Abstract
Oxygen tension is essential for cellular activities and plays an important role in clinical cancer treatment. In this paper, we constructed a single-layer microfluidic device with oxygen gradients to study oxygen-dependent cytotoxicity of anticancer drugs. Oxygen concentrations in the cell culture chamber are controlled by spatially confined oxygen-scavenging chemical reaction in the neighboring microfluidic channel. The groove-shaped structure of PDMS membrane is designed to increase its bonding ability to the bottom layer, which helps avoid the leakage of liquid while facilitating the exchange of gas between the cell culture chamber and the reaction channel. For demonstration, A549 cells were cultured in the microfluidic device and treated with tirapazamine (TPZ) or cisplatin under various oxygen contents. An increase in apoptosis of A549 cells was observed due to the hypoxia-induced cytotoxicity of TPZ, which provided evidence that anticancer efficiency of TPZ is influenced by oxygen tensions directly and have great promise in selectively killing tumor cells in hypoxic microenvironment. Conversely, cisplatin led to an increased cell death in the relatively higher oxygen contents because of the cell resistance in hypoxic conditions. The drug testing results showed that it is necessary to take the oxygen tensions in microenvironment into consideration to get a better standing in cellular responses after drug exposure. In summary, the developed microfluidic cell culture device displayed great cell compatibility and gradient controllability, and also provided a useful tool for drug-screening studies with meaningful oxygen tensions in various biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tumor microenvironment has important functions for modulating cell fates and plays an essential role in clinical cancer therapy (Tredan et al. 2007). Tissue oxygen tension is known to have the ability to modulate the sensitivity of cancer cells to certain nonsurgical treatment modalities (Durand 1993). Well-oxygenated cells and tissues are demonstrated to be more sensitive to most anticancer drugs than those under hypoxic conditions (Aquino-Parsons et al. 1999). Tumor blood vessels are often found to be chaotic, leaky, and disorganized. These characteristics result in reduced oxygen and nutrients delivery to cancer cells, and also induce chronic hypoxia. In turn, this may causes low oxygen concentrations in most tumor tissues, with many areas extremely hypoxic (Brown and Wilson 2004). Hypoxia is the most common tumor microenvironment and represents an independent prognostic factor for different cancer types (Vaupel et al. 1989). Low overall levels of oxygen in a variety of human solid tumors are already confirmed by oxygen electrode measurements, including brain tumors, head and neck tumors, and breast tumors (Brown and Wilson 2004). Although many chemotherapeutic agents have good pharmacological activity during screening research in vitro, poor efficiency or severe rejection reactions are observed in clinical trials due to the reduced oxygen contents in tumor tissues. It is known that the majority of anticancer drugs are only effective against rapidly proliferating cells. As the level of oxygen concentration decreases with increasing distance from blood vessel, cell proliferation decreases. The relative poor cell proliferation may increase the likelihood of cell resistance. Some chemotherapeutic agents such as cisplatin, 5-fluorouracil, and melphalan have already been demonstrated to reduce in cytotoxicity under relative hypoxic conditions (Strese et al. 2013). Moreover, the decrease of oxygen concentrations in tumor tissues tends to select cells with stronger malignant phenotype and make tumor cells highly unresponsive to most anticancer drugs (Carreau et al. 2011). Such hypoxia-activated cell resistant has been experimentally observed with a number of tumors and tumor models (Ledoux et al. 2003; Graceber et al. 1996; Kondo et al. 2001; Gray et al. 2005). Many approaches to circumventing the therapeutic resistance induced by hypoxia have been examined in laboratory studies, and better agents and regimens are also developed for the application in hypoxia-directed cancer therapy (Rockwell et al. 2009). Thus, it is important to take the effects of oxygen tensions into consideration for further studies of anticancer drug screening and clinical chemotherapy.
Microfluidic has clearly advantages in spatiotemporal manipulations at microscale level (Park et al. 2006; Zhang et al. 2010; Mu et al. 2010; Lam et al. 2009). It is used widely as a technique for its great controllability in microenvironments (Zhang et al. 2011; Ren et al. 2009; Khanal et al. 2011; He et al. 2011). This method can perform excellent characteristics in the precise regulation of fluid perfusion and biological sample analysis (Zhang et al. 2009; Skolimowski et al. 2010). Recently, microfluidic chips developed to produce oxygen concentration gradient have been reported in several studies (Mehta et al. 2007; Chen et al. 2011; Lo et al. 2010; Verbridge et al. 2010; Funamoto et al. 2012; Chang et al. 2014). For example, Yang et al. (2013) created in vivo-like 3D microenvironments with reduced oxygen contents by specific ECM proteins with a microfluidic array. Sud et al. (2006) developed a fluorescence-lifetime-based approach for oxygen sensing in PDMS bioreactors with high spatial discrimination for oxygen monitoring in continuous cell culture systems. Adler et al. (2010) presented a system consisting of a microfluidic device made of gas-permeable PDMS with two layers of microchannels and a computer-controlled multi-channel gas mixer. However, these microfluidic platforms could only achieve a certain range of oxygen gradient and the oxygen tensions could not get to very high or low level. Moreover, these devices often have sophisticated chip structure and external gas cylinder with precise and complex operations. Now, a better way to form oxygen gradient is to scavenge oxygen from cell culture wells by using chemical reactions. Grasselli et al. (2005) developed a device which can generate oxygen gradients on a chip by using a pair of chemical reactions. The microfluidic platform not only avoided the employments of pressurized gas cylinders and sophisticated gas flow control devices, but also had no cellular toxicity.
In this paper, a microfluidic chip was designed to establish an oxygen gradient and applied to study hypoxia-dependent cytotoxicity of two anticancer drugs. In the field of cancer chemotherapy, there is a long-standing requirement to illustrate the cellular behavior and response for a variety of anticancer agents under multiple oxygen tensions in a high-throughput manner. The microfluidic device was constructed in a single-layer chip. The oxygen gradient was generated in the cell culture chamber by spatially confined oxygen-scavenging chemical reaction in the neighboring microfluidic channel. The groove-shaped structure of PDMS membrane is designed to increase its bonding ability to the bottom layer, which helps avoid the leakage of liquid while facilitating the exchange of gas between the cell culture chamber and the reaction channel. This method could control the oxygen content precisely and provided a promising platform to study cellular responses under various anticancer drugs and oxygen concentrations. The results indicated that the oxygen gradients can be stably generated in the microfluidic device, and the exploited platform can also be used as a powerful tool for further drug testing and cell migration studies in various biomedical applications.
2 Method and material
2.1 Reagents and apparatus
Sylard 184 polydimethylsiloxane (PDMS, Dow Corning), SU-8 2050 (Microchem), pyrogallol (Sinopharm Group Chemical Reagent Co.), NaOH (Beijing Chemical Plant), tris (4,7-diphenyl-1,10 -phenanthroline) ruthenium dichloride (II) complexes (Sigma), fibronectin (Solarbio), tirapazamine (Sigma), cisplatin (Sigma), RPMI medium 1640 (Invitrogen), fetal bovine serum (Gibicol), antibiotic–antimycotic solution (Gibco1), trichloro-(1H, 1H, 2H, 2H-perfluorooctyl) silane (Sigma), Cell Counting Kit-8 (Beyotime), Live&Dead viability/cytotoxicity assay kit (Keygen Biotech), Microinjection pump (Harvard), Plasma cleaner PDC-32G (Harrick Plasma), and IX81 fluorescence inverted microscope (Olympus) were used in the experiment, and water used to prepare solution in the experiments was supplied by Milli-Q ultrapure water system (Millipore).
2.2 Microfluidic device design and fabrication
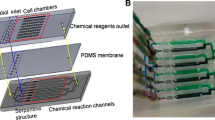
It is shown in Fig. 1 that the microfluidic chamber for chemical reagents is 100 μm width × 100 μm height and the oxygen gradient is generated by chemical reactions to scavenge oxygen in the given areas. The main channel used for cell culture is 800 μm width × 100 μm height. The cell culture channel and chemical reaction channel are separated by thin PDMS membrane with the thickness of 100 μm. Oxygen diffuses through the PDMS membrane due to its high diffusivity. The reactants are completely mixed in the curved path and then react to scavenge oxygen. Groove-shaped structure with the length of 150 μm and the width of 60 μm is shown in Fig. 1a, and the distance between two groove-shaped structures is 500 μm. The PDMS membrane between the cell culture chamber and chemical reaction channel is very thin and might easily be broken to lead to liquid leakage between the cell chamber and reaction channel. Therefore, the groove-shaped structure of PDMS membrane is designed to increase bonding ability between the upper PDMS film and the bottom glass without increase in the distance of gas diffusion, which helps avoid the leakage of liquid while facilitating the exchange of gas between the cell culture chamber and the reaction channel. The sectional view of this developed device is illustrated in Fig. 1b. For developing oxygen gradient stably, we cover a tape on top surface of the microfluidic chip to block the oxygen diffusion between the microchannels and the top atmosphere.
The microfluidic device is fabricated using PDMS by soft lithography and replica molding techniques (Ai et al. 2012). First, SU-8 2050 negative photoresist is spin-coated on a cleaned silicon wafer, and then, the wafer is placed in the oven for 6 min at 65 °C and then 3 min at 95 °C. After cooling down to room temperature, the wafer with SU-8 is exposed to UV light for 120 s and then immerges into developer solution. Finally, the mold is baked for 5 min at 65 °C and silanized with trichloro-(1H, 1H, 2H, 2H-perfluorooctyl) silane in a desiccator for 3 h to prevent undesired bonding between PDMS layer and the mold. After that, the PDMS prepolymer and curing agent mixed in a mass ratio of 10:1 are poured onto the silicon mold and baked in the oven at 80 °C for 2 h. Then, the PDMS layer is peeled off from the wafer mold. Connection holes are punched by needle with the diameter of 1.2 mm, and then, the PDMS layer is bonded to the glass surface by using an oxygen plasma surface treatment at 90 W for 40 s.
2.3 Cell culture
A549 cells are cultured in RPMI medium 1640 with 10 % v/v fetal bovine serum and a 1 % v/v antibiotic–antimycotic solution. The cell culture chamber is coated with fibronectin for 1 h before cell seeding. The cell suspension is injected into the channel with a concentration of 4 × 106 cells/mL by a syringe pump. Then, the chip is put into a cell culture dish and incubated for 24 h at 37 °C under 5 % CO2 to allow cell adhesion and spreading before drug testing.
2.4 Oxygen gradient generation and characterization
The oxygen scavenging is accomplished by using NaOH and pyrogallol. Pyrogallol is a powerful agent which absorbed oxygen from the ambient environment in the presence of alkaline solution. To generate oxygen gradients, NaOH and pyrogallol are pumped into the chemical reaction channel at the concentration of 100 mg/mL and 1 mol/L, respectively. The flow rates of two regents are both set as 0.04 mL/h. Two chemicals are completely mixed in the serpentine channel and start to scavenge oxygen when the mixtures flowed into the region close to the cell culture well. Then, oxygen diffuses through the PDMS membrane to generate an oxygen gradient.
To measure the oxygen concentration accurately, the oxygen concentration is characterized by using oxygen-sensitive fluorescent indicator tris(4,7-diphenyl 1,10-phenanthroline) ruthenium dichloride (II) complexes with the concentration of 250 μM (Polinkovsky et al. 2009). The fluorescence intensity of the dye is quenched by the presence of oxygen. So the oxygen gradient profile can be illustrated by determining the fluorescence intensity of different regions in cell culture channel after oxygen-scavenging reactants are introduced into the chemical reaction channels. As shown in Fig. 1b, oxygen diffuses from one side to another side in cell culture channel. We cover a tape on the top of the chip to block the exchange of gas. The fluorescence measurement is accomplished by an inverted fluorescence microscope. The excitation wavelength for the fluorescent indicator is 455 nm, and fluorescence microscopy exposure time is set as 30 ms. Then, the chip is placed on the fluorescence microscope, and real-time measurements are taken by taking pictures at the different positions of the cell culture channel.
The calibration curve is conducted by measuring the fluorescence intensity of the dye solution which is exposed under the pure N2 ([O2] = 0 %) and air ([O2] = 21 %) condition by converting the fluorescence intensity into oxygen concentration (Zhong et al. 2003). The oxygen concentration is calculated by using the obtained fluorescence intensity of different positions and the Stern–Volmer equation. According to formula (1), the fluorescence intensity is reduced when the oxygen is scavenged by the chemical reaction. I refer to the fluorescence intensity in the presence of oxygen, and I 0 is the fluorescence intensity when the oxygen content is 0 %. K q is the quenching coefficient.
2.5 Cytotoxicity analysis by traditional method
The A549 cells were seeded into 96-well plate at the density of 5000 cells/100 μL. After 12-h cell culture, the growth media is changed by new media with TPZ or cisplatin at the concentration of 100 μM. The growth media without anticancer drug is set as the control group. Then, the 96-well plate is taken into normoxia or hypoxia conditions for 12-h culture. Hypoxic treatment is carried out in an incubator with 1 % O2, 5 % CO2, and 94 % N2. Cell Counting Kit-8 assay is used to determine the cell viability for assessing the drug effects of TPZ and cisplatin under different oxygen conditions.
2.6 Drug treatment in the microfluidic chip
The A549 cells are injected into the microfluidic device and cultured with RPMI medium 1640 for 12 h. Then, cells are treated with TPZ or cisplatin for 12 h at the concentration of 100 μM under different oxygen conditions. The cell viability is determined by a Live&Dead viability/cytotoxicity assay kit, and fluorescence images are analyzed by the software Meta Morph.
3 Results and discussion
3.1 The modification of microfluidic chip for cell culture
Cell attachment was mainly affected by the physical and chemical properties of cell growth substrate. Glass is used in this study due to its permanent bonding force to PDMS microfluidic network. Cell adhesion to the surface and subsequent cell survival and proliferation were mediated via extracellular matrix (ECM) adhesion proteins which were printed on the glass surface. ECM proteins can be selected based on the cell type and the study. Fibronectin is chosen for surface modification by its good adhesive properties for most commonly used cell lines in surface functionalization. The interactions of fibronectin with other matrix components can provide binding sites for cells to the matrix. We made a further study to see whether fibronectin had any positive effects on cell attachment and viability in the microchannel. Specifically, the inner wall of cell culture chamber was coated with fibronectin for 1 h at the concentration of 50 μg/mL until cell was seeded. Cells in the control group grew on glass surface directly with no alterations. Figure 2 shows the microscopy images of A549 cells cultured in the microfluidic channel modified with or without fibronectin after 12 h. From Fig. 2a, we found cells were fully spread and attached on glass surface with fibronectin alternation. In contrast, poor cell spreading is observed in Fig. 2b without fibronectin. The cell morphology differences suggested that fibronectin-coated surfaces enhanced cell adhesion and growth on glass slides.
3.2 Characterization of oxygen gradient
To develop a method for simulating the in vivo oxygen microenvironment of tumor cells, NaOH and pyrogallol were introduced into the reaction channels for oxygen scavenging. Oxygen gradient established in the cell culture chamber was characterized by using oxygen-sensitive dye. The fluorescence of the dye can be quenched by the presence of oxygen. So oxygen tensions were determined by observing the fluorescence intensity in cell culture channel.
The relationship between oxygen content and fluorescence intensity of the dye was calibrated by two known oxygen tensions, and then, the quenching constant Kq was obtained. Oxygen content was calculated using the Stern–Volmer equation. The experiment was carried out with three independent devices. The oxygen distribution curve is illustrated in Fig. 3. The range of oxygen concentration gradient was from approximately 2.3 to 11 %, which was suitable for in vitro studies. From the results, we found the oxygen gradient was successfully generated by using the spatially confined chemical reaction method, and the entire microfluidic device can be placed inside a conventional cell incubator without disturbing global gaseous compositions. The developed microfluidic device was then exploited for cell culture with a simple, robust, and straightforward setup by taking the oxygen gradient into consideration for drug screening.
3.3 Cell viability under oxygen gradient
Changes in oxygen availability often have drastic effects in cell bioactivities. Significant contributions have been made in understanding how tumor cells tend to adapt and survive in low-oxygen environments. Previous studies reported that low-oxygen conditions induce cell apoptosis when oxygen level decreased to 0.5 % or less (Snyder and Chandel 2009). However, cells do not undergo apoptosis when the range of oxygen tensions ranged from 0.5 to 3 %. Hypoxia activated a variety of cellular events to keep cell alive instead of leading to cell death. Therefore, we investigated the effects of oxygen gradient on tumor cell growth and cell viability in this research to get a better understanding in hypoxia-activated cell activities.
Cell chambers (800 μm width) were divided into four regions, and the width of each region was 200 μm. The four regions shown in Fig. 4 were marked as R1 (0–200 μm), R2 (200–400 μm), R3 (400–600 μm), and R4 (600–800 μm). The average oxygen level of each region was calculated for comparing the cytotoxicity of anticancer drugs under corresponding oxygen content. The number of average oxygen level in R1, R2, R3, and R4 is 3, 4.6, 6.4, and 8.5 %, respectively. The viability of A549 cells was investigated for the quantitative assessment of cell activity under certain oxygen content.
The fluorescence image of the A549 cells a treated under normoxic or b oxygen gradient condition. Four zones (R1, R2, R3, and R4) are separated in the chamber for the quantitative evaluation of cell activity. c The viability of cells in the four regions under normoxia or hypoxia conditions. The data are expressed as the mean ± SD
The fluorescence images of A549 cells cultured under normoxic (21 % O2) and hypoxic (1 % O2) conditions are shown in Fig. 4a and b, respectively. Cell viability was assessed using live/dead cell staining. From the results, we found A549 cells were incubated with good cell adhesion and proliferation properties in the cell culture microchamber. In addition, the presence of oxygen gradient had no significantly effects on cell viability during the 12-h treatment. Thus, according to the data, it can be obviously seen that moderate hypoxia does not lead to cell death and the viability has no observed differences between normal and hypoxia conditions.
3.4 Cell responses to anticancer drugs under oxygen gradient
The hypoxic microenvironment in solid tumors is considered as one of the principal reasons for the failure of cancer therapy (Baguley 2010). Tumor cells often make different biological responses based on the different oxygen contents around the tissue. Besides, hypoxia caused intrinsically resistant to cancer therapy due to the increased distance from the vasculature. Moreover, cell proliferation is inhibited with reduced oxygen availability, and hypoxic cancer cells are protected from chemotherapeutic agents. In order to investigate cell response under different oxygen conditions, the on-chip antitumor drug testing was performed under the developed oxygen gradient. A549 cells were cultured for at least 12 h in the cell culture channel to reach 60–80 % confluences before drug treatment. Cisplatin and TPZ were chosen for drug testing in this research. Figure 5 shows the fluorescence images of the stained A549 cells treated with pure growth media, cisplatin, or TPZ under oxygen gradient. A live/dead stain solution containing calcein AM (2 mM) and PI (8 mM) from Live/Dead Viability/Cytotoxicity Kit was used to assess the cell viability in the hypoxia microenvironment. Live cells were stained green (calcein AM stained), while dead cells red (PI stained). As we can see in Fig. 5a, live cells spread to entire microchannel and expressed great cell activities. No significant differences were found in four regions at various oxygen concentrations based on the live/dead stained results. Then, we explored the cell viability after drug exposure. To assess drug cytotoxicity accurately, we calculated the percentage of live cells in three dependent measurements. Unpaired two-tailed Student’s t tests were used to perform the comparative analysis under different oxygen concentrations.
The fluorescence image of the A549 cells treated with pure growth media (a), cisplatin (b), and TPZ (c) under oxygen gradient; d viability of A549 cells treated with pure growth media, TPZ and cisplatin with the concentration 100 μM. Four zones marked dashed line are designated in cell culture channel for the quantitative evaluation of cell activity. *Indicates significant differences from the group treated with TPZ under hypoxia condition, **means P ≤ 0.01; #Indicates significant difference from treated with cisplatin group under hypoxia condition, #means P ≤ 0.05; the data of significant difference of both groups are attained by comparing with the data of R1 region. The data are expressed as the mean ± SD
The A549 cells treated with cisplatin and TPZ showed different drug responses. As shown in Fig. 5c, when the oxygen tensions increased from R1 (3 % O2) to R4 (8.5 % O2), the number of live cells after cisplatin exposure decreased from 65.3 to 37.9 %. The highest percentage of live cells was observed in R4 region with the lowest oxygen content. It is because that the cells at low oxygen level divide less rapidly than those which are fully oxygenated. This relatively low rate of cell proliferation limits the effectiveness of cisplatin which active against rapidly proliferating cells and may lead to cell resistance. In contract, cells showed more sensitivity to TPZ than cisplatin when oxygen concentration decreased. The different results can be explained by its hypoxia-selective cytotoxicity. TPZ is the first compound to be developed specifically as a hypoxic anticancer drug, which gains medicinal activity from its ability to selectively damage DNA in the hypoxic cells found inside solid tumors (Junnotula et al. 2008). At low oxygen level, the TPZ radical intermediate produced by intracellular reduction leads to extensive DNA damage which can finally lead to cell death. According to Fig. 5b, the viability of A549 cells were changed under different oxygen conditions after TPZ treatment. When the oxygen concentration gradually reduced, the cell death rate increased from 43.1 to 63.2 %. According to the data in Fig. 5d, both cisplatin and TPZ showed cytotoxicity to A549 cells under hypoxia microenvironment in the microfluidic chip, but the viability changes in different regions showed opposite trend. The percentage of live cells with TPZ treatment was 1.7 times higher than cisplatin in R4 region, indicated that TPZ is more effective to hypoxic cells than cisplatin. Since tumor hypoxia represents a unique and attractive target to develop strategies for cancer therapy, a large number of studies were conducted on drugs which are selectively toxic against hypoxic cells. For example, Sartorelli and Rockwell have reported that mitomycin C preferentially kills hypoxic compared with normoxic cells in vitro (Rockwell et al. 1982). Another hypoxic-selective drug AQ4N has been proved to have a high affinity for DNA and substantial activity against hypoxic cells in various transplanted tumors (Patterson 1993, 2002). As described above, hypoxic cells are demonstrated to be associated with poor treatment outcome regardless of the modality, and the changes in oxygen content have direct impaction on the function of anticancer drugs, suggesting that the oxygen content in the microenvironment of solid tumors should be considered in the studies of drug screening and clinical cancer treatment.
3.5 Traditional analysis of cell viability
Further studies were accomplished by using conventional well plates in an oxygen-tension-controlled cell incubator under normoxic or hypoxic conditions to investigate the cytotoxicity of cisplatin and TPZ. The results shown in Fig. 6 indicated the cell resistance to cisplatin. However, TPZ caused a large number of cell death under the low oxygen level (1 % O2). These data showed similar trends with the results obtained from microfluidic chip, which gave direct evidence that the developed microfluidic chip expressed great cell compatibility and were suitable for drug testing in further studies. Based on this microfluidic platform, we constructed an oxygen gradient which was similar to in vivo situation and showed the oxygen-dependent cytotoxicity of TPZ and cisplatin. The microfluidic technology showed great promise for drug-screening studies, which can mimic the situation of tumor tissues due to its great controllability in both spatial and temporal domains.
The viability of A549 cells treated with TPZ and cisplatin with the concentration of 100 μM under normoxic and hypoxia conditions in 96-well plate, respectively. Hypoxic treatment is carried out in an incubator with 1 % O2, 5 % CO2, and 94 % N2 for 12 h. *Indicates significant differences from treated with TPZ group and cisplatin group under hypoxia condition. The data are expressed as the mean ± SD
4 Conclusion
In this research, we developed a microfluidic device capable of generating oxygen gradient for in vitro cytotoxicity studies. Controlling of the oxygen tensions was accomplished by using spatially confined chemical reaction to scavenge oxygen in neighboring cell culture channel. The present data indicated that the device can be easily operated and also provided more reliable data for in vitro studies of cell responses to anticancer drugs under different oxygen concentrations. We separated the oxygen gradient into four regions and calculated the average oxygen concentration for each region. The obtained data revealed that both cisplatin and TPZ showed oxygen-dependent cytotoxicity to A549 cells. TPZ caused higher cell death rate under lower oxygen content, while cisplatin performed a converse outcome with increased cell resistance. The developed microfluidic platform showed great promise and advantages for cell studies in various biomedical applications under different oxygen concentrations. It is also noted that the oxygen level in cell microenvironment is necessary to be taken into consideration for drug screening and clinical cancer therapy in further studies.
References
Adler M, Polinkovsky M, Gutierrez E, Groisman A (2010) Generation of oxygen gradients with arbitrary shapes in a microfluidic device. Lab Chip 10:388–391
Ai XN, Liang QL, Luo MN, Zhang K, Pan JM, Luo GA (2012) Controlling gas/liquid exchange using microfluidics for real-time monitoring of flagellar length in living Chlamydomonas at the single-cell level. Lab Chip 12:4516–4522
Aquino-Parsons C, Luo C, Vikse CM, Olive PL (1999) Comparison between the comet assay and the oxygen microelectrode for measurement of tumor hypoxia. Radiother Oncol 51:179–185
Baguley BC (2010) Multiple drug resistance mechanisms in cancer. Mol Biotechnol 46:308–316
Brown JM, Wilson WR (2004) Exploiting tumor hypoxia in cancer treatment. Nat Rev Cancer 4:437–447
Carreau A, Hafny-Rahbi BE, Matejuk A, Grillon C, Kieda C (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15:1239–1253
Chang CW, Cheng YJ, Tu M, Chen YH, Peng CC, Liao WH, Tung YC (2014) A polydimethylsiloxane-polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Lab Chip 14:3762–3772
Chen YA, King AD, Shih HC, Peng CC, Wu CY, Liao WH, Tung YC (2011) Generation of oxygen gradients in microfluidic devices for cell culture using spatially confined chemical reactions. Lab Chip 11:3626–3633
Durand RE (1993) The influence of microenvironmental factors during cancer therapy. In Vivo 8:691–702
Funamoto K, Zervantonakis IK, Liu Y, Ochs CJ, Kim C, Kamm RD (2012) A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab Chip 12:4855–4863
Graceber TG, Smanian CO, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ (1996) Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature 379:88–91
Grasselli F, Basini G, Bussolati S, Bianco F (2005) Cobalt chloride, a hypoxia-mimicking agent, modulates redox status and functional parameters of cultured swine granulosa cells. Reprod Fertil Dev 17:715–720
Gray MD, Mann M, Nitiss JL, Hendershot LM (2005) Activation of the unfolded protein response is necessary and sufficient for reducing topoisomerase IIα protein levels and decreasing sensitivity to topoisomerase-targeted drugs. Mol Pharmacol 68:1699–1707
He TX, Liang QL, Zhang K, Mu X, Luo TT, Wang YM, Luo GA (2011) A modified microfluidic chip for fabrication of paclitaxel-loaded poly (l-lactic acid) microspheres. Microfluid Nanofluid 10:1289–1298
Junnotula V, Sarkar U, Sinha S, Gates KS (2008) Initiation of DNA strand cleavage by 1, 2, 4-benzotriazine 1, 4-dioxide antitumor agents: mechanistic insight from studies of 3-methyl-1, 2, 4-benzotriazine 1, 4-dioxide. J Am Chem Soc 131:1015–1024
Khanal G, Chung K, Solis-Wever X, Johnson B, Pappas D (2011) Ischemia/reperfusion injury of primary porcine cardiomyocytes in a low-shear microfluidic culture and analysis device. Analyst 136:3519–3526
Kondo A, Safaei R, Mishima M, Niedner H, Lin X, Howell SB (2001) Hypoxia-induced enrichment and mutagenesis of cells that have lost DNA mismatch repair. Cancer Res 61:7603–7607
Lam RH, Kim MC, Thorsen T (2009) Culturing aerobic and anaerobic bacteria and mammalian cells with a microfluidic differential oxygenator. Anal Chem 81:5918–5924
Ledoux S, Yang R, Friedlander G, Laouari D (2003) Glucose depletion enhances P-glycoprotein expression in hepatoma cells role of endoplasmic reticulum stress response. Cancer Res 63:7284–7290
Lo JF, Sinkala E, Eddington DT (2010) Oxygen gradients for open well cellular cultures via microfluidic substrates. Lab Chip 10:2394–2401
Mehta G, Mehta K, Sud D, Song JW, Bersano-Begey T, Futai N, Takayama S (2007) Quantitative measurement and control of oxygen levels in microfluidic poly (dimethylsiloxane) bioreactors during cell culture. Biomed Microdevices 9:123–134
Mu X, Liang QL, Hu P, Ren KN, Wang YM, Luo GA (2010) Selectively modified microfluidic chip for solvent extraction of Radix Salvia miltiorrhiza using three-phase laminar flow to provide double liquid–liquid interface area. Microfluid Nanofluid 9:365–373
Park J, Bansal T, Pinelis M, Maharbiz MM (2006) A microsystem for sensing and patterning oxidative microgradients during cell culture. Lab Chip 6:611–622
Patterson LH (1993) Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metast Rev 12:119–134
Patterson LH (2002) Bioreductively activated antitumor N-oxides: the case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy. Drug Metab Rev 34:581–592
Polinkovsky M, Gutierrez E, Levchenko A, Groisman A (2009) Fine temporal control of the medium gas content and acidity and on-chip generation of series of oxygen concentrations for cell cultures. Lab Chip 9:1073–1084
Ren KN, Liang QL, Mu X, Luo GA, Wang YM (2009) Miniaturized high throughput detection system for capillary array electrophoresis on chip with integrated light emitting diode array as addressed ring-shaped light source. Lab Chip 9:733–736
Rockwell S, Kennedy KA, Sartorelli AC (1982) Mitomycin-C as a prototype bioreductive alkylating agent: in vitro studies of metabolism and cytotoxicity. Int J Radiat Oncol 8:753–755
Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT (2009) Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med 9:442–458
Skolimowski M, Nielsen MW, Emnéus J, Molin S, Taboryski R, Sternberg C, Geschke O (2010) Microfluidic dissolved oxygen gradient generator biochip as a useful tool in bacterial biofilm studies. Lab Chip 10:2162–2169
Snyder CM, Chandel NS (2009) Mitochondrial regulation of cell survival and death during low-oxygen conditions. Antioxid Redox Sign 11:2673–2683
Strese S, Fryknas M, Larsson R, Gullbo J (2013) Effects of hypoxia on human cancer cell line. BMC Cancer 13:331–342
Sud D, Mehta G, Mehta K, Linderman J, Takayama S, Mycek M (2006) Optical imaging in microfluidic bioreactors enables oxygen monitoring for continuous cell culture. J Biomed Opt 11:50504–50506
Tredan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer I 99:1441–1454
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465
Verbridge SS, Choi NW, Zheng Y, Brooks DJ, Stroock AD, Fischbach C (2010) Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng Part A 16:2133–2141
Yang K, Han S, Shin Y, Ko E, Kim J, Park KI, Cho SW (2013) A microfluidic array for quantitative analysis of human neural stem cell self-renewal and differentiation in three-dimensional hypoxic microenvironment. Biomaterials 34:6607–6614
Zhang K, Liang QL, Ma S, Mu X, Hu P, Wang YM, Luo GA (2009) On-chip manipulation of continuous picoliter-volume superparamagnetic droplets using a magnetic force. Lab Chip 9:2992–2999
Zhang K, Liang QL, Ma S, He TX, Ai XN, Hu P, Luo GA (2010) A gravity-actuated technique for flexible and portable microfluidic droplet manipulation. Microfluid Nanofluid 9:995–1001
Zhang K, Liang QL, Ai XN, Hu P, Wang YM, Luo GA (2011) Comprehensive two-dimensional manipulations of picoliter microfluidic droplets sampled from nanoliter samples. Anal Chem 83:8029–8034
Zhong W, Urayama P, Mycek MA (2003) Imaging fluorescence lifetime modulation of a ruthenium-based dye in living cells: the potential for oxygen sensing. J Phys D Appl Phys 36:1689–1695
Acknowledgments
We greatly appreciate the financial support from the Ministry of Science and Technology (2013ZX09507005), National Natural Science Foundation of China (21175080, 21235004, 21475071, 21275082), and Beijing Municipality (Z131100006513009).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Z., Liu, Z., Li, L. et al. Investigation into the hypoxia-dependent cytotoxicity of anticancer drugs under oxygen gradient in a microfluidic device. Microfluid Nanofluid 19, 1271–1279 (2015). https://doi.org/10.1007/s10404-015-1637-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-015-1637-6