Abstract
Applied electric fields (EFs) have previously been presented as a potential method of inducing functional recovery after neural trauma. To date, most of this research has focused on the application of a direct current (DC) stimulus to produce the desired EF and induce neuronal growth. We propose that high duty-cycle alternating current (AC) stimulation is capable of inducing similar EFs within the spinal cord and eliciting a neural response with the added benefits of increased field propagation and lower power consumption. Through ex vivo tissue testing of porcine spinal columns and Xenopus laevis cell cultures, 80% duty-cycle AC stimulation was compared to DC stimulation for efficacy in field generation and induction of neurite growth. Results from ex vivo measurement show that AC stimulation is capable of producing EFs of greater magnitudes over an increased distance in the spinal cord than DC stimulation at the same current magnitude. Furthermore, stimulation of Xenopus laevis neuronal cultures with 80% duty-cycle rectangular waves indicated a significant increase in neurite length as compared to non-stimulated controls and cathodal preference, growth that was statistically similar to DC-stimulated cells. These results suggest high duty-cycle stimulation modalities to be applicable and perhaps preferable to DC stimulation in electrically mediated neuronal therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the National Spinal Cord Injury Statistical Center, Birmingham, AL, the annual incidence of spinal cord injury is 12,000 new cases each year.11 The effect of these injuries is often physically and emotionally devastating, affecting not only the lives of the victims but also of their families. Unfortunately, less than 1% of injured individuals experience complete neurological recovery, with the remainder suffering from an array of symptoms, including paralysis.11 In an effort to increase neurological recovery in afflicted patients after injury, and thereby enhance the quality of life of the individual, numerous modalities have been identified as possible therapeutic measures. One of the investigated treatment modalities has been the application of external electrical fields across the injury site to drive neurite outgrowth through the lesion, reestablishing neural connectivity distal to the injury.
Electric fields (EFs) have long been known to influence neuronal guidance and developmental growth in vivo. Experiments injecting Xenopus laevis embryos with a current sufficient to alter the endogenous blastopore current resulted in numerous developmental abnormalities.8 These findings are further supported by the deformation of chick embryos under similar experimental parameters.7,9 Metcalf et al. expanded on these studies by demonstrating that embryonic EFs establish the rostral–caudal orientation of the central nervous system in developing organisms, and disruption of this field results in deformation of the developing embryo with defects occurring more frequently at the cathode.10 Additional evidence for field-directed development lies in the finding that skin potentials vary across a developing embryo with electrical potential gradients presenting in rostrocaudal and mediolateral orientations.16 On a cellular level, studies conducted using neurons from Xenopus laevis embryos indicate a strong galvanotropic response toward the cathode of an externally applied field with neurons demonstrating preferential growth and turning.6,13
Based on these findings, induced EFs were previously studied as a means for therapeutic neural regeneration, recovered neurological function and histological neurite regrowth through an injury site, with positive results. Borgens et al. applied switched-polarity direct current (DC) EFs across transected spinal cord lesions in guinea pigs and naturally occurring spinal cord injuries in dogs by means of an implanted stimulator and demonstrated functional neurological recovery in both animal sets.1,2 Histological examination of guinea pig spinal tissue revealed increased axonal growth through the injury site toward the cathode of the implanted stimulator.1 The stimulator used in the previous guinea pig and dog studies was later adapted for human implantation as the oscillating field stimulator. A phase one clinical trial of the device indicated marked recovery of neurological function in a number of study participants.15
To date, nearly all research in electrically mediated neuronal guidance has centered on using DC stimulation in the establishment of EFs, but stimulation with alternating current (AC) provides certain benefits for implantable therapeutic devices. Biological tissue is known to have a capacitive component (from cell membranes) and a resistive component. Thus, tissue is often modeled as a series resistor–capacitor circuit in parallel with a resistor. At higher frequencies, the capacitance of the tissue is diminished, decreasing the overall impedance of the tissue. In support of this notion, lower encountered tissue impedances have been reported at increased stimulation frequencies.4 According to Ohm’s law, this reduction in impedance allows better propagation of the injected charge into the biological tissue enabling the establishment of stronger EFs and lower power consumption. In a step toward the use of AC stimulation, Patel et al. demonstrated that it was possible to use pulsatile DC stimulation to produce a cathodal response similar to that of the equivalent time-averaged steady DC field.14 Owing to the additive effect of off duty-cycle waves, we hypothesize that high duty-cycle AC stimulation is capable of inducing therapeutic EFs within the spinal cord and eliciting a neural response with the added benefits of increased field propagation and lower power consumption.
In this study, we compare the establishment of therapeutic EFs utilizing AC and DC stimulation in a bulk, porcine tissue sample approximating that of human cervical vertebrae. We then verify the effectiveness of AC stimulation in establishing neurite outgrowth as compared to DC-stimulated neurons using unstimulated cells as a negative control. This experiment is being conducted for the purpose of advancing the effectiveness of electrically mediated neuronal therapies.
Materials and Methods
Ex Vivo Tissue Testing
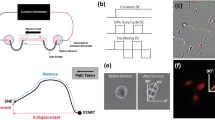
Ex vivo tissue testing provides a comparison of induced EFs from applied AC and DC stimulations of a bulk sample in the context of a therapeutic stimulation device. Five-vertebral segment sections (~140 mm in length) of porcine vertebrae from the lower-cervical/upper-thoracic spine and the surrounding musculature, chosen for its dimensional similarity to human cervical vertebrae, were excised immediately following euthanasia to mitigate postmortem change in tissue impedance.5 To further preserve tissue impedance from fluctuations due to temperature,3 the sample was bathed in a tissue-equivalent saline solution heated to 37 °C. One stainless steel pacemaker electrode (surface area 12.5 mm2) was inserted into the tissue immediately adjacent to the spinous process of the sample’s second vertebral segment (Fig. 1, electrode b), a location similar to the placement of electrodes in the human trial device described by Shapiro et al.15 A second pacemaker electrode was placed in an equivalent position three vertebral segments (~45 mm) inferior to the top electrode (not visible in Fig. 1). Stimulation parameters included 200 μA DC (16 μA/mm2) as in the therapeutic device described above, and 200 μA rectangle waves at 1 kHz and 20 MHz and 80% duty-cycle (pulse width 800 and 40 μs, respectively), to explore higher frequency, high duty-cycle modalities. DC stimulation was via the lower electrode and established by a constant current stimulator with the top electrode serving as electrical ground. AC stimulation was accomplished by utilizing an analog switch controlled by a waveform function generator providing an 80% duty-cycle waveform at the stated frequencies to switch constant current stimulation and ground between the electrodes. This circuit allowed the lower electrode to serve as the stimulating electrode during 80% of the wavelength before switching to electrical ground for 20%. The top electrode did the reverse, thus creating a true biphasic AC stimulus. An ammeter and oscilloscope provided continuous monitoring of amplitude and waveform of the stimuli.
To achieve voltage readings inside the spinal cord, a stainless steel surgical stereotactic device was employed to secure a monopolar silver silver-chloride electrode. By attaching the electrode to an adjustable manipulator arm via a clamp, it was possible to lower the electrode into the sample and record the voltage at 5 mm intervals to a depth of 60 mm (Fig. 2). Time-averaged DC voltage was recorded in the spinal cord through the use of a non-inverting amplifier with a gain of 100 and a voltmeter with input impedance of 2 MΩ. A second monopolar silver silver-chloride electrode placed in the tissue-equivalent saline served as the reference electrode throughout the testing. No filtering was performed. The voltage recorded when the electrode was placed at depth zero (resting on top of the intact spinal cord) was considered the offset voltage of the recording system and was subtracted from each subsequent voltage recording of the trial. We then calculated EF magnitudes by determining the voltage difference between the peak voltage (near the electrode) and minimum appreciable voltage above the stabilized baseline on the plot (when existent) and dividing by the distance between the two points. The distance between the two points was also recorded as the effective field distance. Voltage recordings in the spinal cord were performed six times and averaged.
The recording apparatus employed for ex vivo stimulation and recording. The central, straight recording electrode is shown secured to the stereotactic frame by a stainless steel band around the insulated portion and entering into the spinal canal. This enables controlled vertical manipulation of the electrode through the spinal cord. The top pacemaker lead is also seen looping to the right
Neuronal Cell Culture
Xenopus laevis neuronal cell cultures were employed to determine the effects of AC current stimulation at the cellular level and compare the response to DC-stimulated and non-stimulated cells. This protocol was adapted from those presented by Hinkle et al.6 and Patel and Poo.13 Sexually mature female Xenopus laevis frogs were injected with 700 IUs of human chorionic gonadotropin to induce ovulation. Upon ovulating, the eggs were collected in a 10% Steinberg’s solution (5.8 mM NaCl, 0.04 mM Ca(NO3)2, 0.07 mM KCl, 0.13 mM MgSO4, 0.46 Tris), artificially fertilized, and allowed to progress to Stage 20 of Xenopus embryonic development.12 At this time, the vitelline membrane was removed from the embryos, and the dorsal portion of the embryo was removed and transferred to a 1-mg/mL collagenase solution for 20 min. The neural tube was then dissected from the surrounding tissue and placed in Ca++, Mg++ free Steinberg’s solution for 20 min after which the cells were dissociated in a culture medium consisting of 74% Steinberg’s solution, 20% L-15 media, 2% penicillin–streptomycin, and 4% fetal bovine serum. The dissociated cells were seeded onto polystyrene petri dishes containing 30 mm × 10 mm × 0.15 mm rectangular chambers constructed of No. 1 glass coverslips (Corning) fixed to the petri dish. After seeding, the cells were covered by another coverslip that was affixed to the chamber with high-vacuum grease (Fig. 3). Vacuum grease was also employed to create reservoirs on either side of the chamber for excess media to prevent drying. Agar bridges, consisting of Masterflex pulsatile-pump tubing (Cole-Parmer Instrument Co., Vernon Hills, IL) filled with 0.5 g of agar in 25 mL of Steinberg’s solution, were placed at both ends of the long axis of the chamber. The agar bridges established electrical connectivity between the cell chamber and basins of Steinberg’s solution containing 24 gauge copper wire stimulating electrodes (Fig. 4) while chemically isolating the cells from the electrodes.
Cell culture stimulation was conducted chronologically after the bulk ex vivo tissue testing showed statistically significant similarity in EF production from both 1 kHz and 20 MHz stimulation (see “Results” section). Due to this finding, only 1 kHz, 80% duty-cycle stimulation was tested in cell culture to determine neuronal response to AC stimuli. The 1-kHz 80% duty-cycle AC and DC currents were applied to separate groups of chambers (n = three and four neuronal culture chambers for AC and DC stimulations, respectively). A third group received no electrical stimulus, serving as the control (n = three cultures). Current for each stimulated group was provided via National Semiconductor LM334 constant current sources (Santa Clara, CA). As in the ex vivo tissue testing, the DC stimulus was provided directly from the current source. Accordingly, the AC waveform was accomplished utilizing a waveform generator-controlled, analog-switching circuit to AC and ground between the appropriate electrodes. In this setting, the “cathodal” electrode served as the stimulating electrode for 80% of the wavelength and as electrical ground for 20%, while the “anodal” electrode did the reverse.
To fully test the hypothesis that AC stimulation is capable of inducing preferential neurite growth to a comparable degree as DC stimulation, applied field strengths must be sufficient to induce turning at all. However, variations in chamber, electrode, and agar bridge impedance produce subsequent variability in stimulation-established EFs if current stimulation is kept at constant amplitude across all trials. To enhance the probability of observed preferential neurite growth in this setting and to ensure consistency of EF strength, the current amplitude was adjusted by potentiometer at the onset of each experiment to produce a DC EF of 30 mV/mm across the length of the chamber, within the established effective range for DC-induced turning,6 for both AC- (a time-averaged DC field is made possible by the high duty-cycle waveform) and DC-stimulated scenarios. Cells were stimulated for 4 h, after which stimulation was terminated and the cultures were imaged under 100× magnification with a Zeiss (Thornwood, NY) Axio Observer.Z1 inverted microscope.
Growth was analyzed with vector analysis using Axiovision software from Zeiss Microscopes (Fig. 5). Since this study was conducted with clinical ramifications in mind, the neurites were analyzed to determine longitudinal growth as would be desired to promote neurite excursion through an injury site in therapeutic applications. Neurite length was measured and recorded as a vector from the center of the soma to the neurite terminus. Although this measurement scheme may not measure the entire arc length of individual neurites, it does return clinically relevant information regarding the neurite outgrowth away from soma. The angle of terminal neurite growth was measured along this vector with respect to the long axis of the applied field with the direction facing the cathode defined as 0° and that facing the anode as 180°. The vectors were resolved into their component vectors parallel and normal to the axis of the EF for comparison of cathodal and anodal growth. To measure asymmetry in neurons, the neurite growth asymmetry (NGA) index presented by Patel was employed13: \( NGA = {\frac{{\bar{X}_{\text{c}} - \bar{X}_{\text{a}} }}{{\bar{X}_{\text{c}} + \bar{X}_{\text{a}} }}}, \) where \( \bar{X}_{\text{c}} \) is the average cathodal neurite component and \( \bar{X}_{\text{a}} \) is the average anodal neurite component. NGA indices over 0.10 are considered significantly asymmetric.
Vector analysis of Xenopus laevis neuronal cultures. (a) The approximated growth vector of the neurite. The circle demarcates the soma of the neuron and origin of the vector. The neurite terminus serves as the head of the vector. An angular coordinate system was established with 0° toward the cathodal end of the chamber. As such, (b) serves as the field-parallel component of the vector, whereas (c) is the field-normal component
Results
Ex Vivo Tissue Testing
Figure 6 displays the average recorded voltages of the ex vivo tissue testing as a function of recording depth, with the stimulator residing at ~45 mm. It can be seen that elevated AC-induced voltages, and therefore EFs, were established over a significantly greater distance than DC-induced fields within the spinal cord (p < 0.05). 20 MHz and 1 kHz rectangle currents established EFs over distances greater than 40 mm (41.7 ± 1.0 and 40.8 ± 2.7 mm, respectively), while DC stimulation was effective over a range of 24.2 ± 4.7 mm. The effective field distance at 20 MHz and 1 kHz stimulation was found to be statistically similar (p = 0.78). It should be noted that the effective distance may be even greater for AC stimulation as some of the voltage recordings did not plateau throughout the recording range.
Voltage recordings in stimulated, excised porcine spinal cord as a function of recording depth. The stimulating electrode was placed near 45 mm in all three samples. 200 μA of current was supplied for each stimulation type. Error bars represent the standard error of the data (n = 6 trials for all modalities)
In addition to increased effective distance, AC stimulation of the porcine bulk tissue produced profoundly higher voltages than DC stimulation at various recording depths (Fig. 7). This corresponded with significantly greater calculated EFs in AC stimulation than DC stimulation using the same stimulus current magnitude (Table 1, p < 0.05, student’s t test). Evidence of enhanced EF strength can be seen in Fig. 6 as the increased slope of the voltage curve for both AC waveforms. The average EF established by 200 μA, 20 MHz, 80% duty-cycle stimulation over the previously stated effective field distance (41.7 mm) was found to be 18.3 ± 0.6 μV/mm [mean ± standard error of mean (SEM), n = 6]. The average 1 kHz, 80% duty-cycle, 200 μA AC stimulus-induced field over the effective field distance (40.8 mm) was measured at 15.6 ± 0.9 μV/mm (n = 6). The field strengths of both AC stimulation modalities were found to be statistically similar (p = 0.47). 200 μA of DC stimulation was only able to produce an EF of 11.3 ± 1.3 μV/mm (n = 6) over a reduced effective field (24.2 mm) thereby implying that for the same power consumption, AC stimulation is capable of producing stronger EFs. Post hoc analysis utilizing one-way analysis of variance (ANOVA) and Tukey’s test confirmed that frequency of stimulation indeed had an effect on both length and magnitude of EFs with Tukey’s test showing a significant difference (α = 0.01) between fields induced by both AC stimulation modalities and DC fields with no significant difference between both AC frequencies.
Neuronal Cell Culture
Electrical stimulation of Xenopus laevis neurons by both AC and DC means produced significantly greater neurite outgrowth in direct comparison to non-stimulated controls (p < 0.05) as shown in Table 2. Post hoc analysis of neurite lengths with one-way ANOVA (p = 0.001) and Tukey’s test (α = 0.01) confirmed a statistical difference among stimulated and unstimulated populations with AC- and DC-stimulated neurons showing no statistical difference in average neurite length. Neurons experiencing no external electrical field (study control) produced neurites of 26.2 ± 2.4 μm in length on average (n = 13 neurons, three cultures). Neurons stimulated with 80% duty-cycle rectangle waves, however, exhibited an average neurite length of 53.9 ± 4 μm (n = 21 neurons, three cultures) while DC-stimulated neurons were 59.6 ± 10.2 μm in length on average (n = 16 neurons, four cultures). Furthermore, it has been previously reported that only a subset of stimulated neurons react to externally applied fields.6 In light of this, when disregarding all neurites with process lengths not exceeding two standard deviations of the average non-stimulated neurite length, the average process length of “responding” neurons increases to 89.5 ± 6.8 μm for AC stimulation (12 of 21 neurons responding) and 98.9 ± 11.2 μm DC stimulation (10 of 16 neurons responding). Again, the average neurite lengths of DC and AC responding neurons were found to be statistically similar (p = 0.58).
In evaluating field-parallel vector components of neurites, AC- and DC-stimulated neurons were found to exhibit preferential growth toward the cathode. As seen in Table 3, AC-stimulated cells, on average, grew upwards of 17 μm more toward the cathode than the anode, while DC-stimulated cells demonstrated a cathode–anode growth difference of 9.5 μm toward the cathode (see Fig. 8 for micrographs). In contrast, control cells exhibited a cathode–anode growth difference of less than 2 µm. When evaluating responsive neurons alone, the average cathodal neurite growth component was measured at 78.4 ± 39.6 μm in DC-stimulated cultures and 75.1 ± 14.8 μm in AC-stimulated cultures. Anodal growth was considerably less in both populations (45.2 ± 13.1 μm and 47.6 ± 14.8 μm for DC and AC stimulations, respectively), although statistically significant differences of preferential neurite lengths could not be established utilizing Student’s t test nor through post hoc analysis via ANOVA and Tukey’s test. Nonetheless, DC- and AC-stimulated cells produced NGA indices of 0.27 (DC) and 0.22 (AC) indicating asymmetrical neurite growth, while control cells had an NGA of 0.04 indicating symmetrical growth. When considering only responding neurons, DC- and AC-stimulated cathodal growth components were found to be statistically dissimilar to the control (p < 0.05) with no statistical difference to each other (p = 0.93).
Micrographs of Xenopus laevis cell culture with neurons indicated by an asterisk. (a) Neuron with no external stimulus exhibiting non-preferential growth. (b) Neuron after DC current stimulus. The neuron shows pronounced preferential growth and branching toward the cathode. (c) Neuron after 80% duty-cycle, AC rectangular-wave stimulus also demonstrates preferential cathodal growth. Scale bar for all images is 50 μm. (+) indicates anodal direction, (−) indicates cathodal direction, and arrows represent field-parallel growth direction
Discussion
This study was intended to begin exploration of alternative stimulation modalities, namely high duty-cycle AC waveforms, in the area of electrically driven therapeutic neuronal growth as most research to date has focused primarily on DC stimulation. Stimulation frequencies of 1 kHz and 20 MHz were chosen as representative frequencies of moderate and high frequency AC stimulation within the realm of published tissue impedance data for testing purposes, but we by no means imply that these are the best frequencies to stimulate. As it stands, further research must be conducted to determine the optimal frequency, duty-cycle, and waveform of AC stimulation to maximize both neuronal response and device efficiency. Nonetheless, the results from these tests demonstrate potential for advancement of current therapeutic devices.
Ex vivo testing of porcine spinal column samples revealed that AC stimulation is capable of producing significantly higher EFs in the spinal cord than DC stimulation at the same current magnitudes. In addition to this finding, the effective distance of the applied field was found to be more than 66% greater in AC-stimulated tissue than DC-stimulated tissue. This increase in field strength and distribution is made possible due to the lower encountered tissue impedance at higher stimulation frequencies as described in the “Introduction” of this paper. As frequency rises, the capacitive nature of the Helmholtz layer at the electrode site and cell membranes is diminished. However, different tissues respond to frequency changes at different rates. The differences in decreased impedance among tissue types allow the current supplied by the stimulator to better propagate through the tissue to the spinal cord. This is especially significant in the spinal canal itself. For instance, approximating DC conductivity values at 10 Hz due to the limited availability of DC tissue properties, conductivity of the spinal cord nearly doubles when stimulation frequency is increased from DC to 1 kHz (0.017–0.029 S/m), while the conductivity of cerebrospinal fluid (CSF) and dura remain unchanged (2 and 0.5 S/m, respectively).4 Therefore, according to Ohm’s law, a greater percentage of the current entering the spinal column will reach the spinal cord under AC conditions than DC conditions. Furthermore, although CSF and dura conductivities are unchanged from DC to 1 kHz, the conductivities of both muscle and bone do increase allowing further current propagation into the spinal column.4 With this increase in muscle and bone conductivity, however, comes a concomitant increase in current dispersion to other reduced impedance areas outside the spinal column, limiting the extent of field increase in the target tissue. We are currently conducting in vivo studies to determine the extent of this current dispersion and the electrical field distribution around the stimulation site at increased frequency. Nonetheless, the overall result of these frequency changes is the apparent increased field strength and effective field distance in the spinal cord.
These benefits of AC stimulation may translate into a more effective therapeutic device. Increases in effective field distance, as shown in the bulk tissue trials, conceivably translate to greater therapeutic coverage clinically. At the tested current levels, the AC stimulation modalities could be effective clinically in lesions of about 40 mm in length as compared to lesions of ~24 mm in length by conventional DC stimulation. These distances may be scaled up or down depending on the magnitude of current stimulation. Additionally, it has been shown that neurons in vitro respond more robustly to greater electrical fields.6 It is plausible that neurons in vivo will react in a similar manner to an increase in applied EF provided by an implantable AC-stimulator, possibly resulting in increased functional recovery. Conversely, since greater EFs are established by AC stimulation at equal current magnitudes to DC stimulation, it follows that equal fields can be achieved with less AC stimulation than DC and therefore less power. This decrease in power consumption serves two ends. First, it allows for a decrease in the size of the implantable device due to smaller battery requirements. Secondly, reduced power requirements allow for longer battery life (if battery capacity of the stimulator remains unchanged) and greater treatment periods when implanted. Nevertheless, these potential benefits are contingent on the cellular response of the neurons.
In cell culture stimulation, AC-stimulated Xenopus laevis neurons did respond consistently with DC-stimulated neurons in two key aspects: neurite length and preferential growth. AC- and DC-stimulated neurons had significantly longer neurites than their non-stimulated counterparts, indicating the induction of growth in stimulated cells. More importantly, AC-stimulated cells exhibited preferential cathodal growth under applied fields to a similar degree as DC-stimulated cells. Although statistical significance could not be reached between cathodal and anodal neurite lengths for either population of stimulated cells using the Student’s t test, the NGA index indicated cathodal preference in both DC and AC cultures. No preference was found for non-stimulated control cells. Based on these findings, AC stimulation was found to be as effective as DC stimulation for neuronal guidance in vitro. At this point, however, questions still remain as to the safety of the AC stimulation scheme of the device in vivo. Although DC stimulation of a similar magnitude has proven safe in human trials,15 high frequency stimulation of this type for neuronal recovery has yet to be examined. It is possible that AC stimulation may pose more of a safety concern than DC stimulation due to the rapid switching of EF polarity. This is currently an area of ongoing research.
Conclusion
The results of the cell culture study, in conjunction with the ex vivo tissue data, suggest high duty-cycle AC stimulation to be an attractive alternative to the current DC methods of electrically mediated neuronal therapy. By taking advantage of lower tissue impedances at higher frequencies, implantable devices can be constructed that consume less power while still providing appropriate EFs for neuronal guidance. Conversely, at the same power levels as DC stimulation, these devices would be capable of producing greater EFs, possibly leading to greater neuronal response. Both scenarios present potential improvements to existing stimulation modalities and provide an impetus for further study of AC-stimulating implantable devices in neuronal therapies.
References
Borgens, R. B., A. R. Blight, D. J. Murphy, and L. Stewart. Transected dorsal column axons within the guinea pig spinal cord regenerate in the presence of an applied electric field. J. Comp. Neurol. 250:168–180, 1986.
Borgens, R. B., J. P. Toombs, G. Breur, W. R. Widmer, D. Waters, A. M. Harbath, P. March, and L. G. Adams. An imposed oscillating electric field improves the recovery of function of neurologically complete paraplegic dogs. J. Neurotrauma 16:639–657, 1999.
Chin, L., and M. Sherar. Changes in the dielectric properties of rat prostate ex vivo at 915 MHz during heating. Int. J. Hyperth. 20:517–527, 2004.
Gabriel, S., R. W. Lau, and C. Gabriel. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 41:2251–2269, 1996.
Haemmerich, D., O. R. Ozkan, J. Z. Tsai, S. T. Staelin, S. Tungjitkusolmun, D. M. Mahvi, and J. G. Webster. Changes in electrical resistivity of swine liver after occlusion and postmortem. Med. Biol. Eng. Comput. 40:29–33, 2002.
Hinkle, L., C. D. McCaig, and K. R. Robinson. The direction of growth of differentiating neurons and myoblasts from frog embryos in an applied electric field. J. Physiol. 314:121–135, 1981.
Hotary, K. B., and K. R. Robinson. Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev. Biol. 140:149–160, 1990.
Hotary, K. B., and K. R. Robinson. The neural tube of the Xenopus embryo maintains a potential difference across itself. Dev. Brain Res. 59:65–73, 1991.
Hotary, K. B., and K. R. Robinson. Evidence for a role of endogenous electrical fields in chick embryo development. Development 114:985–996, 1992.
Metcalf, M. E., and R. B. Borgens. Weak applied voltages interfere with amphibian morphogenesis and pattern. J. Exp. Zool. 268:322–338, 1994.
National Spinal Cord Injury Statistical Center (NSCISC). Spinal Cord Injury Facts and Figures at a Glance. Birmingham, AL: National Spinal Cord Injury Statistical Center, 2008.
Nieuwkoop, P. D., and J. Faber. Normal Table of Xenopus laevis (Daudin). New York: Garland Publishing, Inc., 1994.
Patel, N., and M. Poo. Orientation of neurite growth by extracellular electric fields. J. Neurosci. 2:483–496, 1982.
Patel, N. B., and M. Poo. Perturbation of the direction of neurite growth by pulsed and focal electric fields. J. Neurosci. 4:2939–2947, 1984.
Shapiro, S., R. Pascuzzi, K. Roos, M. Groff, S. Purvines, S. Hagy, and P. Nelson. Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J. Neurosurg. Spine 2:3–10, 2005.
Shi, R., and R. B. Borgens. Three dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of the embryonic pattern. Dev. Dyn. 202:101–114, 1995.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Leonidas D Iasemidis oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Graves, M.S., Hassell, T., Beier, B.L. et al. Electrically Mediated Neuronal Guidance with Applied Alternating Current Electric Fields. Ann Biomed Eng 39, 1759–1767 (2011). https://doi.org/10.1007/s10439-011-0259-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0259-8