Abstract
Phenotype transformation of vascular smooth muscle cells (VSMCs) has been reported to be directly influenced by the frequency of mechanical strain. This study explored the effects of different frequencies of mechanical strain on expression of phenotype marker h1-calponin and the possible mechanism. VSMCs were subjected to cyclic strains of 10% elongation at 1 and 2 Hz for 24 h by using a Flexercell strain unit. The protein expression of h1-calponin was assessed by Western blotting and the possible protein kinases involved were evaluated by their specific inhibitor or targeted siRNA ‘knock-down.’ The results showed that cyclic strains modulated the expressions of h1-calponin, phospho-p38, Rac and Rho-guanine nucleotide dissociation inhibitor alpha (Rho-GDIα) in nonlinear frequency-dependent manners. This nonlinear frequency-dependent change of h1-calponin expression could be blocked by a specific p38 inhibitor, SB202190. The changed expression of phospho-p38 induced by the frequencies of cyclic strain was reversed by targeted siRNA ‘knock-down’ of Rac, while enhanced by targeted siRNA ‘knock-down’ of Rho-GDIα. These results suggest that the frequency-dependent expression of h1-calponin under cyclic strain is mediated at least partly by the regulation of Rac and Rho-GDIα expression on the activation of p38 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanical strain has been recognized as an important factor in the regulation of vascular modeling and remodeling.20 At least three kinds of information are included within a cyclic strain loading, i.e., magnitude, frequency, and duration. Up to now, there are only several studies that have been reported about the effects of magnitude and frequency of mechanical strain on different cell types.9,18,31 These studies suggest that strain frequency may play an important role in the pathophysiological processes of cells. Moreover, the frequency-dependent phenotype modulation is also important in the field of vascular tissue engineering, since the control of cell phenotype and function could be used to tailor the properties of tissues in vitro.23,30 Although our previous work revealed that the frequencies of mechanical strain have a great influence on the phenotype transformation of vascular smooth muscle cells (VSMCs),19 it just scratched the surface and more work needs to be done to unveil the nature of this mechanism.
Calponin is a family of actin-associated protein which was first found in chicken gizzard smooth muscle.24 H1-calponin, a number of this family, has been proved to be a specifically differentiated marker in smooth muscle cells, and many studies demonstrated its role in the regulation of smooth muscle contractility.15,16 It has been reported that mechanical strain can regulate expression of h1-calponin in VSMCs.16,19 However, it remains unclear how the mechanical strain induces its expression and then influences vascular remodeling. Therefore, studies on the molecular mechanism of how mechanical strain modulates the expression of h1-calponin might give some lights on its possible function under pulsatile blood flow in vivo.
Mechanical strain can evoke various intracellular signaling pathways, such as Rho family of small GTP-binding proteins, Rho, Rac, and Cdc42, and p38 mitogen-activated protein kinase (MAPK) cascades for example, which could initiate differentiation, migration, and proliferation of VSMCs.12,27 Rho-guanine nucleotide dissociation inhibitor (Rho-GDI), consisting of Rho-GDI-alpha, -beta, and -gamma, is expressed in all cell types and can interact with several Ras-like GTP-binding proteins, including Rho A, Rho B, and Rac to regulate their activities.10 Although various intracellular signals have been identified, for example, p38 MAPK, the activation of which is dependent on the Ras/Rac signal pathway, it is not yet clear whether Rac and Rho-GDIα contribute to the activation of p38 and the differentiation of VSMCs under different strain frequencies.
In order to find out the roles of Rac, Rho-GDIα, and p38 pathway in the frequency-induced VSMC differentiation under mechanical strains, we investigated how different frequencies of mechanical strains affected expression of differentiated VSMC marker h1-calponin, and further studied whether Rac, Rho-GDIα, and p38 MAPK contributed to the signal transduction pathways that mediate cellular responses to the strains of different frequencies.
Materials and methods
VSMCs Isolation and Culture
Primary cultures of VSMCs were isolated from the thoracic aorta of male Sprague–Dawley rats, 250–300 g, by the explant method. Cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco Grand Island, NY) containing 20% fetal calf serum (FCS, Gibco), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified incubator of 95% air and 5% CO2. VSMCs were identified by their characteristic ‘peak and valley’ morphology and more than 98% positive immunostaining of smooth muscle (SM) α-actin monoclonal antibody (Sigma, St. Louis, Mo). VSMCs monolayers were passaged every 3–4 days after trypsinization and were used for experiment from passages 6 to 10.
Application of Mechanical Strain
For the application of mechanical strain, VSMCs were plated on six-well silicone elastomer-bottomed culture plates (Flexcell International, NC) at low and high densities, initial density = 3 × 105 cells and 6 × 105 cells per 9.32 cm2 well, respectively. After 24 h, the media of cells were replaced with 1% FCS/DMEM and VSMCs were then subjected to the cyclic mechanical strain which produced by computer-controlled vacuum (FX-4000T Strain Unit, Flexcell International, NC) as previously described.19 With respect to our current experiments, the following mechanical parameters were applied: frequencies of 1 and 2 Hz at a constant strain magnitude, 10%, and a constant duration, 24 h. Cells cultured on the same kind of plates without stretch loading, i.e., a static group, were considered as a time-matched control.
Western Blotting Analysis
Lysates were harvested for Western blotting as previously described.19 Protein concentration was determined by the Bradford method (Beckman Coulter, DU800, CA). Proteins, 30 μg/lane, were immunoblotted with antibodies against Rac1/2/3 (Cell Signaling Technology, Beverly, Mass), Rho-GDIα (Santa Cruz Biotechnology, Santa Cruz, CA), h1-calponin (Sigma) and phospho-p38 (Thr180/Tyr182, Cell Signaling Technology, Beverly, Mass), respectively. Blots were then stripped and reprobed with antibodies which can recognize GAPDH (Santa Cruz) or total p38 (Cell Signaling) to normalize for equal loading. After incubated with alkaline phosphatase-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA), the signals were detected by nitroblue tetrazolium–bromochloroindolyl phosphate (Bio Basic Inc., Mississauga, Ontario, Canada).
siRNA Transfection
For mRNA ‘knock-down’ studies, Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA) was used for transient transfection of VSMCs with gene-specific siRNA duplexes. Briefly, 2 × 105 cells were seeded into each well of a 6-well plate and cultured to 40–50% confluence. Duplexes of Rac (sense: 5′-CAAA CAGA CGUG UUCU UAAT T-3′; anti-sense: 5′-UUAA GAAC ACGU CUGU UUGC G-3′) and Rho-GDIα (sense: 5′-AGCA CUCU GUGA ACUA CAAdT dT-3′; anti-sense: 5′-UUGU AGUU CACA GAGU GCUC dG-3′) were diluted in RNase-free water to a final concentration of 20 μM. For each well, siRNA stock, 100 pmol, was mixed with 5 μL Lipofectamine 2000 in OPTI-MEM, serum- and antibiotic-free, to a final volume of 800 μL. After 20 min, the siRNA/Lipofectamine 2000 complex was added to the well and incubated with cells for 4 h at 37 °C in a humidified CO2 incubator. Following incubation, media were replaced with 1% FCS/DMEM and cells were allowed to recover for up to 24 h prior to experimentation. The rat-specific siRNA duplexes of Rac and Rho-GDIα were supplied by Shanghai GenePharma Co. Nonsilencing siRNA that does not recognize any known homology to rat genes was also synthesized as a negative control.
Immunocytochemistry
The attached SMCs were fixed in 4% paraformaldehyde for 20 min, permeabilized with 0.4% Triton X-100 for 5 min, then blocked with PBS containing 1% BSA for 30 min. Rho-GDIα was stained with a specific antibody and a FITC-conjugated secondary antibody (Jackson Immunoresearch, PA), and filamentous actin was stained with rhodamine phalloidin (Molecular Probes, NY). The cell was visualized and photographed under a fluorescence microscope (Olympus IX71, Japan).
Inhibition Studies
DMSO (Sigma) was used as a solvent for SB202190 (Sigma), an inhibitor of p38. The confluence cells were incubated with SB202190, 10 μmol/L, for an additional hour before application of mechanical strain12 to enable the compound to penetrate the cells and block the p38 pathway.
Statistical Analysis
All experiments were performed at least in triplicate. All data were presented as means ± SD. Statistical significance was assessed by one-way analysis of variance followed by Student’s t-test. A value of p < 0.05 was considered significant.
Results
Expression of h1-calponin was Dependent on the Frequency of Mechanical Strain and Independent of Cell Density in VSMCs
VSMCs were subjected to 10% elongation mechanical strains at 1 and 2 Hz for 24 h, respectively, and then protein expression of h1-calponin was analyzed by Western blotting. The cells cultured on the same kind of plates without stretch loading were examined as a static control. The result showed that the frequency of strain had a nonlinear dose dependent effect on the expression of h1-calponin. Compared with the static control, only 1 Hz-strain could increase the expression of h1-calponin, while 2 Hz-strain had no remarkable effect (Fig. 1). Furthermore, this frequency-dependent upregulation of h1-calponin was independent of cell density since high- and low-density cultures showed similar expression patterns. These results suggest that the expression level of h1-calponin is related to the specific frequency of strain and independent of cell–cell contacts.
Western blotting for protein expression of h1-calponin at the different strain frequencies. VSMCs were cultured on collagen I-coated elastic membranes and subjected to 10% mechanical strains at 1, 2 Hz and static group (considered as a control) for 24 h, respectively. The cell lysates were prepared and h1-calponin protein levels were evaluated by immunoblotting and quantitated by densitometry. Results are means ± SD. * p < 0.05 vs. the control, # p < 0.05 vs. 1 Hz, n = 3
Mechanical Strain Resulted in a Frequency-dependent Phosphorylation of p38
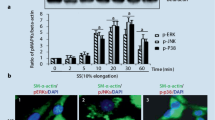
Numerous reports have illustrated that mechanical strain can result in the activation of p38 MAPK with subsequent induction of SM-α-actin, which is the most widely used marker to define the VSMC phenotype.27 To assess the possible role of p38 pathway in different frequencies of mechanical strains, VSMCs in 1% FCS/DMEM were exposed to 10% mechanical strains at 1, 2 Hz and the static control for 24 h, respectively, and then lysed. The lysates were immunoblotted with phospho-specific antibodies for determination of activated p38 MAPK, the phosphorylation of p38 at Thr180/Tyr182. As shown in Fig. 2, the phosphorylation of p38 was activated significantly in a nonlinear frequency-dependent manner. The 1 Hz-strain treatment resulted in a significant increase in p38 MAPK phosphorylation compared with the static control and 2 Hz in VSMCs. There was no distinct up- or downregulation of p38 phosphorylation in 2 Hz-strain compared with the static control. No significant differences were seen in total kinase proteins.
Effects of mechanical strains at different frequencies on the activation of the p38 pathway. VSMCs were treated with 10% elongation mechanical strains at 1 and 2 Hz for 24 h, respectively. Lysates were prepared, matched for protein and immunoblotted with antibodies which can recognize the phosphorylated form or total p38 and the trend of activation of phosphorylated p38 was shown from the corresponding blot. Means ± SD. * p < 0.05 vs. the control, # p < 0.05 vs. 1 Hz, n = 4
Induction of h1-calponin was Counteracted by Blockade of Strain Frequency-induced p38 Activation
It had been demonstrated that the frequency-dependent activation of p38 MAPK was induced by mechanical strain. Then, we examined whether the corresponding pathway mediated the protein expression of h1-calponin using specific inhibitor of p38 MAPK. As shown in Fig. 3, the pretreatment of VSMCs with specific inhibitor of p38 MAPK, SB202190 (10 μmol/L), could significantly reduce the strain-mediated activation of p38 measured by the phosphorylated form. And the frequency strain upregulated protein expression of h1-calponin was also blocked by SB202190. These results demonstrate that p38 MAPK plays an important role in the regulation of h1-calponin expression under the different strain frequencies.
SB202190 inhibited the strain frequency-induced the expression of h1-calponin. Confluent VSMCs were exposed to 10% strains at frequencies of 1 and 2 Hz for 24 h in the presence of 10 μM SB202190 or vehicle (0.1% DMSO-control), respectively. Cell extracts were prepared, matched for protein and immunoblotted with antibodies which can recognize h1-calponin and GAPDH. The protein expression of h1-calponin was found remarkably reduced by the inhibition of SB202190. Means ± SD. * p < 0.05 vs. DMSO control, # p < 0.05 vs. 1 Hz (DMSO), n = 3
Frequency-dependent Regulation of Rac and Rho-GDIα Under Mechanical Strains
In order to determine whether Rac and Rho-GDIα are involved in mechanical strain signal transduction, we performed Western blotting experiments using the specific antibody of Rac or Rho-GDIα. VSMCs in 1% FCS/DMEM were treated with 10% elongation mechanical strains at frequencies of 1, 2 Hz and the static control for 24 h, respectively, and the cell lysates were prepared for Western blotting. As seen in Fig. 4, the mechanical strain regulated the protein expression of Rac and Rho-GDIα in a frequency-dependent manner. The expression of Rac was enhanced under cyclic strain with frequency, and higher expression could be found at 1 Hz-strain. On the contrary, Rho-GDIα expression was inhibited at 1 Hz-strain compared with the static control and 2 Hz, and no obvious difference could be found between 2 Hz-strain and the static control in the Rho-GDIα expression.
Effect of mechanical strains at different frequencies on expression of Rho-GDIα and Rac. VSMCs were cultured on collagen I-coated elastic membranes and subjected to 10% elongation mechanical strains at 1, 2 Hz and static group (considered as a control) for 24 h, respectively. The cell lysates were prepared and the protein expression of Rho-GDIα and Rac was evaluated by immunoblotting and quantitated by densitometry. Means ± SD. * p < 0.05 vs. the control, # p < 0.05 vs. 2 Hz, n = 4
As shown in Fig. 6, after VSMCs were stained by immunocytochemistry, higher expression of Rho-GDIα could be found near the nucleus in the control, which VSMCs maintained a normal shape, and the cell oriented randomly. While after stretched at 1 Hz for 24 h, most of VSMCs changed to a more spindle-shaped morphology, and F-actin aligned corresponded to cellular long axis, which was nearly perpendicular to the stretch direction. The expression of Rho-GDIα decreased, but still some accumulation points of Rho-GDIα were found at the ring of local adhesion.
siRNA-targeted ‘Knock-down’ of Rac or Rho-GDIα Affected Phosphorylation of p38 and h1-calponin Expression
It has been shown that the expressions of Rac and Rho-GDIα could be regulated by mechanical strain. To assess the possible roles of Rac and Rho-GDIα in the modulation of p38 MAPK activation, we used target siRNA to ‘knock down’ their expressions in VSMCs, respectively, and then examined the change of p38 phosphorylation. As shown in Fig. 5, the expression levels of Rac and Rho-GDIα protein were both decreased after siRNA treatment compared with the mock control. The siRNA ‘knock-down’ to Rac decreased p38 phosphorylation while siRNA ‘knock-down’ to Rho-GDIα significantly enhanced the activation of p38 MAPK in VSMCs.
The activation of p38 was enhanced by targeted siRNA knock-down of Rho-GDIα but inhibited by Rac siRNA. VSMCs were transfected with Rac or Rho-GDIα-specific siRNA and the effect on the phosphorylation of p38 was monitored. Histogram shows the relative expression of Rac, Rho-GDIα and phosphorylated p38. Means ± SD. * p < 0.05, ** p < 0.01 vs. the mock, n = 4
As shown in Fig. 6, after transfected with siRNA to Rho-GDIα and stretched at 1 Hz for 24 h, fewer Rho-GDIα expression could be seen in VSMCs, most of which were in a more spindle-shaped morphology, and cells were also nearly perpendicular to the stretch direction.
Immunocytochemistry results of actin and Rho-GDIα. VSMCs of each group were fixed, and actin filament was stained with red rhodamine-phalloidin and Rho-GDIα was stained with an anti-Rho-GDIα antibody and green FITC-IgG, respectively. The a and b showed that Rho-GDIα accumulated at the ring of focal adhesion. Photographs were taken with the fluorescence microscope. Bar = 100 μm, and the arrow indicates the radial direction
As seen in Fig. 7, VSMCs transfected with siRNA to Rho-GDIα showed an increase in the expression of h1-calponin compared with the mock transfected control, while transfected with siRNA to Rac resulted in decreasing of h1-calponin expression. These results indicated that Rac upregulation and Rho-GDIα downregulation were both necessary for the activation of p38 MAPK and the induction of VSMC differentiated marker, h1-calponin, which may contribute to the induction of the differentiated VSMC phenotype.
Rho-GDIα specific siRNA transfection increased the expression of h1-calponin while Rac siRNA did contrarily. VSMCs were transfected with Rac or Rho-GDIα specific siRNA and the effect on the expression of h1-calponin was monitored. Histogram shows the relative expression of h1-calponin. Means ± SD. * p < 0.05, ** p < 0.01 vs. the mock, n = 4
Discussion
VSMC phenotype transformation is regarded as an important event during the progress of vascular diseases.3,17 Understanding the mechanism of VSMC differentiation will add to the foundation for elucidating VSMC-related diseases such as atherosclerosis, restenosis, and asthma. Mechanical strain plays an important role in regulating VSMC phenotype. The effect of mechanical strain on the phenotypic state of VSMCs is regarded as an important field of investigation.20 It is generally accepted that the magnitude of applied strain seriously influenced cell responses in several cell lines,22,26 and numerous studies have shown that the mechanical strain can induce the expression of smooth muscle markers in VSMCs.16,27 However, the responses of VSMCs under the different strain frequencies have been less well characterized. We have reported that the frequency of cyclic strain could affect phenotype of VSMCs, which suggested that apart from the amplitude, the frequency may play an important role in VSMC phenotype alterations in the sense of vascular diseases.19 Here we explored the potential role of Rac and its negative regulator Rho-GDIα in frequency of cyclic strain-induced VSMC differentiation.
In our current study, the strain treatment of VSMCs modulated protein expression of h1-calponin, which indicating the differentiation of VSMCs, in a frequency-dependent manner. The mechanical strain at frequency of 1 Hz could increase protein expression of h1-calponin compared with the static control, while the 2 Hz-strain had no remarkable effect. The frequency-dependent regulation of h1-calponin was independent of cell density since high- and low-density cultures showed similar expression patterns. These results suggest that the expression level of h1-calponin is more sensitive under a specific frequency of cyclic mechanical strain, and this sensitivity is independent of cell–cell contacts. We can also infer from these results that the frequency is an important parameter in mechanical strain.
Numerous reports have been proposed about intracellular signaling mechanisms that strain can regulate cells phenotype. One model pathway illustrated that stretch could result in the activation of the p38 MAPK with subsequent modulation of cell morphology,13 and the induction of SM-α-actin.27 Other models pointed out that the expression of SM22α, desmin, and tropomyosin was dependent on ERK phosphorylation.29 Previous researches have demonstrated that mechanical strain could resulted in a rapid time-dependent activation of p38 MAP kinase, and returned to baseline in 1 h.12,19 Our data here indicated that using the same amplitude of mechanical strain, the p38 activity was induced in a nonlinear frequency-dependent pattern which was consistent with the frequency-dependent expression of h1-calponin. SB202190 could significantly reduce the strain-mediated activation of p38 and upregulate expression of h1-calponin. These findings demonstrate that p38 pathway is crucial in the long-term frequency-dependent phenotype modulation of VSMCs. Interestingly, here we found that without serum-starving prior to the experiment, VSMCs in 1% FCS/DMEM applied to the mechanical strain at 1 Hz could induce a sustained increase phosphorylation of p38 compared with the static control and 2 Hz at 24 h, which was similar to that Wang et al. 28 and Brown et al.4 had reported about the phosphorylation of Akt. Thus, we hypothesize that a certain quantity of serum in medium might conduce to prolonging the duration of p38 activation in VSMCs under mechanical strain. Further studies are needed to explore the complicated modulation mechanism.
Recent findings have implicated the Rho-proteins, including Rac, Rho, and Cdc42 et al., as key regulators of many vascular diseases such as angiogenesis. They can modulate a diversity of cellular processes, including vascular permeability, extracellular matrix remodeling, migration, proliferation, morphogenesis, and survival.5,6 Rac is one of the Rho-family molecules involved in the control of the actin cytoskeleton in response to various signals,14 and it is also an important modulator of cell differentiation.1,11,32 The Rho-GDI family is an inhibitory exchange factor regulator for members of the Rho subfamily, which binds preferentially to the GDP-bound form of the GTPase.8 It is reported to be a regulator for cell migration,21 and could most strongly inhibited the transcriptional activity of the skeletal α-actin gene during muscle differentiation.25 In this regard, our current data indicated that mechanical strain could regulate the protein expression of both Rac and Rho-GDIα in a frequency-dependent manner. VSMC transfection with siRNA to Rho-GDIα resulted in p38 activation and an evident increasing expression of h1-calponin while siRNA to Rac did contrarily. These results suggest that the upregulation of h1-calponin at 1 Hz might need the increasing of Rac and decreasing of Rho-GDIα expression, and further indicate that the frequency-dependent h1-calponin expression under mechanical strain is related to the frequency-dependent regulation of Rac and Rho-GDIα.
Rac has been reported to regulate the activity of c-Jun NH2-terminal kinase and p38 MAPK in COS-7 cells and VSMCs with or without the strain treatment.7,12 Furthermore, Arozaren et al.2 have shown that retaining Cdc42 in its GDP-bound state by overexpressing Rho-GDI can inhibit Ras-GRF-mediated MAPK activation. Based on these findings, we hypothesized that Rho-GDIα might be an upstream regulator which could influence the activation of p38 through Rho family molecule under mechanical cyclic strain. In order to confirm this, we subsequently used siRNA to knock down the expression of Rac and Rho-GDIα in VSMCs, and examined the activity of p38. As siRNA transfection blocked Rho-GDIα expression in VSMCs and the activation of p38 was significantly elevated, and blocking Rac inhibited on the contrary. The results suggest that mechanical strain at 1 Hz activates the p38 pathway probably through increasing of Rac expression and Rho-GDIα deceasing. Further study is still needed about the interaction between Rac and Rho-GDIα in the different frequencies of cyclic strain-induced VSMC phenotype transformation.
How to control the phenotype transformation of VSMCs and regulate the structure and function of tissue-engineered blood vessel more similar to normal in vitro, is still a difficult mission in vascular tissue engineering. In this study, we explored the influence of Rac and Rho-GDIα in frequency of cyclic strain-induced VSMC phenotype transformation, and tried to control the phenotype of VSMCs with RNAi. There may be an appropriate frequency for cardiovascular system in vivo, which could protect the contractile phenotype of VSMCs and against transforming to synthetic phenotype abnormally, keep the stable structure and function of vascular well, and slow down the process of atherogenesis. Our findings about the importance of frequency of cyclic strain and Rho-GDIα in VSMC phenotype transformation will help to understand the “Stress-Remodeling” process, and, though still many problems should be resolved, might help for the vascular tissue engineering.
In summary, the main results reported in this study demonstrate that mechanical cyclic strain can upregulate the expression of Rac and downregulate its negative regulator Rho-GDIα in a nonlinear frequency-dependent pattern, then cause the activation of p38 pathway followed by increasing expression of h1-calponin, which marked VSMC differentiation. As some Rho-GDIα was prone to gathering at local adhesion, further studies are needed to better elucidate the effect of Rho-GDIα on VSMCs under the different strain frequencies. These future endeavors may lead to new breakthroughs in the effective prevention and control of vascular disorders.
References
Akhtar N., C. H. Streuli. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J. Cell Biol. 173:781–793, 2006 doi:10.1083/jcb.200601059
Arozarena I., D. Matallanas, P. Crespo Maintenance of CDC42 GDP-bound state by Rho-GDI inhibits MAP kinase activation by the exchange factor Ras-GRF evidence for Ras-GRF function being inhibited by Cdc42-GDP but unaffected by CDC42-GTP. J. Biol. Chem. 276:21878–21884, 2001 doi:10.1074/jbc.M011383200
Bochaton-Piallat M.-L., P. Ropraz, F. Gabbiani, G. Gabbiani. Phenotypic heterogeneity of rat arterial smooth muscle cell clones: implications for the development of experimental intimal thickening. Arterioscler Thromb. Vasc. Biol. 16:815–820, 1996
Brown D. J., E. M. Rzucidlo, B. L. Merenick, R. J. Wagner, K. A. Martin, R. J. Powell. Endothelial cell activation of the smooth muscle cell phosphoinositide 3-kinase/Akt pathway promotes differentiation. J. Vasc. Surg. 41:509–516, 2005 doi:10.1016/j.jvs.2004.12.024
Bryan B. A., P. A. D’Amore. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol. Life Sci. 64:2053–2065, 2007 doi:10.1007/s00018-007-7008-z
Cernuda-Morollón E., A. J. Ridley. Rho GTPases and leukocyte adhesion receptor expression and function in endothelial cells. Circ. Res. 98:757–767, 2006 doi:10.1161/01.RES.0000210579.35304.d3
Coso O. A., H. Teramoto, W. F. Simonds, J. S. Gutkind. Signaling from G protein-coupled receptors to c-Jun kinase involves beta gamma subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J. Biol. Chem. 271:3963–3966 1996 doi:10.1074/jbc.271.8.3963
Hori Y., A. Kikuchi, M. Isomura, M. Katayama, Y. Miura, H. Fujioka, K. Kaibuchi, Y. Takai. Post-translational modifications of the C-terminal region of the rho protein are important for its interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene 6:515–522, 1991
Kaspar D., W. Seidl, C. Neidlinger-Wilke, A. Beck, L. Claes, A. Ignatius. Proliferation of human-derived osteoblast-like cells depends on the cycle number and frequency of uniaxial strain. J. Biomech. 35:873–880, 2002 doi:10.1016/S0021-9290(02)00058-1
Kasper B., N. Tidow, D. Grothues, K. Welte. Differential expression and regulation of GTPases (RhoA and Rac2) and GDIs (LyGDI and RhoGDI) in neutrophils from patients with severe congenital neutropenia. Blood 95:2947–2953, 2000
B. A. Kerr, T. Otani, E. Koyama, T. A. Freeman, M. Enomoto-Iwamoto. Small GTPase protein Rac-1 is activated with maturation and regulates cell morphology and function in chondrocytes. Exp. Cell Res. 314: 1301–1312 (2008) doi:10.1016/j.yexcr.2007.12.029
Li C., Y. Hu, G. Sturm, G. Wick, Q. Xu. Ras/Rac-dependent activation of p38 mitogen-activated protein kinases in smooth muscle cells stimulated by cyclic strain stress. Arterioscler Thromb. Vasc. Biol. 20:E1–E9, 2000
Li W., Q. Chen, I. Mills, B. E. Sumpio. Involvement of S6 kinase and p38 mitogen activated protein kinase pathways in strain-induced alignment and proliferation of bovine aortic smooth muscle cells. J. Cell Physiol. 195:202–209, 2003 doi:10.1002/jcp.10230
Moldovan L., K. Mythreye, P. J. Goldschmidt-Chermont, L. L. Satterwhite. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc. Res. 71:236–246, 2006 doi:10.1016/j.cardiores.2006.05.003
Morgan K.G., S. S. Gangopadhyay. Invited review: cross-bridge regulation by thin filament-associated proteins. J. Appl. Physiol. 91:953–962, 2001
Morrow D., A. Scheller, Y. A. Birney, C. Sweeney, S. Guha, P. M. Cummins, R. Murphy, D. Walls, E. M. Redmond, P. A. Cahill. Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am. J. Physiol. Cell Physiol. 289:C1188–C1196, 2005 doi:10.1152/ajpcell.00198.2005
Neuville P., A. Geinoz, G. Benzonana, M. Redard, F. Gabbiani, P. Ropraz, G. Gabbiani. Cellular retinol-binding protein-1 is expressed by distinct subsets of rat arterial smooth muscle cells in vitro and in vivo. Am. J. Pathol. 150:509–521, 1997
Nishimura K., W. Li, Y. Hoshino, T. Kadohama, H. Asada, S. Ohgi, B. E. Sumpio. 2006 Role of AKT in cyclic strain-induced endothelial cell proliferation and survival. Am. J. Physiol. Cell Physiol. 290:C812–C821 doi:10.1152/ajpcell.00347.2005
Qu M. J., B. Liu, H. Q. Wang, Z. Q Yan, B. R. Shen, Z. L. Jiang. Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J. Vasc. Res. 44:345–353, 2007 doi:10.1159/000102278
Riha G. M., P. H. Lin, A. B. Lumsden, Q. Yao, C. Chen. Roles of hemodynamic forces in vascular cell differentiation. Ann. Biomed. Eng. 33:772–779, 2005 doi:10.1007/s10439-005-3310-9
Santos M. F., S. A. McCormack, Z. Guo, J. Okolicany, Y. Zheng, L. R. Johnson, G. Tigyi. Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J. Clin. Invest. 100:216–225, 1997 doi:10.1172/JCI119515
Sotoudeh M., Y. S. Li, N. Yajima, C. C. Chang, T. C. Tsou, Y. Wang, S. Usami, A. Ratcliffe, S. Chien, J. Y. Shyy. Induction of apoptosis in vascular smooth muscle cells by mechanical stretch. Am. J. Physiol. Heart Circ. Physiol. 282: H1709–H1716, 2002
Stegemann J. P., R. M. Nerem. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann. Biomed. Eng. 31:391–402, 2003 doi:10.1114/1.1558031
Takahashi K., K. Hiwada, T. Kokubu. Isolation and characterization of a 34,000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem. Biophys. Res. Commun. 141:20–26. 1986 doi:10.1016/S0006-291X(86)80328-X
Takano H., I. Komuro, T. Oka, I. Shiojima, Y. Hiroi, T. Mizuno, Y. Yazaki. The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell Biol. 18:1580–1589 1998
Tang L., Z. Lin, Y. M. Li. Effects of different magnitudes of mechanical strain on osteoblasts in vitro. Biochem. Biophys. Res. Commun. 344:122–128. 2006 doi:10.1016/j.bbrc.2006.03.123
Tock J., V. Van Putten, K. R. Stenmark, R. A. Nemenoff. Induction of SM-alpha-actin expression by mechanical strain in adult vascular smooth muscle cells is mediated through activation of JNK and p38 MAP kinase. Biochem. Biophys. Res. Commun. 301:1116–1121 2003 doi:10.1016/S0006-291X(03)00087-1
Wang H. Q., L. X. Huang, M. J. Qu, Z. Q. Yan, B. Liu, B. R. Shen, Z. L. Jiang. Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothelium 2006 13:171–180 doi:10.1080/10623320600760282
Wang J., H. Chen, A. Seth, C. A. McCulloch. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 285:H1871–H1881, 2003
Wille J. J., E. L. Elson, R. J. Okamoto. Cellular and matrix mechanics of bioartificial tissues during continuous cyclic stretch. Ann. Biomed. Eng. 34:1678–1690. 2006 doi:10.1007/s10439-006-9153-1
Wolf A., B. Ackermann, J. Steinmeyer. Collagen synthesis of articular cartilage explants in response to frequency of cyclic mechanical loading. Cell. Tissue. Res. 327:155–166 2007 doi:10.1007/s00441-006-0251-z
Woods A., G. Wang, H. Dupuis, Z. Shao, F. Beier. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J. Biol. Chem. 282:23500–23508, 2007 doi:10.1074/jbc.M700680200
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China, Nos. 30570459 and 10732070.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ming-Juan Qu and Bo Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qu, MJ., Liu, B., Qi, YX. et al. Role of Rac and Rho-GDI Alpha in the Frequency-dependent Expression of h1-calponin in Vascular Smooth Muscle Cells under Cyclic Mechanical Strain. Ann Biomed Eng 36, 1481–1488 (2008). https://doi.org/10.1007/s10439-008-9521-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9521-0