Abstract
A molecular-dynamics (MD) study of non-reacting and disparate mass binary gas mixture is conducted. The interaction properties of a typical mixture of gases on the walls of a nanochannel, at moderately rarefied conditions, are investigated with MD. The study discusses the method of calculation of the energy and the momentum accommodation coefficients for a binary gas mixture confined in a nanochannel. The thermal distributions are obtained from the imposed thermal wall conditions using MD. While keeping the bulk number density a constant, the interaction properties of gas mixtures at different molar concentration of individual components are determined. The accommodation properties are found to increase with an increase in concentration of heavier gas component in the gas mixture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The main purpose of this article is to report the method of calculation of the interaction properties of a disparate mass binary gas mixture on the walls of a nanochannel using molecular-dynamics (MD) simulation. The interaction properties of a gaseous or liquid mixture in a micro/nano-sized structure, is a subject of great interest in the present time. The thermo-physical properties of a gas mixture confined in a nano-sized structure are rarely studied either experimentally or by numerical simulation due to the complex nature of physics involved in such systems. One of the difficulties in the determination of accommodation properties of a gas mixture is the calculation of thermal wall properties for a prescribed condition. Nevertheless, significant advances have been made in the overall understanding of the gas–surface interaction properties through extensive computer simulation and comparing it with the experiment (Harley et al. 1995; Arkilic et al. 2001). The theoretical methods, simulation studies and experimental results of flow through micro/nanochannel are extensively discussed in recent studies (Cao et al. 2009; Morini et al. 2011).
The rarefied gas flow can be analyzed by solving Boltzmann equation, direct simulation, MD etc. (Bhattacharya and Lie, 1989; Jenkins and Mancini 1989; Siewert and Valougeorgis 2004; Naris et al. 2005; Heffelfinger and van Swol 1994). The MD method enables a deeper understanding of different mechanisms involved in the gas–surface interaction, which greatly affect the transport properties of a gas in a nano-sized channel. The majority of the MD simulation studies on gas mixtures have been restricted to separation of gas mixtures through nanopores (Xu et al. 1998; Firouzi et al. 2007), diffusion of gases (Kandemir and Sevilgen 2008; Malek and Sahimi 2010), interaction of single gas on gas-adsorbed solid surfaces (Black and Bopp 1987; Yamamoto et al. 2007) etc. In addition, there have been a few MD implementations which report the calculation of accommodation coefficients (ACs) for a single gas species (Yamamoto 2002; Spijker et al. 2010; Prabha and Sathian 2012).
In the recent past, there have been attempts to explore the accommodation properties of a single gas and gas mixture at moderately rarefied conditions by experimental means (Trott et al. 2011; Pitakarnnop et al. 2010; Yamaguchi et al. 2011). They report incomplete or diffuse accommodation depending on different surface conditions, type of gas, and rarefaction levels. However, it is observed that the incomplete accommodation can considerably affect the non-equilibrium heat transfer properties and flow parameters (John et al. 2011; Prabha and Sathian 2012; Zhang et al. 2010, 2012). Consequently, additional efforts for the calculation of the ACs is well justified by the theoretical and the practical significance of these coefficients.
In the present study, MD simulations have been performed to investigate the gas–solid interaction properties of a binary gas mixture at non-equilibrium conditions in a nanochannel. The energy accommodation coefficient (EAC) and the momentum accommodation coefficient (MAC), along with its partial components, are obtained for different molar proportions. The thermal wall properties are obtained from the thermal wall distributions using the kinetic theory approximation of equivalent mass, as well as using the imposed thermal wall conditions. The details of the simulation are presented in the next section and the results in the later sections.
2 Details of simulation
The thermal accommodation can be measured from a parallel plate assembly, wherein the plates are kept at different temperatures (Trott et al. 2011). The non-equilibrium accommodation properties can be modeled in a similar way by keeping the walls at different temperatures (Yamamoto 2002; Spijker et al. 2010). This non-equilibrium conditions can also be effectively modeled by controlling the gas and solid surfaces (platinum) of the nanochannel at different temperatures. In the present study, the temperature of both the walls are maintained at 300 K using a Berendsen thermostat (Berendsen et al. 1984). In this method, the temperature control is achieved by a weak coupling to an external heat bath. The method is ideally suited to NEMD simulations, as in the present study.
A three-dimensional system in which a mixture of monatomic gases (argon and xenon) confined between infinite parallel surfaces is selected for the present study. The infinite parallel solid walls of the nano-channel are assumed to be flat, smooth and without any defects. The dimensions of the simulation box are 10 × 12 × 10 nm. Initially, the solid atoms are packed with FCC structure and the gas atoms randomly with a number density of 1.27 nm−3. The spacing between the solid walls (L) is 10 nm and, hence, the rarefaction level of the gas is in the transition regime (Bird 1994). The kinetic theory model for hard sphere molecules is used to calculate the mean free path and the Knudsen number is found to vary from 0.114 (at C = 0) to 0.152 (at C = 1).
The interactions are modeled using Lennard–Jones (LJ) potential. The potential is defined as
wherein σ and \(\epsilon\) represents the characteristic length and energy parameters, respectively. The LJ hard sphere model with a cut-off distance equal to 2.5σ is used for all interactions and the parameters used in the simulation are listed in Table 1 (Yamamoto 2002; Spijker et al. 2010). The cut-off distance is selected by considering mainly the force field strength, rarefaction levels, the accuracy of parameters calculated, and the computational cost associated with it. The cross type non-bonded interaction parameters for Ar–Xe interaction are computed by Lorentz–Berthelot mixing rules (Allen and Tildesley 1994). The trajectories of the atoms are calculated using velocity-verlet algorithm with a time step of 2 fs. The production run is for 1 ns duration. Periodic boundary conditions are employed in all directions. Parallel algorithms for classical molecular dynamics are used for implementing MD simulations with short range forces (LAMMPS) (Plimpton 1995).
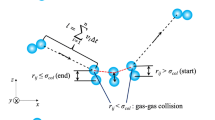
When an atom is moving towards the wall and crosses the plane, the incident components of collision are recorded and when the atom crosses the plane while moving away from the wall, the reflected components are recorded. Thus a collision data contain information regarding the incident components and reflected components of a collision. The location of the plane is determined by considering the influence of the wall on a gas atom and the mean free path of the gas atoms. In the present study, the gas is so rarefied that the possibility of collisions with another gas molecule near the wall is rare. The location of the collision tracking plane is fixed to be at a wall–gas interaction cut-off distance, which is at 0.8 nm from the solid wall (Spijker et al. 2010; Prabha and Sathian 2012). It is the 2.5 times the characteristic diameter (σ) for the largest of the two gas atoms (xenon). Nevertheless, it is a larger domain for argon when compared to xenon. However, while considering the strength of interaction force field of argon and the rarefaction levels, this extra distance (2.7 σ) will not cause any trouble to the collision data and subsequent calculation of interaction properties.
3 Accommodation coefficients
The accommodation coefficient (AC) is a measure to quantify the interaction between a wall and a fluid. It indicates the degree to which molecules are accommodated to the surface. The AC can be defined as
wherein K denotes any quantity such as energy or momentum; or its components in different directions. The subscripts i, r are for the incident, reflected conditions, respectively. The energy and the MACs are identified with subscripts E and p, respectively. The quantities with subscript W stand for thermal wall conditions and are determined from the thermal wall distributions (Spijker et al. 2010). In thermal wall model, the parallel and perpendicular components of velocities are assigned separately. The distributions are given as
in which f x and f y are the reflected velocity distribution functions in the tangential and normal directions (Fig. 1). In equations, the variants m, k, T, and v represent the mass, Boltzmann constant, temperature, and velocity of the particles, respectively.
4 Results and discussion
In an effort to study the gas–surface interaction properties, some of the general results for gases are presented first to demonstrate the credibility of the present MD model and the results for gas mixtures are presented subsequently. Unless specified, the results reported are for gas at 400 K and the mixture at equal molar concentrations. The temperature of the gas is controlled in the region between collision tracking planes, so as not to influence the accommodation mechanism. We have carried out simulation for different proportion of gas mixtures, keeping the bulk number density of the gas mixture a constant. In the beginning, some preliminary results are presented to check the robustness of simulation model and the efficiency of thermostat. The gas–surface interaction properties are presented in the subsequent sections.
4.1 Density profile
Figure 2 shows the variation of density with respect to the distance from the wall. The density distributions are normalized with the bulk number density(n 0). As seen from the figure, a density build up is observed near the wall. The variation of the density distribution for the gas mixture attenuates as the distance from the wall increases and it oscillates near the value of unity. This behavior is because of the presence of the attractive interaction potential. As it moves from the wall, the influence of wall on the fluid atom decreases and has no effect on the fluid atom the beyond the cut-off distance. The density accumulation near the wall slightly reduces the density in the bulk. This phenomenon has been reported in studies where single gas is confined between the solid walls (Barisik and Beskok 2011; Markvoort et al. 2005).
The density variation for the individual components of the gas mixture is also studied. As seen from Fig. 2, the distribution of heavier gas has a higher peak indicating that it has greater affinity towards the wall, due to a higher value of the interaction potential. This is in good agreement with the study on density profiles with different wall–fluid interaction parameters (Barisik and Beskok 2012). These differences in the mass and interaction potentials can make differences in the interaction properties of the individual components, and hence the accommodation properties.
4.2 Energy distribution
Consider, the two gases at the same number density and temperature but not mixed. The two gases will be having same energy (kinetic) distribution. To validate this, energy distribution of the atoms approaching the wall are plotted as shown in Fig. 3. The two distributions for the single gases follow the same path. Furthermore, the energy distribution of the mixture of gases also traces the same path. This is true for the individual components of the gas mixture, too. This validates the results from the present simulation with the well-established laws for the mixture of gases. In contrast, the individual components of a gas mixture at a constant temperature will be having the same energy distribution as that of bulk gas, and this, in turn, shows that the temperature control technique is efficiently mimics the physical conditions.
4.3 Calculation of accommodation coefficients
The mean molecular (or the effective) mass m of a gas mixture is calculated using the kinetic theory model as
where C is the molar concentration (Present and Debethune 1949; Naris et al. 2005). C is calculated as
where n is the number density and the subscripts 1, 2 denotes argon, xenon respectively. The energy and the momentum accommodation coefficients, and its partial components, are calculated using Eq. 2 and are tabulated in Table 2. The ACs are calculated for average incident and reflected properties. To determine the consistency of calculation, as reported in our previous study (Prabha and Sathian 2012), the collision data (the incident and reflected components) are divided into an equal number of classes and the standard deviation of ACs obtained from different classes is given in parenthesis. Nearly 150,000 collisions are sufficient to yield a statistically stable calculated value for different molar proportions. For the calculation of normal momentum accommodation coefficient (NMAC), the absolute values of velocity components are used. To calculate tangential momentum accommodation coefficient (TMAC), the incident direction is taken as positive, and if the direction changes on collision, it is taken as negative.
Note that, the EAC first decreases (up to case C3) with an increase in the concentration of argon, and then increases (Fig. 4). It is perhaps surprising to see such a atypical variation for EACs. However, for the single gas (C = 0.0, 1.0), the results obtained are as expected; the heavier mass species is well accommodated than the lighter one. In addition, the NMAC and the MAC show a similar variation as that of EAC with a change in molar concentration. In all these cases, the thermal wall distributions take a non-zero average value. It should be noted that the thermal wall momentum distribution in the tangential direction, which is Gaussian distribution (Eq. 3), is averaged to zero value. Accordingly, in the calculation of TMAC only the incident and reflected momentum are the variants (Eq. 2), and the variation of which shows a smooth transition as the molar concentration of the constituent gas changes from 0 to 1. The mixture of gases differ from single species gases mainly in the effective mass and the law of inter atomic interaction of gases. In the prescribed simulation conditions, the validity of the kinetic theory model based on effective mass can be validated by imposing the thermal wall condition in the simulation.
4.4 Thermal wall properties
For a moderately rarefied gas interacting with a solid surface, the thermal wall distribution applies to the surface that is near to thermodynamic equilibrium with the gas surrounding it. When a gas and a solid surface are in thermodynamic equilibrium, the reflected distributions from the solid surface are considered to have the properties of a thermal wall. This condition can be achieved through MD method by keeping the solid surface and the fluid at the same temperature. At the thermal equilibrium conditions, the expected values of both the incident and reflected velocity distributions will be equal in magnitude. This is true for both the tangential and normal components of velocity distributions.
4.4.1 Thermal equilibrium for a single gas
For the thermal wall simulations of a single gas (argon, C = 1.0), the gas is kept at a constant temperature (300 K) which is on par with the wall temperature. The properties of the reflected distributions are obtained from the collision data. The reflected velocity distributions at this condition in the normal and tangential directions are depicted in Fig. 5. For simplicity and clarity, only the positive axis is shown for the tangential component and the absolute values are considered for the normal component. At thermal equilibrium, the incident and reflected distributions are identical for both the tangential and normal components. The average values of the reflected energy and momentum can be calculated from the reflected velocity distributions. The expected values of average momentum and energy components thus obtained from the MD simulations are in very good agreement with the expected values calculated from the thermal wall distributions (Eqs. 3, 4). The comparison of energy components are given in Table 3 and it shows that the MD simulation data are in consistent with the analytical results. Consequently, it can be concluded that the imposed thermal wall conditions fairly mimics the thermal equilibrium conditions.
The velocity distributions at thermal wall condition for a single species gas are given (C = 1.0). The points represent the distributions obtained from MD simulations and lines represent the theoretical prediction. The positive axis is shown for the tangential component and the absolute values are considered for the normal component
4.4.2 Thermal equilibrium for mixture of gases
An identical approach is adopted for the mixture of gases. The reflected distributions of the gas mixture (C = 0.5), when the gas temperature is controlled closely to the surface temperature, are shown in Fig. 6. At the thermal wall condition, the velocity distribution of the reflected molecules from the surface is identical with the distribution of molecules approaching the surface (Fig. 6). The thermal wall distributions obtained from Eqs. 3 and 4 are now compared with the distributions obtained from the imposed thermal wall conditions in MD. Figure 6 shows that both the tangential and normal distributions obtained from MD deviates from the analytical prediction using mean molar mass. This suggests that use of the kinetic theory approximation of the effective mass is unrealistic for the thermal wall distributions of a disparate mass gas mixture.
The velocity distributions at thermal wall condition for a mixture of gases are given (C = 0.5). The points represent the reflected distributions and the continuous lines represent incident distributions obtained from MD simulations. The dotted lines represent the theoretical prediction. The absolute values are considered for the normal component and only the positive axis is shown for the tangential component
4.4.3 AC using thermal wall simulation data
The average values of the thermal wall configuration for the gas mixture can now be obtained from the collision data. Using these expected values and Eq. 2, different components of the energy accommodation are re-calculated. The EACs are calculated for a non-equilibrium condition in which gas temperature is maintained at 400 K. Figure 7 shows the variation of EAC and its partial components with different molar concentration. For EACs, the heavier gas as a single species takes the maximum value. The gas mixture shows a decreasing value of accommodation as the concentration of the lighter gas increases, which is anticipated. This trend that the accommodation properties decrease with a decrease in molecular weight, provided the other conditions remain the same, is consistent with the experimental as well as numerical studies (Trott et al. 2011; Spijker et al. 2010).
For EACs, the highest value is obtained for the normal component and the lowest value is for the tangential component. The value for the total energy accommodation lies between the parallel and perpendicular components. This shows that the accommodation properties increases if there is change in the direction of motion after collision. For normal components, the change in direction is definite as the collisions are at the scattering conditions.
When MAC calculated with the thermal wall properties obtained from the MD simulation, a similar variation, a decreasing value of accommodation as the concentration of the lighter gas increases, is observed for momentum components too (not shown for brevity). This reiterates the fact that the accommodation properties increase with an increase in molecular weight. For the MACs, the tangential components take higher values (Table 2). For the calculation of TMAC the incident direction was taken as positive and if the direction changes after collision it was considered as negative. Thus, for a stagnant gas, the change in direction after collision is more likely to happen when compared to a flowing fluid. Thus, the magnitude of tangential components take higher values. The momentum accommodation for the total velocity is found to be lower than its partial components, and its value lies between the values of the tangential and normal components. This result is in good agreement with the behavior of ACs of a single species gas at moderately rarefied condition (Spijker et al. 2010). The calculated values of ACs primarily depend on the values of the potential parameters whereby the intensity of interactions are defined.
The surface conditions, orientation of crystallographic planes, material properties etc., significantly affect the accommodation properties in nanoscale systems. In addition, the simulation model that represent a physical system can have different approximations such as the potential parameters (e.g., Lorentz–Berthelot mixing rules). All these conditions can influence the calculated values of accommodation properties. Nevertheless, the analytical model that describe the thermal wall properties do not accommodate these effects. Apparently this work suggests that the error, if any, in the calculation of thermal wall properties can be inadvertently nullified by calculating the thermal equilibrium properties from the same physical model. Thus, with the method implemented in the present work, the thermal wall properties and the accommodation properties of a gas mixture in a nano-sized structure can be effectively calculated using MD.
5 Conclusion
In summary, we have analyzed the gas–surface interaction properties of typical monatomic gases, argon and xenon, separately and their mixture in different proportions. The density variation for the individual components of gas mixture show that the heavier gas has a greater accumulation near the wall than the lighter one. The thermal wall properties of the binary gas mixture were obtained from the imposed thermal wall. The comparison of thermal wall velocity distributions obtained from MD with the kinetic theory approximation showed that the parameters obtained from MD from the imposed equilibrium conditions is appropriate to calculate accommodation coefficients. The thermal wall properties thus obtained were used to calculate the accommodation coefficients for the mixture. The accommodation properties are found to increase with an increase in the mole fraction of heavier gas. This method can be employed for the determination of the momentum and energy interactions of a mixture of gases in nanoscale systems.
References
Allen MP, Tildesley DJ (1994) Computer simulation of liquids. Oxford University Press, New York
Arkilic EB, Breuer KS, Schmidt MA (2001) Mass flow and tangential momentum accommodation in silicon micromachined channels. J Fluid Mech 437:29–43
Barisik M, Beskok A (2011) Equilibrium molecular dynamics studies on nanoscale-confined fluids. Microfluid Nanofluid 11(3):269–282
Barisik M, Beskok A (2012) Surface–gas interaction effects on nanoscale gas flows. Microfluid Nanofluid 1–10. doi:10.1007/s10404-012-1000-0
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Bhattacharya DK, Lie GC (1989) Molecular-dynamics simulations of nonequilibrium heat and momentum transport in very dilute gases. Phys Rev Lett 62(8):897–900
Bird GA (1994) Molecular gas dynamics and the direct simulation of gas flows. Oxford University Press, New York
Black JE, Bopp P (1987) A molecular dynamics study of the behaviour of xenon physisorbed on Pt(111): Coverages less than one monolayer. Surf Sci 182(1–2):98–124
Cao BY, Sun J, Chen M, Guo ZY (2009) Molecular momentum transport at fluid-solid interfaces in MEMS/NEMS: a review. Int J Mol Sci 10(11):4638–4706
Firouzi M, Tsotsis TT, Sahimi M (2007) Molecular dynamics simulations of transport and separation of supercritical carbon dioxide-alkane mixtures in supported membranes. Chem Eng Sci 62(10):2777–2789
Harley JC, Huang Y, Bau HH, Zemel JN (1995) Gas flow in micro-channels. J Fluid Mech 284:257–274
Heffelfinger GS, van Swol F (1994) Diffusion in Lennard-Jones fluids using dual control volume grand canonical molecular dynamics simulation (DCV-GCMD). J Chem Phys 100(10):7548–7552
Jenkins JT, Mancini F (1989) Kinetic theory for binary mixtures of smooth, nearly elastic spheres. Phys Fluids A Fluid Dyn 1(12):2050–2057
John B, Gu XJ, Emerson DR (2011) Effects of incomplete surface accommodation on non-equilibrium heat transfer in cavity flow: a parallel DSMC study. Comput Fluids 45(1):197–201
Kandemir I, Sevilgen FE (2008) Molecular dynamics simulation of helium–argon gas mixture under various wall conditions. Mol Simul 34(8):795–808
Malek K, Sahimi M (2010) Molecular dynamics simulations of adsorption and diffusion of gases in silicon-carbide nanotubes. J Chem Phys 132(1):014,310
Markvoort AJ, Hilbers PAJ, Nedea SV (2005) Molecular dynamics study of the influence of wall-gas interactions on heat flow in nanochannels. Phys Rev E 71(6):066702
Morini GL, Yang Y, Chalabi H, Lorenzini M (2011) A critical review of the measurement techniques for the analysis of gas microflows through microchannels. Exp Thermal Fluid Sci 35(6):849–865
Naris S, Valougeorgis D, Kalempa D, Sharipov F (2005) Flow of gaseous mixtures through rectangular microchannels driven by pressure, temperature, and concentration gradients. Phys Fluids 17(10):100607
Pitakarnnop J, Varoutis S, Valougeorgis D, Geoffroy S, Baldas L, Colin S (2010) A novel experimental setup for gas microflows. Microfluid Nanofluid 8(1):57–72
Plimpton SJ (1995) Fast parallel algorithms for short-range molecular dynamics. J Comp Phys 117(1):1–19
Prabha SK, Sathian SP (2012) Molecular-dynamics study of poiseuille flow in a nanochannel and calculation of energy and momentum accommodation coefficients. Phys Rev E 85(4):041201
Present RD, Debethune AJ (1949) Separation of a gas mixture flowing through a long tube at low pressure. Phys Rev 75(7):1050–1057
Siewert C, Valougeorgis D (2004) The McCormack model: channel flow of a binary gas mixture driven by temperature, pressure and density gradients. Eur J Mech B Fluids 23(4):645–664
Spijker P, Markvoort AJ, Nedea SV, Hilbers PAJ (2010) Computation of accommodation coefficients and the use of velocity correlation profiles in molecular dynamics simulations. Phys Rev E 81(1):011203
Trott WM, neda JNC, Torczynski JR, Gallis MA, Rader DJ (2011) An experimental assembly for precise measurement of thermal accommodation coefficients. Rev Sci Instrum 82(3):035120
Xu L, Sedigh MG, Sahimi M, Tsotsis TT (1998) Nonequilibrium molecular dynamics simulation of transport of gas mixtures in nanopores. Phys Rev Lett 80(16):3511–3514
Yamaguchi H, Hanawa T, Yamamoto O, Matsuda Y, Egami Y, Niimi T (2011) Experimental measurement on tangential momentum accommodation coefficient in a single microtube. Microfluid Nanofluid 11(1):57–64
Yamamoto K (2002) Slip flow over a smooth platinum surface. JSME Int J Ser B Fluids Therm Eng 45(4):788–795
Yamamoto K, Takeuchi H, Hyakutake T (2007) Scattering properties and scattering kernel based on the molecular dynamics analysis of gas-wall interaction. Phys Fluids 19(8):087102
Zhang H, Zhang Z, Zheng Y, Ye H (2010) Corrected second-order slip boundary condition for fluid flows in nanochannels. Phys Rev E 81(6):066303
Zhang H, Zhang Z, Ye H (2012) Molecular dynamics-based prediction of boundary slip of fluids in nanochannels. Microfluid Nanofluid 12(1):107–115
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prabha, S.K., Sathian, S.P. Determination of accommodation coefficients of a gas mixture in a nanochannel with molecular dynamics. Microfluid Nanofluid 13, 883–890 (2012). https://doi.org/10.1007/s10404-012-1020-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-012-1020-9