleration deserves a second look, however, due to increasing concerns about chronic cognitive and emotional deficiencies resulting from mild TBI (i.e., concussion). This is particularly relevant in the setting of contact and collision sports, wherein participants may be exposed to hundreds of exposures over the course of a season.89 Another recent study indicated that a majority of concussions (>90%) resulting from participation in college football were graded as relatively minor according to multiple clinical assessments.66 Recent reports have indicated that chronic cognitive deficiencies may occur in the absence of frank neurologic dysfunction.7 These reports are particularly concerning given the long-term implications of repeated exposures to concussive injuries, including increased risk of neuropsychiatric disorders, cognitive dysfunction, and neurodegenerative disease.36–38 From an injury biomechanics standpoint, these human studies have clearly outlined a gap in the understanding of cumulative head exposure on brain injury tolerance and severity. Studies highlighting neurocognitive decline in athletes following repetitive head impact exposure imply some level of cumulative brain injury that may acutely decrease injury tolerance and/or contribute to chronic cognitive and emotional changes. These issues are being explored from a clinical standpoint.7,36,67 However, preclinical studies incorporating biofidelic experimental models are required to outline biomechanical mechanisms and consequences of cumulative exposures.
The present study provides a valid experimental model and forms the basis for continued examination of rotational acceleration-based injury tolerance for mild TBI and subconcussive exposure. Furthermore, these findings have major translational relevance to our understanding of mechanisms underlying mild TBI in humans, particularly at-risk populations of athletes participating in contact or collision sports67 and military personnel exposed to rotational dynamics from combat blast incidents or other combat related incidents.21 These results have demonstrated a complex relationship between the magnitude and duration of insults contributing to acute cognitive and emotional impairments. Further investigation will continue to define the independent roles of these biomechanical factors and the influence of repetitive insults. The current model is one of a limited number of experimental models capable of this type of investigation due to the realistic biomechanics and the ability to examine the acute response (<1 h) of animals subjected to single or multiple exposures.18,100 Translation of biomechanical metrics from the rodent to the human is another clear benefit of this experimental model. Scaling biomechanical tolerance from the rodent to the human, given consistent cognitive and behavioral outcomes, can help to provide more effective safety enhancement for motor vehicle occupants, athletes, and military personnel and further elucidate the relationship between injury biomechanics, pathophysiology, and behavioral outcomes.
References
Abdel Baki, S. G., H. Y. Kao, E. Kelemen, et al. A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Res 1280:98–106, 2009.
Abel, J. M., T. A. Gennarelli, and H. Segawa. Incidence and severity of cerebral concussion in the rhesus monkey following sagittal plane angular acceleration. In: 22nd Stapp Car Crash Conference, Ann Arbor, MI, 1978, pp. 35–53.
Bao, F., S. R. Shultz, J. D. Hepburn, et al. A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats. J. Neurotrauma 29:2375–2392, 2012.
Baykara, B., F. Cetin, B. Baykara, et al. Anxiety caused by traumatic brain injury correlates to decreased prefrontal cortex VEGF immunoreactivity and neuron density in immature rats. Turk. Neurosurg. 22:604–610, 2012.
Bolouri, H., A. Saljo, D. C. Viano, et al. Animal model for sport-related concussion; ICP and cognitive function. Acta Neurol. Scand. 125:241–247, 2012.
Bortolato, M., S. C. Godar, S. Davarian, et al. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology 34:2746–2757, 2009.
Breedlove, E. L., M. Robinson, T. M. Talavage, et al. Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J. Biomech. 45:1265–1272, 2012.
Broglio, S. P., J. J. Sosnoff, S. Shin, et al. Head impacts during high school football: a biomechanical assessment. J. Athl. Train. 44:342–349, 2009.
Budde, M. D., and J. Annese. Quantification of anisotropy and fiber orientation in human brain histological sections. Front. Integr. Neurosci. 7:3, 2013.
Budde, M. D., and J. A. Frank. Examining brain microstructure using structure tensor analysis of histological sections. Neuroimage 63:1–10, 2012.
Budde, M. D., L. Janes, E. Gold, et al. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 134:2248–2260, 2011.
Camarillo, D. B., P. B. Shull, J. Mattson, et al. An instrumented mouthguard for measuring linear and angular head impact kinematics in American football. Ann. Biomed. Eng. 41:1939–1949, 2013.
Carobrez, A. P., and L. J. Bertoglio. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 29:1193–1205, 2005.
Cassidy, J. D., C. Cancelliere, L. J. Carroll, et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95:S132–S151, 2014.
Cheney, J. A., A. L. Brown, F. M. Bareyre, et al. The novel compound LOE 908 attenuates acute neuromotor dysfunction but not cognitive impairment or cortical tissue loss following traumatic brain injury in rats. J. Neurotrauma 17:83–91, 2000.
Collins, M. W., S. H. Grindel, M. R. Lovell, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA 282:964–970, 1999.
Davenport, E. M., C. T. Whitlow, J. E. Urban, et al. Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J. Neurotrauma 31:1617–1624, 2014.
Davidsson, J., and M. Risling. A new model to produce sagittal plane rotational induced diffuse axonal injuries. Front. Neurol. 2:41, 2011.
Decker, M. W., P. Curzon, and J. D. Brioni. Influence of separate and combined septal and amygdala lesions on memory, acoustic startle, anxiety, and locomotor activity in rats. Neurobiol. Learn. Mem. 64:156–168, 1995.
Denny-Brown, D., and W. R. Russell. Experimental cerebral concussion. J. Physiol. 99:153, 1940.
Elder, G. A., and A. Cristian. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt. Sinai J. Med. 76:111–118, 2009.
Erlanger, D., D. Feldman, K. Kutner, et al. Development and validation of a web-based neuropsychological test protocol for sports-related return-to-play decision-making. Arch. Clin. Neuropsychol. 18:293–316, 2003.
Fan, L. W., L. T. Tien, H. J. Mitchell, et al. Alpha-phenyl-n-tert-butyl-nitrone ameliorates hippocampal injury and improves learning and memory in juvenile rats following neonatal exposure to lipopolysaccharide. Eur. J. Neurosci. 27:1475–1484, 2008.
Fijalkowski, R. J., B. M. Ellingson, B. D. Stemper, et al. Interface parameters of impact-induced mild traumatic brain injury. Biomed. Sci. Instrum. 42:108–113, 2006.
Fijalkowski, R. J., K. M. Ropella, and B. D. Stemper. Determination of low-pass filter cutoff frequencies for high-rate biomechanical signals obtained using videographic analysis. J. Biomech. Eng. 131:054502, 2009.
Fijalkowski, R. J., B. D. Stemper, F. A. Pintar, et al. New rat model for diffuse brain injury using coronal plane angular acceleration. J. Neurotrauma 24:1387–1398, 2007.
Fijalkowski, R. J., B. D. Stemper, F. A. Pintar, et al. Influence of insult duration on unconscious time following concussion. In: Proceedings of the International IRCOBI Conference on the Biomechanics of Impact, Maastricht, The Netherlands, 2007, pp. 161–171.
Fijalkowski, R. J., B. D. Stemper, F. A. Pintar, et al. Biomechanical correlates of mild diffuse brain injury in the rat. Biomed. Sci. Instrum. 43:18–23, 2007.
Fijalkowski, R. J., N. Yoganandan, J. Zhang, et al. A finite element model of region-specific response for mild diffuse brain injury. Stapp Car Crash J. 53:193–213, 2009.
Fromm, L., D. L. Heath, R. Vink, et al. Magnesium attenuates post-traumatic depression/anxiety following diffuse traumatic brain injury in rats. J. Am. Coll. Nutr. 23:529S–533S, 2004.
Gao, W., H. Xu, M. Liang, et al. Association between reduced expression of hippocampal glucocorticoid receptors and cognitive dysfunction in a rat model of traumatic brain injury due to lateral head acceleration. Neurosci. Lett. 533:50–54, 2013.
Gennarelli, T. A., F. A. Pintar, and N. Yoganandan. Biomechanical tolerances for diffuse brain injury and a hypothesis for genotypic variability in response to trauma. In: 47th Proceedings of the Association for the Advancement of Automotive Medicine, 2003, pp. 624–628.
Gennarelli, T. A., and L. E. Thibault. Biomechanics of acute subdural hematoma. J. Trauma 22:680–686, 1982.
Gennarelli, T. A., L. E. Thibault, and A. K. Ommaya. Pathophysiologic response to rotational and translational acceleration of the head. In: 16th Stapp Car Crash Conference, New York, NY, 1972, pp. 296–308.
Gurdjian, E. S., V. L. Roberts, and L. M. Thomas. Tolerance curves of acceleration and intracranial pressure and protective index in experimental head injury. J. Trauma 6:600–604, 1966.
Guskiewicz, K. M., S. W. Marshall, J. Bailes, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57:719–726, 2005; discussion-26.
Guskiewicz, K. M., S. W. Marshall, J. Bailes, et al. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 39:903–909, 2007.
Guskiewicz, K. M., M. McCrea, S. W. Marshall, et al. Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. JAMA 290:2549–2555, 2003.
Guskiewicz, K. M., J. P. Mihalik, V. Shankar, et al. Measurement of head impacts in collegiate football players: relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurgery 61:1244–1252, 2007; discussion 52–53.
Hammoud, D. A., and B. A. Wasserman. Diffuse axonal injuries: pathophysiology and imaging. Neuroimaging Clin. N. Am. 12:205–216, 2002.
Hart, T., J. M. Hoffman, C. Pretz, et al. A longitudinal study of major and minor depression following traumatic brain injury. Arch. Phys. Med. Rehabil. 93:1343–1349, 2012.
Hasan, K. M., D. L. Parker, and A. L. Alexander. Comparison of gradient encoding schemes for diffusion-tensor MRI. J. Magn. Reson. Imaging 13:769–780, 2001.
Hayasaka, S., and T. E. Nichols. Validating cluster size inference: random field and permutation methods. Neuroimage 20:2343–2356, 2003.
Higgens, L. S., and F. Unterharnscheidt. Pathomorphology of experimental head injury due to rotational acceleration. Acta Neuropathol. 12:200–204, 1969.
Higgins, L. S., and R. A. Schmall. Device for investigation of head injury effected by non-deforming head accelerations. In: 11th Stapp Car Crash Conference, Anaheim, CA, 1967, pp. 57–72.
Hirsch, A. E., and A. K. Ommaya. Protection from brain injury: the relative significance of translational and rotational motions of the head after impact. In: 14th Stapp Car Crash Conference, 1970, pp. 144–151.
Hogg, S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 54:21–30, 1996.
Ibrahim, N. G., J. Ralston, C. Smith, et al. Physiological and pathological responses to head rotations in toddler piglets. J. Neurotrauma 27:1021–1035, 2010.
Jenkinson, M., C. F. Beckmann, T. E. Behrens, et al. FSL. Neuroimage 62:782–790, 2012.
Jespersen, S. N., H. Lundell, C. K. Sonderby, et al. Orientationally invariant metrics of apparent compartment eccentricity from double pulsed field gradient diffusion experiments. NMR Biomed. 26:1647–1662, 2013.
Jones, N. C., L. Cardamone, J. P. Williams, et al. Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J. Neurotrauma 25:1367–1374, 2008.
Jones, D. K., T. R. Knosche, and R. Turner. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 73:239–254, 2013.
Kelly, J. P., and J. H. Rosenberg. Diagnosis and management of concussion in sports. Neurology 48:575–580, 1997.
King, P. R., and L. O. Wray. Managing behavioral health needs of veterans with traumatic brain injury (TBI) in primary care. J. Clin. Psychol. Med. Settings 19:376–392, 2012.
Koenigs, M., E. D. Huey, V. Raymont, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat. Neurosci. 11:232–237, 2008.
Kovesdi, E., A. Kamnaksh, D. Wingo, et al. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front. Neurol. 3:111, 2012.
Kwon, S. K., E. Kovesdi, A. B. Gyorgy, et al. Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Front. Neurol. 2:12, 2011.
Lamy, M., D. Baumgartner, N. Yoganandan, et al. Experimentally validated three-dimensional finite element model of the rat for mild traumatic brain injury. Med. Biol. Eng. Comput. 51:353–365, 2013.
Lindemann, S., M. Gernert, M. Bennay, et al. Comparative analysis of anxiety-like behaviors and sensorimotor functions in two rat mutants, ci2 and ci3, with lateralized rotational behavior. Physiol. Behav. 91:551–560, 2007.
Ling, J. M., A. Pena, R. A. Yeo, et al. Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 135:1281–1292, 2012.
Macciocchi, S. N., J. T. Barth, W. Alves, et al. Neuropsychological functioning and recovery after mild head injury in collegiate athletes. Neurosurgery 39:510–514, 1996.
Margulies, S. S., and L. E. Thibault. A proposed tolerance criterion for diffuse axonal injury in man. J. Biomech. 25:917–923, 1992.
Margulies, S. S., L. E. Thibault, and T. A. Gennarelli. Physical model simulations of brain injury in the primate. J. Biomech. 23:823–836, 1990.
McAllister, T. W., J. C. Ford, L. A. Flashman, et al. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology 82:63–69, 2014.
McAllister, T. W., J. C. Ford, S. Ji, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann. Biomed. Eng. 40:127–140, 2012.
McCrea, M., K. M. Guskiewicz, S. W. Marshall, et al. Acute effects and recovery time following concussion in collegiate football players: The NCAA Concussion Study. JAMA 290:2556–2563, 2003.
McCrea, M., K. Guskiewicz, C. Randolph, et al. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J. Int. Neuropsychol. Soc. 19:22–33, 2013.
McCrea, M., C. Randolph, and J. P. Kelly. The standardized assessment of concussion (SAC): manual for administration, scoring and interpretation (2nd ed.). Waukesha, WI: CNS Inc, 2000.
Meyer, J. S., B. J. Piper, and V. E. Vancollie. Development and characterization of a novel animal model of intermittent MDMA (“Ecstasy”) exposure during adolescence. Ann. NY Acad. Sci. 1139:151–163, 2008.
Moller, C., L. Wiklund, W. Sommer, et al. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 760:94–101, 1997.
Moser, E., M. B. Moser, and P. Andersen. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J. Neurosci. 13:3916–3925, 1993.
Moser, M. B., E. I. Moser, E. Forrest, et al. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl. Acad. Sci. USA 92:9697–9701, 1995.
Napieralski, J. A., R. J. Banks, and M. F. Chesselet. Motor and somatosensory deficits following uni- and bilateral lesions of the cortex induced by aspiration or thermocoagulation in the adult rat. Exp. Neurol. 154:80–88, 1998.
Nelson, W. E., J. A. Jane, and J. H. Gieck. Minor head injury in sports: a new system of classification and management. Phys. Sportsmed. 12:103–107, 1984.
Ommaya, A. K., P. Corrao, and F. S. Letcher. Head injury in the chimpanzee. 1. Biodynamics of traumatic unconsciousness. J. Neurosurg. 39:152–166, 1973.
Ommaya, A. K., and A. E. Hirsch. Tolerances for cerebral concussion from head impact and whiplash in primates. J. Biomech. 4:13–21, 1971.
Ommaya, A. K., A. E. Hirsch, E. S. Flamm, et al. Cerebral concussion in the monkey: an experimental model. Science 153:211–212, 1966.
Ommaya, A. K., S. D. Rockoff, and M. Baldwin. Experimental concussion: a first report. J. Neurosurg. 21:249–265, 1964.
Ommaya, A. K., P. Yarnell, and A. E. Hirsch. Scaling of experimental data on cerebral concussion in subhuman primates to concussion threshold for man. In: 11th Stapp Car Crash Conference. Anaheim, CA, 1967, pp. 73–80.
Ono, K., A. Kikuchi, M. Nakamura, et al. Human head tolerance to sagittal impact reliable estimation deduced from experimental head injury using subhuman primates and human cadaver skulls. In: 24th Stapp Car Crash Conference. Troy, MI, 1980, pp. 103–160.
Pandey, D. K., S. K. Yadav, R. Mahesh, et al. Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav. Brain Res. 205:436–442, 2009.
Pellman, E. J., D. C. Viano, A. M. Tucker, et al. Concussion in professional football: location and direction of helmet impacts-Part 2. Neurosurgery 53:1328–1340, 2003; discussion 40–41.
Primeaux, S. D., and P. V. Holmes. Role of aversively motivated behavior in the olfactory bulbectomy syndrome. Physiol. Behav. 67:41–47, 1999.
Ramamoorthy, R., M. Radhakrishnan, and M. Borah. Antidepressant-like effects of serotonin type-3 antagonist, ondansetron: an investigation in behaviour-based rodent models. Behav. Pharmacol. 19:29–40, 2008.
Rapoport, M. J. Depression following traumatic brain injury: epidemiology, risk factors and management. CNS Drugs 26:111–121, 2012.
Roberts, W. O. Who plays? Who sits? Managing concussions on the side-lines. Phys. Sportsmed. 20:66–76, 1992.
Rostami, E., J. Davidsson, K. C. Ng, et al. A model for mild traumatic brain injury that induces limited transient memory impairment and increased levels of axon related serum biomarkers. Front. Neurol. 3:115, 2012.
Rowson, S., J. G. Beckwith, J. J. Chu, et al. A six degree of freedom head acceleration measurement device for use in football. J. Appl. Biomech. 27:8–14, 2011.
Rowson, S., S. M. Duma, J. G. Beckwith, et al. Rotational head kinematics in football impacts: an injury risk function for concussion. Ann. Biomed. Eng. 40:1–13, 2012.
Saatman, K. E., P. C. Contreras, D. H. Smith, et al. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp. Neurol. 147:418–427, 1997.
Shultz, S. R., D. F. MacFabe, K. A. Foley, et al. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behav. Brain Res. 224:326–335, 2011.
Simpson, D. A. Clinical examination and grading. In: Head injury: pathophysiology and management 2nd, edited by P. L. Reilly, and R. Bullock. Great Britain: Hodder Education, 2005, pp. 143–163.
Sun, S. W., J. J. Neil, and S. K. Song. Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains. Magn. Reson. Med. 50:743–748, 2003.
Thibault, L. E., and T. A. Gennarelli. Brain injury: an analysis of neural and neurovascular trauma in the nonhuman primate. In: 34th Annual Proceedings of the Association for the Advancement of Automotive Medicine, 1990, pp. 337–351.
Ventura-Silva, A. P., A. Melo, A. C. Ferreira, et al. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front. Behav. Neurosci. 7:32, 2013.
Versace, J. A review of the severity index. In: 15th Stapp Car Crash Conference. San Diego, CA, 1971, pp. 771–796.
Viano, D. C., and E. J. Pellman. Concussion in professional football: biomechanics of the striking player–Part 8. Neurosurgery 56:266–280, 2005; discussion-80.
Wiest-Daessle, N., S. Prima, P. Coupe, et al. Non-local means variants for denoising of diffusion-weighted and diffusion tensor MRI. Med. Image Comput. Comput. Assist. Interv. 10:344–351, 2007.
Wilde, E. A., S. R. McCauley, J. V. Hunter, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70:948–955, 2008.
Xiao-Sheng, H., Y. Sheng-Yu, Z. Xiang, et al. Diffuse axonal injury due to lateral head rotation in a rat model. J. Neurosurg. 93:626–633, 2000.
Zhang, J., N. Yoganandan, F. A. Pintar, et al. Role of translational and rotational accelerations on brain strain in lateral head impact. Biomed. Sci. Instrum. 42:501–506, 2006.
Zhang, H., P. A. Yushkevich, D. Rueckert, et al. Unbiased white matter atlas construction using diffusion tensor images. Med. Image Comput. Comput. Assist. Interv. 10:211–218, 2007.
Acknowledgments
This work was supported in part by Merit Review Award number I01 RX000380 from the United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development Service Program (PI: FAP), the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin (5520207 to MDB), and the Department of Neurosurgery, Medical College of Wisconsin. The authors acknowledge the considerable contributions of Rachel Chiariello, Natasha Wilkins, and Andrea Winegar.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M Duma oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Stemper, B.D., Shah, A.S., Pintar, F.A. et al. Head Rotational Acceleration Characteristics Influence Behavioral and Diffusion Tensor Imaging Outcomes Following Concussion. Ann Biomed Eng 43, 1071–1088 (2015). https://doi.org/10.1007/s10439-014-1171-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1171-9