Abstract
Development of in vitro models of native and injured vasculature is crucial for better understanding altered wound healing in disease, device implantation, or tissue engineering. Conditions were optimized using polyethyleneteraphalate transwell filters for human aortic endothelial cell (HAEC)/smooth muscle cell (HASMC) co-cultures with divergent HASMC phenotypes (‘more or less secretory’) while maintaining quiescent HAECs. Resulting HASMC phenotype was studied at 48 and 72 h following co-culture initiation, and compared to serum and growth factor starved monocultured ‘forced contractile’ HASMCs. Forced contractile HASMCs demonstrated organized α-smooth muscle actin filaments, minimal interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1) secretion, and low intracellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and tissue factor expression. Organization of α-smooth muscle actin was lost in ‘more secretory’ HASMCs in co-culture with HAECs, and IL-8 and MCP-1 secretion, as well as ICAM-1, VCAM-1, and tissue factor expression were significantly upregulated at both time points. Alternately, ‘less secretory’ HASMCs in co-culture with HAECs showed similar characteristics to forced contractile HASMCs at the 48 h time point, while by the 72 h time point they behaved similarly to ‘more secretory’ HASMCs. These co-culture systems could be useful in better understanding vascular healing, however there remain time constraint considerations for maintaining culture integrity/cell phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development of in vitro endothelial cell (EC)/vascular smooth muscle cell (SMC) co-culture systems is crucial to better understand EC/SMC cross-talk and model vascular healing. In a healthy, mature blood vessel, SMCs exhibit a latent differentiated contractile phenotype, characterized by expression of several smooth muscle specific contractile proteins, contractile agonist receptors, and signaling molecules, as well as the lack of migration, proliferation, and extracellular matrix (ECM) protein synthesis.9 This phenotype allows SMCs to perform their primary function: contraction to aid in vessel tone regulation, blood pressure, and flow.24 During angiogenesis, or following loss of an intact EC monolayer (due to injury, device implantation, or disease states), SMCs undergo a shift to a proliferative and synthetic secretory phenotype32 characterized by enriched endoplasmic reticulum, increased ECM synthesis, reduced levels of actin and myosin (and thus, contraction), increased proliferation,1 induction of migration, elaboration and degradation of ECM proteins, growth factor secretion, and expression of cell adhesion molecules.18
Despite this widespread knowledge of SMC bidirectional phenotypic modulation, cell culture systems to model this behavior, and the resulting interactions with ECs, are limited. The primary limitation is the induction of a secretory SMC phenotype during in vitro culture, and the inability to induce and maintain a quiescent SMC phenotype in in vitro EC/SMC co-culture conditions. It is well known that SMC growth can be reversibly arrested in vitro by removing serum under defined conditions.19 However, ECs require serum to grow, survive, and maintain a quiescent phenotype. Thus, a balance must be found to induce contractility in SMCs while maintaining the desired EC phenotype. Because of this balance, SMCs in co-culture with ECs are somewhere along the spectrum from fully secretory to fully contractile, however truly contractile SMCs cannot be co-cultured in vitro with current technology.
Several different co-culture systems have been developed, including direct co-culture of ECs over SMCs,12 culturing ECs or SMC with conditioned media from the other cell type,2,37 microcarrier cultures of ECs with SMCs,7,16 and EC culture on collagen gels containing SMCs.33,38 Also, several groups have developed co-culture systems wherein ECs and SMCs are grown on opposite sides of a thin permeable transwell membrane with 0.4 μm pores.4,5,11,22,23,26 The later co-culture system has several benefits, including correct luminal/abluminal EC/SMC orientation, and the ability to easily and quickly separate ECs from SMCs for assay. Also, the short distance (∼10 μm) between EC and SMC layers allows for contact between ECs and SMCs by formation of cytoplasmic projections from SMCs which penetrate the pores in the membrane.10,11 For the work described herein, specific modifications and media formulations have been developed and used in a transwell membrane EC/SMC co-culture system.
The major caveat to EC/SMC co-culture systems is the inability to control or modulate SMC phenotype, specifically to induce a contractile SMC phenotype and maintain a quiescent EC monolayer at the same time. In fact, in the vast majority of co-culture systems used, SMCs are assumed to be in a secretory state based on culture conditions, and very little characterization of SMC phenotype is performed. Co-culture with secretory SMCs alters EC phenotype in a number of ways. Several groups have found that static co-culture of ECs with SMCs induced upregulation of EC tissue factor,37 ICAM-1, VCAM-1, and E-selectin expression,26 inducement of growth-related oncogene-α (GRO-α) and MCP-14 secretion, and upregulation of vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF)-AA, PRGF-BB, and transforming growth factor-β secretion.12 In addition, SMCs in co-culture also produce VEGF, resulting in more basic fibroblast growth factor (bFGF) release from ECs, in turn leading to further SMC migration and proliferation, and the reinforcement of a SMC secretory phenotype.6 In addition, co-culture with SMCs has been shown to prime EC responses to tumor necrosis factor-α and thus enhanced lymphocyte adhesion.26
While much data has been gathered on the influence of secretory SMCs on EC phenotype, and the functional consequences of secretory SMCs on EC behavior, far less is understood about cross-talk between quiescent ECs and contractile SMCs, and the functional consequences of their interactions on interaction with blood cells. In addition, to the best of our knowledge, there has been no published data on the use of complimentary EC/SMC co-culture systems with divergent SMC phenotypes. Thus, the aim of this work was to create two alternative co-culture techniques to push SMCs toward a ‘more or less secretory’ phenotype, while maintaining a quiescent EC monolayer. The resulting co-culture systems have been examined to confirm phenotype. These systems have since been used for an in vitro model of vascular healing in the presence of biomaterials and have been presented in a separate publication, examining how SMC phenotype impacts EC interactions with biomaterial activated leukocytes.27 While the co-culture techniques developed herein have timeframe and use limitations, they provide an important step toward better understanding EC/SMC cross-talk and the functional consequences of their interactions. Understanding this complex biological system is crucial for development of cardiovascular devices such as stents, which integrate into the vessel without resulting in in-stent restenosis, and tissue engineered vascular grafts, which mimic a native vessel.
Materials and Methods
Endothelial Cell Culture
Primary passage cryopreserved human aortic endothelial cells (HAECs) (Clonetics, Walkersville, MD) were maintained in culture in MCDB 131 media (Mediatech Cellgro) with 5% (v/v) fetal bovine serum (FBS) containing 1% (v/v) HyQ Penicillin-Streptomyocin (Pen-Strep) (Mediatech Cellgro), 1% (v/v) l-glutamine, 1 μg/mL hydrocortisone (Sigma), 2 ng/mL bFGF (PeproTech), 10 ng/mL epidermal growth factor (EGF) (Gibco), 2 ng/mL insulin-like growth factor-1 (IGF-1) (Gibco), 1 ng/mL VEGF (Sigma), and 50 μg/mL ascorbic acid (Sigma) (termed ‘complete HAEC media’). HAEC were maintained in culture in complete HAEC media using standard technique.15
Smooth Muscle Cell Culture
Primary passage cryopreserved human aortic smooth muscle cells (HASMCs) (Clonetics) were maintained in culture in MCDB 131 media (Mediatech Cellgro) with 5% (v/v) FBS containing 1% (v/v) HyQ Pen-Strep (Mediatech Cellgro), 1% (v/v) l-glutamine, 2 ng/mL bFGF (PeproTech), 0.5 ng/mL EGF (Gibco), and 5 μg/mL insulin (Gibco) (termed ‘complete HASMC media’). HASMC were maintained in culture in complete HASMC media using standard technique.13 For monoculture experimentation, HASMCs were cultured in either MCDB 131 media (Mediatech Cellgro) with 5% (v/v) FBS containing 1% (v/v) HyQ Pen-Strep (Mediatech Cellgro), 1% (v/v) l-glutamine, 2 ng/mL bFGF (PeproTech), 0.5 ng/mL EGF (Gibco) (termed ‘secretory HASMC media’), or secretory HASMC media lacking FBS, bFGF, and EGF (termed ‘forced contractile HASMC media’), each for 48 h prior to assay. Both secretory HASMC media and forced contractile HASMC media were utilized in our co-culture systems as well, as described below.
Endothelial Cell/Smooth Muscle Cell Transwell Co-Cultures
Transwell filters made of polyethyleneterephthalate (PET; 4.2 cm2 surface area, 0.4 μm pore size, BD Biosciences) were incubated in secretory HASMC media overnight to enhance cell attachment. Filters were then removed from plate with sterile forceps, and placed upside down in Petri dishes to access lower surface. Cell suspension (400 μL of HASMCs at 90,000 cells/cm2) was dripped onto the filter and cells were allowed to adhere for 2 h at 37 °C before returning transwell filters back to wells of a 6-well plate containing 3 mL secretory HASMC media in the lower transwell compartment. Then, 1.5 mL secretory HASMC media was added to the cell-free upper compartment. For ‘more secretory’ HASMCs, HAEC cell suspension (40,000 cells/cm2) was then added to the inner compartment, and plates were incubated at 37 °C for 48 h prior to assay. For ‘less secretory’ HASMCs, HASMCs were incubated overnight in secretory HASMC media, and media was then switched out for forced contractile HASMC media (serum and growth factor starved, to induce contractility). Then, media was again switched out for media of the same formulation as secretory media, but containing only 2% FBS (termed ‘quiescent HASMC media’) HAEC cell suspension (40,000 cells/cm2) was added to the inner compartment, and plates were incubated at 37 °C for 48 h prior to assay. Optimization was done to formulate ‘quiescent HASMC media’ such that HASMCs could maintain their forced contractile phenotype as long as possible without causing co-cultured HAEC to lose their ability to maintain a quiescent monolayer. It was found that monocultured HAEC in media with below 1% FBS did not form a monolayer over 48 h, while monocultured HASMCs retained organized α-smooth muscle actin filaments that had been induced by serum and growth factor starvation when cultured in media with up to 2% FBS over 48 h (data not shown). Flow cytometry on HAEC co-cultured with HASMCs demonstrated that media containing 2% FBS (but not 1% FBS) was sufficient for the formation of a quiescent HAEC monolayer in co-culture with HASMCs.
Immunocytochemistry for α-Smooth Muscle Actin in HASMCs and VE-Cadherin in HAECs
Forced contractile (serum and growth factor starved) HASMCs, as well as ‘more or less secretory’ HASMCs (in co-culture with HAECs) or HAECs (in co-culture with ‘more or less secretory’ HASMCs) were washed with PBS, and fixed with 4% paraformaldehyde in PBS at room temperature for 20 min. Then, cells were washed with PBS containing 0.1% bovine serum albumin (BSA), permeabilized and blocked with PBS containing 0.1% Triton X-100, 1% BSA, and 10% normal donkey serum (Jackson Immunoresearch, West Grove, PA) at room temperature for 45 min. Cells were again washed with PBS containing 0.1% BSA and incubated with monoclonal mouse IgG1 anti-human α-smooth muscle actin antibodies (1A4, R&D Systems) for HASMCs or monoclonal mouse IgG2β VE-cadherin (123413, R&D Systems) for HAECs at room temperature in the dark for 1 h. Cells were again washed with PBS containing 0.1% BSA, and then incubated with FITC-coupled donkey anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) at 4 °C overnight. Following a final wash with PBS containing 0.1% BSA, HASMCs or HAECs were visualized at room temperature on a Zeiss LSM 510 confocal microscope, and imported into ImagePro Plus software for analysis. The percentage of cells demonstrating a particular morphology were determined using manual cell counting in 10 randomized fields at 20× original magnification.
Enzyme-Linked Immunosorbant Assays (ELISAs) for Cytokine Release from HAEC or HASMC Monocultures and HAEC/HASMC Co-Cultures
Levels of IL-8 or MCP-1 secretion from HAEC or HASMC monocultures, or HAEC/HASMC co-cultures were measured using commercially available ELISA kits (R&D Systems). Briefly, supernatants were collected at both 48 and 72 h after induction of final culture conditions, and frozen at −20 °C until use. Diluted supernatants were applied to plates coated with IL-8 or MCP-1 antibodies, and allowed 2 h at room temperature to attach. Plates were then washed, and polyclonal antibodies for IL-8 or MCP-1 conjugated to horseradish peroxidase were added for 1 h at room temperature. Following another washing, a substrate solution consisting of hydrogen peroxide and tetramethylbenzidine was added. The reaction was stopped after 20 min (for MCP-1) or 30 min (for IL-8) with 2 N sulfuric acid, and absorbances were read at 450 nm.
Flow Cytometry for Proinflammatory and Pro/Anti-Coagulant Marker Expression on HASMCs in Co-Culture with HAECs
Following removal from wells with cell isolation solution (Sigma) at 48 or 72 h after initiation of co-culture with HAECs, or 48 h after removal of serum and growth factors for monocultured forced contractile HASMCs, HASMCs were labeled with saturating levels of fluorescently labeled monoclonal antibodies specific for cell surface markers including mouse IgG1 anti-human ICAM-1 (BBIG-I1, R&D Systems), mouse IgG1 anti-human VCAM-1 (BBIG-V3, R&D Systems), mouse IgG1κ anti-human tissue factor (CD142, HTF-1, BD Biosciences) or mouse IgG1κ anti-human thrombomodulin (CD141, 1A4, BD Biosciences). Analysis was done by determining the mean fluorescence intensities (MFI) associated with the HASMC populations using a BD LSR flow cytometry (Bectin-Dickinson, Franklin Lakes, NJ) using BD FACSDiVa software.
Data Analysis
Data for ELISAs was presented as pg/well, along with associated standard deviations, and flow cytometry data was presented as MFI, along with the associated standard deviations. Statistics were performed using general ANOVAs to test for significant differences followed by pair wise comparisons and comparisons with a control with a Tukey post-test using the Minitab software (version 13, Minitab, Inc., State College, PA). p-Values of ≤0.05 were considered significant.
Results
Immunocytochemistry
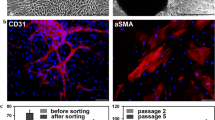
‘More secretory’ (5% FBS) HASMCs (in co-culture with HAECs) showed diffuse, unorganized α-smooth muscle actin staining in >95% of cells at both the 48 and 72 h time point (Fig. 1a). Forced contractile (serum and growth factor starved) monocultured HASMCs showed organized α-smooth muscle actin filaments throughout >90% of cells (Fig. 1b). ‘Less secretory’ (2% FBS) HASMCs (in co-culture with HAECs) at the 48 h time point show organized α-smooth muscle actin filaments throughout >85% of cells (Fig. 1c), however by the 72 h time point under these culture conditions, organized filaments have begun to disappear, with the majority of cells displaying some filaments, and some areas of disorganization (Fig. 1d). Immunocytochemistry was also performed for VE-cadherin staining on HAECs (in co-culture with HASMCs) to confirm the maintenance of a monolayer and proper cell–cell junctions in co-culture conditions resulting in ‘more or less secretory’ HASMCs (representative image shown in Fig. 1e). Images of HAEC were indiscernible between culture conditions and time points used, and demonstrated uniform VE-cadherin around cell borders.
Confocal microscopy images of immunocytochemistry for α-smooth muscle actin or VE-cadherin staining. ‘More secretory’ HASMCs in co-culture with HAECs (5% FBS, data for 48 h representative of the 48 and 72 h time points) (a), monocultured forced contractile HASMCs (0% FBS) (b), or ‘less secretory’ HASMCs (2% FBS) in co-culture with HAECs at the 48 h (c) or 72 h (d) time point were staining for α-smooth muscle actin (original modification 20×). Also, HAEC co-cultured with ‘more secretory’ HASMCs at the 48 h time point is shown as a representative image of immunocytochemistry for VE-cadherin staining of HAECs in our systems (e) (original modification 20×)
Control experiments on HAECs or HASMCs in monoculture were also performed to determine the effects of the culturing conditions alone in the absence of the other cell type (data not shown). It was found that monocultured HASMCs had diffuse, unorganized α-smooth muscle actin filaments in >95% of cells when cultured in secretory HASMC media over 48 h, while they alternately maintained organized α-smooth muscle actin filaments in 85% of cells when cultured in contractile HASMC media over 48 h. Monocultured HAECs demonstrated uniform VE-cadherin staining in both secretory HASMC media and contractile HASMC media over 48 h.
Enzyme-Linked Immunosorbant Assays for Cytokine Release from HASMCs
Secretory SMCs are known to secrete high levels of MCP-1 and IL-8,14 while quiescent SMCs do not.14 To determine the phenotypic state of HASMCs at various time points, as well as the possible impacts of chemokine release on the co-culture, ELISAs for IL-8 (Fig. 2a) and MCP-1 (Fig. 2b) were performed. Monocultured HAEC in secretory HASMC media secreted significantly less IL-8 and MCP-1 than forced contractile HASMCs (serum and growth factor staved) at both time points (bars for HAEC MS 5% 48, 72 h). Monocultured HAEC in quiescent HASMC media secreted significantly less IL-8 and the same level of MCP-1 as forced contractile HASMCs (serum and growth factor staved) at both time points (bars for HAEC LS 2% 48, 72 h). Monocultured HASMCs in secretory HASMC media significantly upregulated secretion of IL-8 and MCP-1 at both time points (bars for HASMC MS 5% 48, 72 h) as compared to forced contractile HASMCs. Alternately, monocultured HASMCs in quiescent HASMC media (bars for HASMC LS 2% 48, 72 h) secreted similar levels of IL-8 and MCP-1 to forced contractile HASMCs at the 48 h time point (bar for HASMC LS 2% 48 h), but significantly upregulated IL-8 and MCP-1 secretion by the 72 h time point (bar for HASMC LS 2% 72 h). Co-culture of HAECs with ‘more or less secretory’ HASMCs (bars for HAEC/HASMC MS 5% or LS 2%, respectively, at 48 and 72 h) significantly upregulated IL-8 and MCP-1 secretion as compared to monocultured forced contractile HASMCs, with the 72 h time point always being significantly higher than the 48 h time point. Additionally, both HAEC/HASMC co-cultures secreted significantly more IL-8 and MCP-1 (Fig. 2) than HASMC monocultures under the same conditions in nearly all conditions. Importantly, levels of IL-8 and MCP-1 secretion were always significantly lower for ‘less secretory’ HASMCs as compared to ‘more secretory’ HASMCs (in co-culture with HAECs). In summary, co-cultures of HAECs with ‘less secretory’ HASMCs maintain relatively low levels of IL-8 and MCP-1 secretion at the 48 h time point, but secrete high levels of MCP-1 similar to monocultured HASMCs in secretory HASMC media (MS, 5%) and moderate levels of IL-8 by the 72 h time point.
Analysis of IL-8 or MCP-1 secretion from HAEC or HASMC monocultures, or HAEC/HASMC co-cultures. ELISA absorbances for IL-8 (a) or MCP-1 (b) secretion into the culture media of HASMC or HAEC monocultures, or HAEC/HASMC co-cultures under varying conditions. The X-axis labels are separated into four lines, with the lower line denoting the cell type(s) in culture (HASMCs, HAEC, or HASMC/HAEC), and the upper three lines denoting HASMC phenotype (FC = forced contractile, MS = more secretory, LS = less secretory), time points in culture (48 or 72 h) of the supernatants collected for IL-8 or MCP-1 analysis, and serum content of media (0, 2, 5%). For all data, n = three independent determinations; mean ± standard determination. *Means statistically different than forced contractile HASMCs (p ≤ 0.05), bracket means statistically different than other time point for same cell type and media conditions (p ≤ 0.05), ^ means statistically different than HASMC monoculture for same culture conditions and time point (p ≤ 0.05)
Flow Cytometry for Proinflammatory and Pro/Anti-Coagulant Marker Expression on HASMCs
Levels of proinflammatory and pro/anti-coagulant markers on HASMCs (in monoculture for forced contractile, or in co-culture with HAECs for ‘less secretory’ (2% FBS) or ‘more secretory’ (5% FBS) HASMCs) were examined to determine the effect of different co-culture techniques and time points (48 or 72 h) on HASMC phenotype (Fig. 3). Thrombomodulin expression was unchanged by all culture conditions (thus has been omitted from graph). Expression of ICAM-1, VCAM-1, and tissue factor was significantly increased on ‘more secretory’ HASMCs in co-culture with HAECs at both time points (bars for MS 5% 48, 72 h) as compared to both monocultured forced contractile HASMCs (bars for FC 0%), and ‘less secretory’ HASMCs in co-culture with HAECs at the 48 h time point (bars for LS 2% 48 h). ‘Less secretory’ HASMCs in co-culture with HAECs at the 48 h time point (bars for LS 2% 48 h) showed similar expression levels of all markers to monocultured forced contractile HASMCs, however both ICAM-1 and tissue factor expression significantly increased on ‘less secretory’ HASMCs in co-culture with HAECs from the 48 to the 72 h time point (bars for LS 2% 72 h). In addition, by the 72 h time point, expression levels of most markers in ‘less secretory’ HASMCs in co-culture with HAECs were similar to ‘more secretory’ HASMCs in co-culture with HAECs, with only VCAM-1 expression remaining significantly lower.
Flow cytometry for HASMC expression of VCAM-1, ICAM-1, and tissue factor for different culture conditions. The Y-axis shows mean fluorescence intensity for each marker examined. X-axis labels are separated into four lines, with the lower line denoting the cell type(s) in culture (HASMCs or HASMC/HAEC), and the upper three lines denoting HASMC phenotype (FC = forced contractile, MS = more secretory, LS = less secretory), time points (48 or 72 h) of culture, and serum content of media (0, 2, 5%). For all data, n = three independent determinations; mean ± standard determination. *Means significantly above forced contractile HASMCs (p ≤ 0.05), + means significantly above ‘less secretory’ HASMCs at the 48 h time point (p ≤ 0.05), # means significantly above ‘less secretory’ HASMCs at the 72 h time point (p ≤ 0.05), and bracket means significantly different than other time point for the same media conditions (p ≤ 0.05)
Expression levels of proinflammatory and pro/anti-coagulant markers were also examined on monocultured HASMCs to determine the effect of culture conditions in the absence of HAECs. It was found that for the four markers examined (VCAM-1, ICAM-1, tissue factor, and thrombomodulin), there were no significant differences between expression on monocultured HASMCs verses HASMCs co-cultured with HAEC over 72 h.
Discussion
The overall hypothesis of this work was that alternate co-culture conditions could be developed to push HASMCs toward a ‘more or less secretory’ phenotype (on the spectrum from secretory to contractile) while maintaining a quiescent HAEC monolayer. It has been demonstrated in unpublished data associated with this paper, as well in previously published work of Rose and Babensee27 that HAEC in co-culture with both ‘more or less secretory’ HASMCs maintain the same levels of proinflammatory and pro/anti-coagulant markers as HAEC in monoculture. Additionally, in this paper it was shown that VE-cadherin staining remained consistent for monoculture, and both co-culture models during the time frame of experimentation. The goal of this work was to characterize the phenotypes of HASMCs in our co-culture conditions, and to determine whether they were also maintained during the time frame of experimentation allowing for subsequent studies using this co-culture system.
Monocultured forced contractile (serum and growth factor starved) HASMCs demonstrated highly organized α-smooth muscle actin filaments, low IL-8 and MCP-1 secretion, and low levels of proinflammatory (ICAM-1, VCAM-1) and procoagulant (tissue factor) markers. At both time points (48 and 72 h), ‘more secretory’ HASMCs (5% FBS) in co-culture with HAECs resulted in the loss of HASMC α-smooth muscle actin organization, upregulation of IL-8 and MCP-1 secretion, and increased HASMC surface expression of ICAM-1, VCAM-1, and tissue factor. Alternately, at the 48 h time point, ‘less secretory’ HASMCs (2% FBS) in co-culture with HAECs showed similar levels of α-smooth muscle actin organization, IL-8 and MCP-1 secretion, and proinflammatory/procoagulant marker expression to monocultured forced contractile HASMCs (serum and growth factor starved). However, by the 72 h time point, HASMC α-smooth muscle actin had become significantly disorganized and IL-8 and MCP-1 secretion, as well as proinflammatory/procoagulant marker expression, had become more similar to that of ‘more secretory’ HASMCs in co-culture with HAECs. Thus, over the time frame of our experimentation, ‘less secretory’ HASMCs are beginning to shift on the spectrum to a ‘more secretory’ HASMC phenotype. Additionally, both HAEC/HASMC co-cultures in general secreted more IL-8 and MCP-1 than HASMC monocultures under the same conditions. These increases could be due to a summation effect with IL-8 and MCP-1 being released from HAECs to some extent, or the presence of HAEC causing further upregulation of IL-8 and MCP-1 release from HASMCs.
It is well documented that in vitro culture of SMCs typically induces a secretory SMC phenotype.28,30,31 In vivo, in healthy and uninjured vessels, SMCs only receive growth factors locally delivered from other SMCs or ECs, and are sequestered from blood. They are highly quiescent, and non-proliferating. However, upon injury or disease, the EC monolayer is lost, exposing the underlying SMCs to higher levels of growth factors, as well as serum components such as PDGF and thrombin.36 Binding of these components leads to activation of nuclear factors-κB3 and GATA-6,17, and the induction of a secretory and proliferative phenotype. Thus, it is not surprising that while growing SMCs in culture, it is actually necessary to induce a secretory phenotype by the presence of growth factors and serum in the culture media so that the SMCs will proliferate. In addition, a secretory phenotype can be beneficial for fabrication of vascular tissue, as SMCs are more able to populate the graft, and produce and remodel matrix. However, the trick is that the cells need to be quiescent upon implantation.
One way to induce SMC contractility in vitro is the removal of growth factors and serum from culture media. Thus, examining contractile-like SMCs in monoculture is quite simple. However, inducing SMC contractility in EC/SMC co-cultures is more complicated. First, ECs require serum and some growth factors in order to survive, grow, and maintain a quiescent monolayer. Second, the presence of ECs in in vitro co-cultures has been shown by some groups to push SMCs toward a secretory and proliferative phenotype6,35 in the absence of any exogenous stimulus. Stimulation of SMC proliferation by ECs in co-culture may be due to EC release of endothelin-1, a potent mitogenic factor for SMC,34 as well as FGF and PDGF.21 Thus, it is likely that the presence of serum and growth factors in the media, as well as the presence of ECs, act to push the ‘less secretory’ SMCs in our system toward a ‘more secretory’ phenotype over time.
Another caveat to our newly developed system is the use of static rather than dynamic culture. However, Chiu et al.4 have developed a system for using PET transwell-based human umbilical cord EC/SMC co-cultures under shear conditions. They found that when the EC surface of their co-cultures were exposed to 12 dynes/cm2 shear stress, ECs oriented parallel to flow, SMCs oriented perpendicular to flow. In addition, GRO-α and MCP-1 secretion4 and ICAM-1, VCAM-1, and E-selectin expression5 induced in ECs by static co-culture with SMCs (assumed to be secretory based on culture conditions) was abolished under shear conditions. Thus, the application of shear stress seems to provide an atheroprotective effect, downregulating pathophysiologically relevant genes seen in static culture. This effect may be due to the recently discovered gene Kruppel-like Factor-2 (KLF-2) which is regulated by steady laminar shear stress in cultured endothelial cells8 and has been implicated in atheroprotected regions in vivo.25 Overexpression of KLF-2 in cultured ECs leads to upregulation of endothelial nitric oxide synthase, and inhibition of interleukin-1β-dependent EC expression of E-selectin, VCAM-1, and tissue factor.20,29 So, while using our co-culture systems under shear stress is more physiologically relevant, it will also make phenotypic changes more subtle, and perhaps difficult to study.
Further examination of the phenotypic states of the SMCs in our co-culture systems would be beneficial to determine the level of quiescence induced. Specifically, expression of a variety of contractile proteins over the course of our co-cultures could be examined by reverse transcriptase-polymerase chain reaction or other methods. However, some groups have found that even in the presence of serum, SMCs expressed several contractile apparatus proteins, including α-smooth muscle actin, smooth muscle myosin heavy chain, calponin, and heavy caldesmon.18 Increases in these markers were seen in serum-starved forced contractile SMCs, however the magnitudes of changes were modest, with most increases seen only after several days of attainment of contractile competence. Thus, changes in expression of these proteins may not be detectable during the time frame of our co-cultures.
The work presented herein demonstrates, to our knowledge, the first example of divergent EC/SMC co-culture systems in which SMC phenotype has been characterized and a quiescent EC phenotype has been maintained. Development of adequate in vitro models of different stages of vascular healing, as well as an improved understanding of cell phenotype is crucial toward the development of vascular tissue grown in vitro, as well as the success of implanted tissues or devices. While understanding the limitations described above, these co-cultures systems have successfully been utilized in a manuscript submitted for publication concurrently.27 We were able to study the effect of biomaterial-activated leukocytes on HAEC phenotype, and the impact of ‘more or less secretory’ HASMCs in co-culture on this HAEC phenotype modulation. Smooth muscle cells in implanted tissue, or neighboring implanted devices, must avoid restenosis, provide a substrate for endothelial growth and formation of a monolayer, and promote normal vessel physiology. The static in vitro co-culture conditions described herein provide a useful system for examining the functional consequences of HAEC/HASMC interactions at short time frames, as well as the starting of point for the development of more adequate and representative perfusion-based co-culture models of vascular healing.
References
Campbell G. R., J. H. Campbell. Development of the vessel wall. In: S. M. Schwartz, F. P. Mechams (eds) The Vascular Smooth Muscle Cell. San Diego, CA: Academic Press, 1995, pp. 3–15
Castellot J. J. Jr., M. L. Addonizio, R. Rosenberg, M. J. Karnovsky. 1981 Cultured endothelial cells produce a heparin-like inhibitor of smooth muscle cell growth. J. Cell Biol. 90:372–379
Chiu J. J., L. J. Chen, S. F. Chang, P. L. Lee, C. I. Lee, M. C. Tsai, D. Y. Lee, H. P. Hsieh, S. Usami, S. Chien 2005 Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: role of NF-κB. Arterioscler. Thromb. Vasc. Biol. 25:963–969
Chiu J. J., L. J. Chen, C. N. Chen, P. L. Lee, C. I. Lee 2004 A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J. Biomech. 37:531–539
Chiu J. J., L. J. Chen, P. L. Lee, L. W. Lo, S. Usami, S. Chien 2003 Shear stress inhibits adhesion molecules expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 101:2667–2674
Cucina A., V. Borrelli, B. Randone, P. Coluccia, P. Sapienza, A. Cavallaro 2003 Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J. Surg. Res. 109:16–23
Davies P. F., G. A. Truskey, H. B. Warren, S. E. O’Conner, B. H. Eisenhaure. 1985 Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J. Cell Biol. 101:871–879
Dekker R. J., S. van Soest, R. D. Fontijn, S. Salamanca, P. G. de Groot, E. Van Bavel, H. Pannekoek, A. J. G. Horrevoets. 2002 Prolonged fluid shear stress induces a distinct set of endothelial genes, most specifically lung Kruppel-like factor (KLF2). Blood 100:1689–1698
Fetalvero K. M., M. Shyu, A. P. Nomikos, Y. F. Chiu, R. J. Wagner, R. J. Powell, J. Hwa, K. A. Martin. 2006 The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am. J. Physiol. Heart Circ. Physiol. 290:H1337–H1346
Fillinger M. F., S. E. O’Conner, R. J. Wagner, J. L. Cronenwett 1993 The effect of endothelial cell coculture on smooth muscle cell proliferation. J. Vasc. Surg. 17:1058–1068
Fillinger M. F., L. N. Sampson, J. L. Cronenwett, R. J. Powell, R. J. Wagner 1997 Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J. Surg. Res. 67:169–178
Heydarkhan-Hagvall S., G. Helenius, B. R. Johansson, J. Y. Li, E. Mattsson, B. Risberg. 2003 Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J. Cell Biochem. 89:1250–1259
Imberti B., S. Seliktar, R. M. Nerem, A. Remuzzi 2002 The response of endothelial cells to fluid shear stress using a co-culture model of the arterial wall. Endothelium 9:11–23
Jordan N. J., M. L. Watson, R. J. Williams, A. G. Roach, T. Yoshimura, J. Westwick 1997 Chemokine production by vascular smooth muscle cells: modulation by IL-13. J. Pharmacol. 122:749–757
Kladakis S. M., R. M. Nerem 2004 Endothelial cell monolayer formation: effect of substrate and fluid shear stress. Endothelium 11:29–44
Korff T., S. Kimmina, G. Martiny-Baron, H. G. Augustin. 2001 Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 15:447–457
Lepore J. J., T. P. Capola, P. M. Mericko, E. E. Morrisey, M. S. Parmacek 2005 GATA-6 regulates genes promoting synthetic functions in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 25:309–314
Li S., S. Sims, Y. Jiao, L. H. Chow, J. G. Pickering 1999 Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ. Res. 85:338–348
Libby P., K. V. O’Brian 1983 Culture of quiescent arterial smooth muscle cells in a define serum-free medium. J. Cell Physiol. 115:217–223
Lin Z., A. Kumar, S. SenBanerjee, K. Staniszewski, K. Parmar, D. E. Vaughan, M. A. Gimbrone, V. Balasubramanian, G. García-Cardeña, M. K. Jain 2005 Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 96:e48–e57
Lin P. H., D. Ren, M. Hirko, S. S. Kang, G. F. Pierce, H. Greisler 1998 Fibroblast growth factor-2-toxin induced cytotoxicity: differential sensitivity of co-cultured vascular smooth muscle cells and endothelial cells. Atherosclerosis 137:277–289
Nackman G. B., F. R. Bech, M. F. Fillinger, R. J. Wagner, J. L. Cronenwett 1996 Endothelial cells modulate smooth muscle cell morphology by inhibition of transforming growth factor-beta1 activation. Surgery 120:418–426
Nackman G. B., M. F. Fillinger, F. Shafritz, T. Wei, A. M. Graham 1998 Flow modulates endothelial regulation of smooth muscle cell proliferation: a new model. Surgery 124:353–361
Owens G. K. 1995 Regulation of differentiation of vascular smooth-muscle cells. Physiol. Rev. 75:487–517
Parmar K. M., H. B. Larman, G. Dai, Y. Zhang, E. T. Wang, S. N. Moorthy, J. R. Kratz, Z. Lin, M. K. Jain, M. A. Gimbrone, G. García-Cardeña 2006 Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116:49–58
Rainger G. E., P. Stone, C. M. Morland, G. B. Nash 2001 A novel system for investigating the ability of smooth muscle cells and fibroblasts to regulate adhesion of flowing leukocytes to endothelial cells. J. Immunol. Methods 255:73–82
Rose, S. L., and J. E. Babensee. Smooth muscle cell phenotype alters co-cultured endothelial cell response to biomaterial-pretreated leukocytes. J. Biomed. Mater. Res. (in press)
Schecter A. D., B. Spirn, M. Rossikhina, P. L. A. Giesen, V. Bogdanov, J. T. Fallon, E. A. Fisher, L. M. Schnapp, Y. Nemerson, M. B. Taubman. 2000 Release of active tissue factor by human arterial smooth muscle cells. Circ. Res. 87:126–132
SenBanerjee S., Z. Lin, B. Atkins, D. M. Greif, R. M. Rao, A. Kumar, M. W. Feinberg, Z. Chen, D. I. Simon, F. W. Luscinskas, T. M. Michel, M. A. Gimbrone, G. García-Cardeña, M. K. Jain 2004 KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 199:1305–1315
Soff G. A., R. W. Jackman, R. D. Rosenberg 1991 Expression of thrombomodulin by smooth muscle cells in culture: different effects of tumor necrosis factor and cyclic adenosine monophosphate on thrombomodulin expression by endothelial cells and smooth muscle cells in culture. Blood 77:515–518
Stegemann J. P., Hong H., Nerem R. M. 2005 Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J. Appl. Physiol. 98:2321–2327
Thyberg J., U. Hedin, M. Sjolund, L. Palmberg, B. A. Bottger. 1990 Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Atherosclerosis 63:99–107
Van Buul-Wortelboer M. F., H. J. M. Brinkman, Z. K. P. Dingemans, P. G. De Groot, W. G. van Aken, J. A. van Mourick 1986 Reconstitution of the vascular wall in vitro: a novel model to study interactions between endothelial and smooth muscle cells. Exp. Cell Res. 162:151–158
Vouyouka A. G., S. S. Salib, S. Cala, J. D. Marsh, M. D. Basson 2003 Chronic high pressure potentiates the antiproliferative effect and abolishes contractile phenotypic changes caused by endothelial cells in cocultured smooth muscle cells. J. Surg. Res. 110:344–351
Waybill P. N., L. J. Hopkins 1999 Arterial and venous smooth muscle cell proliferation in response to co-culture with arterial and venous endothelial cells. J. Vasc. Interv. Radiol. 10:1051–1057
Wilcox J. N., K. M. Smith, S. M. Schwartz, D. Gordon 1997 Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. J. Clin. Invest. 100:2276–2285
Zhang J. C., Q. R. Ruan, L. Paucz, A. Fabry, B. R. Binder, J. Wojta. 1999 Stimulation of tissue factor expression in human microvascular and macrovascular endothelial cells by cultured vascular smooth muscle cells in vitro. J. Vasc. Res. 36:126–132
Ziegler T., R. W. Alexander, R. M. Nerem 1995 An endothelial cell-smooth muscle cell co-culture model for use in the investigation of flow effects on vascular biology. Ann. Biomed. Eng. 23:216–225
Acknowledgments
The authors thank Tiffany Johnson and Dr. Robert Nerem for advice on EC/SMC co-culture, and Johnafel Crowe for flow cytometry assistance (all of the Parker H. Petite Institute for Bioengineering and Bioscience at Georgia Institute of Technology). This work was supported by transitional funding from a Biomedical Engineering Research Grant from the Whitaker Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rose, S.L., Babensee, J.E. Complimentary Endothelial Cell/Smooth Muscle Cell Co-Culture Systems with Alternate Smooth Muscle Cell Phenotypes. Ann Biomed Eng 35, 1382–1390 (2007). https://doi.org/10.1007/s10439-007-9311-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-007-9311-0