Abstract
Radix Salvia Miltiorrhiza, a famous herb medicine is widely used in China and limitedly used in USA, Japan, and other countries for the treatment of cardiovascular and cerebrovascular diseases. This herb medicine has two groups (non-polar and polar) of active ingredients with distinct clinical effects, and thus theses ingredients should be separately used to enhance therapeutic efficacy and reduce side effect. In this article, as an alternative of conventional mechanical shaking and separatory funnel, laminar flow extraction in microfluidic chip is proposed to separate the two kinds of herb ingredients. Compared with conventional methods, microfluidic chip provides continuous extraction, less labor intensity, and better performance. Furthermore, we employ three-phase laminar flow to provide double liquid–liquid interface area, circumventing the low efficiency of two-phase laminar flow. Therefore, the extraction ratio is dramatically improved to 92% (tanshinone IIA). To predict the extraction ratio, a straightforward theoretical model is also established and agrees well with the experimental results. This microfluidic chip would be a powerful technical platform for handling complicated natural products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Radix Salvia Miltiorrhiza, the dried root of Salvia miltiorrhiza Bge. is a famous herb medicine in China for its treatment of cardiovascular and cerebrovascular diseases (Zhou et al. 2005). It also has been used in United States and Japan (Cheng 2007). This herb medicine contains two categories of active ingredients with diverse clinical effects (Hu et al. 2005b, c; Fig. 1). One group consists of non-polar tanshinone IIA, tanshinone I, dihydrotanshinone I and cryptotanshinone and they have a variety of biological activities including anti-ischemic, anti-oxidant, and anti-tumor properties (Cui et al. 2004). Another group is polar phenolics, such as salvianolic acid B, which is recognized to protect myocardium from ischemia-induced derangement and neural cells from anoxia, to reduce hepatic fibrosis, and to inhibit platelet aggregation (Li 1997; Lay et al. 2003). The different components in Radix Salvia Miltiorrhiza with diverse clinical effects should be used separately in order to enhance therapeutic efficacy and reduce adverse effects. For instance, Shuanglongfang therapy, a composite formula, only contains the phenolics (polar components) from Radix Salvia Miltiorrhiza instead of non-polar components, and the ginsenoside from Radix Ginseng (Li et al. 2003). Hereby, it is essential to separate the non-polar and polar compounds of Radix Salvia Miltiorrhiza for clinical treatment.
The extraction of Radix Salvia Miltiorrhiza is usually accomplished by solvent extraction, which is one of the most widely used processes in chemical engineer, chemical and biochemical analysis. The conventional solvent extraction, based on separatory funnels and mechanical shaking, suffers from laborious processes, large amounts of organic solution and emulsification at the interface which results in an obstacle of separating phases (Lo et al. 1983).
In recent years, laminar flow extraction on microfluidic chip has been developed as a kind of miniaturized solvent extraction. Laminar flow is a well-known phenomenon in microfluidic chips and has extensive applications (Sia and Whitesides 2003; Mu et al. 2009a). Laminar flow extraction usually depends on the diffusion and repartitioning of specific molecule between two immiscible and parallel streams (Tokeshi et al. 2000; Hisamoto et al. 2001; Hibara et al. 2002; Maruyama et al. 2004; Aota et al. 2007; Aota et al. 2009; Mata et al. 2008). In micrometer dimension, interface area between water and organic streams is relatively large, and diffusion distance is rather short. Therefore, the solvent extraction is naturally fit to be performed in microfluidic chip with expected high efficiency. This miniaturized extraction also has distinguished advantages including low consumption of organic solvent, continuous process, and possible integration with other functional units. However, the actual extraction ratio is not very high. For example, it is 50% for Y(III) (Maruyama et al. 2004), 26.1–36.1% for methamphetamine (Miyaguchi et al. 2006) and 79.5% for strychnine (Tetala et al. 2009). It may be due to the short extraction time and short length of extracting channel that utilized. While the channel is patterned by laminar flow patterning, the length is usually restricted by the diffusive broadening.
In this work, we rely on the existed technology of laminar flow extraction, but take three steps to promote this technology and distinguish our work from others. (1) Laminar flow extraction is employed to separate the two categories of components in Radix Salvia Miltiorrhiza. Except extracting strychnine in one recent paper (Tetala et al. 2009), as far as we know, there is no any reports about herb medicine (or plant) extraction by laminar flow extraction in microfluidic chip. Laminar flow extraction, as an efficient and important alternative to conventional methods, would benefit the development of herb medicine. (2) The extraction is performed by both two-phase and three-phase laminar flow. Three-phase laminar flow has already been employed in laminar flow extraction for “simultaneous extraction and back-extraction” (Surmeian et al. 2002; Maruyama et al. 2004; Tetala et al. 2009). However, we exploit a previous unrecognized aspect of it; that is, three-phase laminar flow offers double interface area compared with two-phase laminar flow. A large liquid–liquid interface would benefit the diffusion process and lead to a better performance of extraction. (3) A new theoretical model based on diffusion process is also established to predict the extract ratio. This theoretical model is more straightforward than others (Maruyama et al. 2003; Znidarsic-Plazl and Plazl 2007) and still agrees well with the experimental results.
2 Materials and methods
2.1 Chemicals and materials
Toluene, hexanol, dichloromethane and Sudan II were purchased from Yili Fine Chemical Co., Ltd (Beijing, China). Rhodamine B and octadecyltrichlorosilane (ODS) were brought from Sigma-Aldrich (St. Louis, MO, USA). The original solution of Radix Salvia Miltiorrhiza was home-made and filtered through a membrane filter (2-μm pore size). Polyetheretherketone (PEEK) screws and unions, polyterafluoroethylene (PTFE) tubes were bought from Alltech (Deerfield, IL, USA). Fused silica capillary with 100-μm inside diameter (ID) were purchased from Yongnian Photoconductive Fiber Factory (Hebei, China). Two glass syringes (1 ml) coupled with PTFE plungers were brought from GaoKe industry and trading Co., Ltd (Shanghai, China). In all cases, chemicals were of analytical reagent grade and water purified in a Milli-Q system (Millipore, Bedford, MA, USA) was used.
2.2 Instrument
Direct observation of laminar flow is performed through a 37XR biological microscope (Shanghai Optic Instrument Company, China) equipped with a charge-coupled device (CP230, Panasonic, made in China). The fluids were driven by two syringe pumps (Kd Scientific and Harvard, USA).
2.3 The fabrication of chip and interconnector
Photolithographic and wet chemical etching techniques were used for fabricating channels onto a 1.7 mm-thick 63 × 63-mm glass plate with chromium and photoresist coating (Shaoguang Microelectronics Corp., Changsha, China; Yao et al. 2004). After drilling holes on the etched glass plate, we bonded the etched glass plate and a blank glass plate permanently in a programmable furnace.
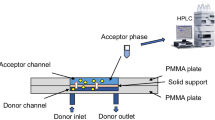
The configurations of a syringe and interconnected glass microfluidic chip are shown in Fig. 2. The interconnector consists of a polymethylmethacrylate (PMMA) scaffold and a metal plate which is to fix the microfluidic chip. PEEK screws in the PMMA plate were properly adjusted to press on an O-ring as well as to align the reservoirs on the chip. The fused silica capillary tube with 360-μm outside diameter (OD) was inserted into a PTFE tube with 300-μm ID to form a compression fitting.
The configurations of interconnector and microfluidic chips. a The detailed setup of interconnections. 1 Capillary, 2 PTFE tube, 3 PEEK screw, 4 metal screw, 5 PMMA plate, 6 O-ring, 7 glass microfluidic chip, 8 metal plate. b The union of glass syringe to capillary tube. c The layout of two-phase microfluidic chip with a channel of 500-μm wide and 30-μm deep. d The layout of three-phase microfluidic chip with a main channel of 600-μm wide and 30-μm deep
2.4 The procedure of modification
We implemented the hydrophobic modification on a glass plate before we selectively modified the microchannel. The glass plate was washed by Piranha solution (H2SO4:H2O2 = 3:1) and then immersed in a ODS solution in toluene (1%) for 1–2 min to complete the modification. After the modification, the surface of glass became hydrophobic. For selectively modifying the microchannel, the streams of toluene and toluene solution of ODS (1%) were pumped into it for 3 min and the two streams flow in parallel. The side of the channel contacting with the toluene solution of ODS was modified into hydrophobicity; yet the another side of the channel contacting the toluene maintained hydrophilicity. The stream of ODS solution was stopped before the toluene stream to confine the modified area. After the selective modification of glass channel, the stream of water could flow side by side with the stream of dichloromethane. If the modification is not implemented, the segmented flow would occur instead of stratified flow.
2.5 Extraction procedure
The original solution of Radix Salvia Miltiorrhiza and dichloromethane solvent were pumped into the modified glass microchannel. The solution of Radix Salvia Miltiorrhiza flowed on the hydrophilic side, which is the unmodified; the dichloromethane solvent flowed on the hydrophobic side, the modified side. Owing to the surface free energy, a stable laminar flow of water and organic phases were generated. Crossing the interface of the two phases, the non-polar components (such as tanshinone IIA) were extracted from original solution into the dichloromethane solvent, because non-polar components have a better solubility in a non-polar solvent than in a polar solvent. And the polar components (phenolics) remained in the water phase. After the extraction, the polar and non-polar fractions were collected from the outlets, respectively.
2.6 HPLC procedure
The results of the extraction were confirmed by off-chip HPLC analysis, which was carried out on an Waters 2695 series HPLC system (Waters Corporation, Milford, MA, USA) by using a C18 analytical column (Alltech, Alltima 250 mm × 5 μm × 4.6 mm) at an ambient temperature with a sample injection volume of 10 μl. Detection was carried out by using a photodiode array detector (Waters model 2996), at an optimized wavelength of 280 nm (Hu et al. 2005a, b, c). The mobile phases were set as follows: water contains 0.5% formic acid (eluent A), and acetonitrile (eluent B). The following linear elution gradient was used (flow rate, 1 ml/min): 0–40 min, 100–70% A; 40–50 min, 70–20% A; 50–70 min, 20–15% A.
3 Results and discussion
3.1 The fabrication and evaluation of interconnections
The extraction of laminar flow is usually required to be compatible with organic reagent. In this sense, PDMS, a prevalent material to fabricate microfluidic chip, is incompatible to organic solution, and thus cannot be used to serve solvent extraction (Lee et al. 2003; Favre 1996). The material for fabricating microfluidic chip should be organic-resistant; therefore, we choose glass as the suitable material to fabricate microfluidic chip. Furthermore, the connections and tubes should also be compatible with organic solution and high pressure. In this study, a detachable scaffold interconnect was adopted for its convenient fabrication and reusability (Tokeshi et al. 2000; Hisamoto et al. 2001). It should also be noted that the alignment of this interconnector to chip requires some technical agility. The mismatch would lead to a remarkable drop of sustained pressure.
The feasibility of this interface is detected both by visual check and by recording the pressure of the liquid. The suddenly drop of pressure on the curve corresponds to the highest pressure sustained by the interface before leakage (Fig. 3). It is notable that the pressure drops to a lower value but do not reach to zero immediately, because interconnects are not completely corrupted. The leakage of the solution is also visible. Owing to the differences of handicraft to assembly the whole system, the max pressure (inflection point) is between ~800 to ~1,100 psi. The scaffold connections were usually employed less than 900 psi corresponding to a flow rate of 0.8 ml/min.
3.2 Selective hydrophobic modification
Although the laminar flow of fluids in the same phase is a natural phenomenon, the co-current flows of aqueous and organic streams, such as water and chloroform, in one channel is not easy to be generated. Since the different phases have diverse interfacial forces to the channel walls, one of these phases would preferentially wet the boundaries and form discontinuous flow of droplets of other phases (Shui et al. 2007). Surface modification is a versatile approach for various applications in microfluidic chip (Swickrath et al. 2008; Woolford et al. 2009; Zhou et al. 2009; Wong and Ho 2009). The modification to partial channel, herein, is essential to generate a stable interface of aqueous and organic streams.
The contact angles of water and toluene droplet in air on modified or untreated glass plates are shown in Fig. 4. After modification, the contact angle of water is improved from 10.0 ± 1.1° to 105.1 ± 2.3°, which indicates the surface of glass becomes hydrophobic and nonwettable to water; the contact angle of toluene is also improved from 11.2 ± 0.9° to 27.1 ± 1.8°. So, the modified surface still remains wettability to toluene.
The hydrophobic modification of glass plates and microchannels. a The contact angle of water on an unmodified glass plate and a modified glass plate. b The contact angle of toluene on an unmodified glass plate and a modified glass plate. c The two-phase laminar flow of water (a) and toluene (dyed by Sudan II 1%w/w) (b) flow through the selectively modified glass channel; D. The three-phase laminar flow of water (a) and dichloromethane (c) flow in the selectively modified glass channel. The three-phase laminar flow provides two-fold liquid–liquid interface. Both in c and d, the interface of immiscible liquids is clear
Furthermore, we selectively modified the inner walls of glass channel by laminar flow patterning (Kenis et al. 1999). After successful modification to the wall of channel, two-phase and three-phase laminar flows of immiscible streams are shown in Fig. 4c and d, respectively. Three-phase laminar flow provides double interface area than two-phase laminar flow at the same volumetric velocity. The laminar flow is stable and reliable, and the interface between two immiscible liquid streams is easily visible.
3.3 The extraction of rhodamine B
To test the capability of the selectively modified microfluidic chip, the extraction of rhodamine B from aqueous solution into hexanol was performed by two-phase laminar flow. Figure 5 shows the two-phase flow (hexanol solution and aqueous solution of rhodamine B) in a channel of 15 mm long. It could be clearly observed that the red rhodamine B gradually moves into hexanol phase across the interface. The dye concentration profiles in the channel before and after extraction are calculated by the gray value in Fig. 5a and b. The curve in hexanol phase rises a little after extraction (Fig. 5c). The curve of water phase near the liquid–liquid interface declines a little, but the rest curve remains as the same value as that before extraction. As a consequence, the extraction of rhodamine B is incomplete.
Two-phase laminar flow extraction of rhodamine B. The upper stream is a hexanol stream at 10 μl/h and the lower stream is a water stream (rhodamine B 1% w/w) at 12 μl/h. a A junction near inlets of chip, b a junction near outlets, and c the gray value profiles of the laminar flows before (a) and after (b) extraction
The laminar flow extraction is based on the diffusing of molecular across an interface transverse to the flow direction. Thus, such diffusion is a one-dimensional diffusion. It could be described as the following equation (Bruus and Henrik 2008)
where t is the diffusion time or residence time (s) in this work, l is the diffusion distance (μm), and D is the diffusion coefficient (m2/s). The diffusion length is the half of width of the channel, 250 μm, and D of rhodamine is 0.39 × 10−9 m2/s (Rani, Pitts, and Stewart 2005). In light of Eq. 1, it needs about 200 s to extract all of the rhodamine B from aqueous phase into hexanol phase. However, the residence time in experiments is far below 200 s. Thus, the fluids would flow out the confluent channel before most of rhodamine B reaching the interface. It is why the completed extraction of rhodamine B is not achieved. Extending the residence time by lowering the flow rate may be helpful. However, the low flow rate cannot guarantee the collection of extract and the processed amounts of the original solution. An alternative method of increasing the liquid–liquid interface is adopted in the following experiments.
3.4 The extraction of Radix Salvia Miltiorrhiza
The aim of this work is to extract ingredients in Radix Salvia Miltiorrhiza by laminar flow extraction. So, on the grounds of the previous experiments, we finally carried out the extraction by two-phase (water/dichloromethane) and three-phase laminar flow (water/dichloromethane/water). HPLC was determined to confirm extraction results, not only because it is a prevalent method to analyze Radix Salvia Miltiorrhiza (Hu et al. 2005a, b, c), but also because some ready and integrated (on-chip) fluorescence or electrochemical methods (Yao et al. 2006; Ren et al. 2009; Mu et al. 2009b) are not capable to detect these drug molecules. Figure 6 shows HPLC chromatograms of original solution and extracted aqueous solution by the extraction of three-phase laminar flow.
The chromatograms clearly show that much of the non-polar components (the peaks after 45 min) are extracted. Comparing the two peaks of tanshinone IIA in Fig. 6, the extraction ratio reaches to 92% at a total flow rate of 39 μl/h. The near-complete extraction ratio is highest among the published laminar flow-based systems. And it is due to the larger liquid–liquid interface provided by three-phase laminar flow and prolonged extraction time. Droplet extraction on microfluidic chip (Chen et al. 2005; Shen et al. 2006; Shen and Fang 2008) might provide higher extraction ratio (or enrichment ratio) but fails to attain off-chip extracted samples.
Although several theoretical models have been developed (Znidarsic-Plazl and Plazl 2007; Maruyama et al. 2003), here, a theoretical model, which is simpler than others, was established to predict the results of the extraction. In this theoretical calculation, the solubility of tanshinone IIA in dichloromethane is assumed to be large enough; therefore, the non-polar molecular would be trapped in the organic phase as long as it comes across the liquid–liquid interface by diffusion. The extraction ratio could be estimated by the diffusive length of the non-polar molecular from Eq. 1 and is expressed as follow
where R is the extraction ratio (%) and l 0 is the longest distance (μm) which the molecular needs to move to the interface. In two-phase condition, l 0 is the half width of the confluent channel, 250 μm. In three-phase condition, because the area of liquid–liquid interface is doubled, the diffusion length should be expressed as follows
And the extraction ratio of three-phase laminar flow is given as follows
where l 1 is 200 μm for the channel used in three-phase laminar flow, D of tanshinone IIA is assumed as 0.1 × 10−9 m2/s. Figure 7 depicts the theoretical and experimental extraction ratio of tanshinone IIA by two-phase and three-phase laminar flows. The theoretical results are based on Eqs. 2 and 4; the experimental results are calculated from the corresponding peaks of HPLC chromatograms. The theoretical values agree well with the experimental data.At the equal volumetric velocity, three-phase laminar flow offers double liquid–liquid interface area compared to two-phase laminar flow. A large interface area would benefit the diffusion progress, and thus the target molecular could be easily extracted to another phase by increased interface and shortened distance. Since the diffusion distance is also shorter in three-phase laminar flow than that in two-phase laminar flow, the improvement of final extraction ratios is larger than two folds in Fig. 7.
The theoretical extraction ratio (R) of tanshinone IIA extracted by two-phase (dash line, b1) and three-phase laminar flow (solid line, a1). It is also shown that the experimental results by three-phase laminar flow (black square, a2) and by two-phase laminar flow (white square, b2). Three-phase laminar flow provides nearly two folds extraction ratio than two-phase laminar flow. The experiment was conducted three times parallel. The extraction ratio is calculated by peak area of tanshinone IIA in HPLC chromatograms before and after extraction
4 Concluding remarks
In this article, a notable herb medicine, Radix Salvia Miltiorrhiza, was extracted by laminar flow in a microfluidic chip. The two groups of non-polar and polar components with different clinical effects were extracted and separated successfully by the three-phase laminar flow, which brings double liquid–liquid interface area to improve the extract ratio. We also combine the experimental results with the theoretical model to describe the process of laminar flow extraction and predict the extraction ratio, which agrees well with experimental results. The current problem is the actual low throughput. It is because that flow rate of liquid in micrometer size is difficult to increase. The high flow rate of liquids would be too finicky for both the power driving the liquid through microchannel and the sustainability of the whole system to prevent leakage. However, this issue regarding the low throughput could be addressed by utilizing multiple parallel channels sharing one set of outlet and inlet (Yung et al. 2009) which just requires the common microfluidic fabrication techniques. Hence, highly arrayed parallel channels based on three-dimensional structure could offer high throughput for large-scale applications. The future work aims at both the separating ingredients of herb medicine in a high throughput manner and other complex systems of natural products.
References
Aota A, Nonaka M, Hibara A, Kitamori T (2007) Countercurrent laminar microflow for highly efficient solvent extraction. Angew Chem Int Ed 46(6):878–880
Aota A, Mawatari K, Takahashi S, Matsumoto T, Kanda K, Anraku R, Hibara A, Tokeshi M, Kitamori T (2009) Phase separation of gas-liquid and liquid-liquid microflows in microchips. Microchim Acta 164(3–4):249–255
Bruus H (2008) Theoretical microfluidics. Oxford University Press, Oxford
Chen H, Fang Q, Yin XF, Fang ZL (2005) Microfluidic chip-based liquid-liquid extraction and preconcentration using a subnanoliter-droplet trapping technique. Lab Chip 5(7):719–725
Cheng TO (2007) Cardiovascular effects of Danshen. Int J Cardiol 121(1):9–22
Cui L, Wu T, Liu YY, Deng YF, Ai CM, Chen HQ (2004) Tanshinone prevents cancellous bone loss induced by ovariectomy in rats. Acta Pharmacol Sin 25(5):678–684
Favre E (1996) Swelling of crosslinked polydimethylsiloxane networks by pure solvents: influence of temperature. Eur Polym J 32(10):1183–1188
Hibara A, Nonaka M, Hisamoto H, Uchiyama K, Kikutani Y, Tokeshi M, Kitamori T (2002) Stabilization of liquid interface and control of two-phase confluence and separation in glass microchips by utilizing octadecylsilane modification of microchannels. Anal Chem 74(7):1724–1728
Hisamoto H, Horiuchi T, Uchiyama K, Tokeshi M, Hibara A, Kitamori T (2001) On-chip integration of sequential ion-sensing system based on intermittent reagent pumping and formation of two-layer flow. Anal Chem 73(22):5551–5556
Hu P, Liang QL, Luo GA, Zhao ZZ, Jiang ZH (2005a) Multi-component HPLC fingerprinting of Radix Salviae Miltiorrhizae and its LC-MS-MS identification. Chem Pharm Bull 53(6):677–683
Hu P, Luo GA, Zhao ZZ, Jiang ZH (2005b) Quantitative determination of four diterpenoids in Radix Salviae Miltiorrhizae using LC-MS-MS. Chem Pharm Bull 53(6):705–709
Hu P, Luo GA, Zhao ZZ, Jiang ZH (2005c) Quality assessment of Radix Salviae Miltiorrhizae. Chem Pharm Bull 53(5):481–486
Kenis PJA, Ismagilov RF, Whitesides GM (1999) Microfabrication inside capillaries using multiphase laminar flow patterning. Science 285(5424):83–85
Lay IS, Chiu JH, Shiao MS, Lu WY, Wu CW (2003) Crude extract of Salvia miltiorrhiza and salvianolic acid B enhance in vitro angiogenesis in murine SVR endothelial cell line. Planta Med 69(1):26–32
Lee JN, Park C, Whitesides GM (2003) Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem 75(23):6544–6554
Li LN (1997) Water soluble active components of Salvia miltiorrhiza and related plants. J Chin Pharm Sci 6:57–64
Li LD, Zhang RL, Liu CY, Ning KY, Li YK, Feng XQ, He J (2003) Establishment of Chinese Miniswine Model of myocardial infarct by catheterization in coronary artery. Chin J New Drugs 12:999–1004
Lo TC, Baird MHI, Carl H (1983) Handbook of solvent extraction. Wiley, New York
Maruyama T, Uchida J, Ohkawa T, Futami T, Katayama K, Nishizawa K, Sotowa K, Kubota F, Kamiyaa N, Goto M (2003) Enzymatic degradation of p-chlorophenol in a two-phase flow microchannel system. Lab Chip 3(4):308–312
Maruyama T, Matsushita H, Uchida J, Kubota F, Kamiya N, Goto M (2004) Liquid membrane operations in a microfluidic device for selective separation of metal ions. Anal Chem 76(15):4495–4500
Mata C, Longmire EK, McKenna DH, Glass KK, Hubel A (2008) Experimental study of diffusion-based extraction from a cell suspension. Microfluid Nanofluid 5(4):529–540
Miyaguchi H, Tokeshi M, Kikutani Y, Hibara A, Inoue H, Kitamori T (2006) Microchip-based liquid-liquid extraction for gas-chromatography analysis of amphetamine-type stimulants in urine. J Chromatogr A 1129(1):105–110
Mu X, Liang QL, Hu P, Ren KN, Wang YM, Luo GA (2009a) Laminar flow used as “liquid etch mask” in wet chemical etching to generate glass microstructures with an improved aspect ratio. Lab Chip 9(14):1994–1996
Mu X, Liang QL, Hu P, Yao B, Ren KN, Wang YM, Luo GA (2009b) Prototypical nonelectrochemical method for surface regeneration of an integrated electrode in a PDMS microfluidic chip. Anal Lett 42(13):1986–1996
Rani SA, Pitts B, Stewart PS (2005) Rapid diffusion of fluorescent tracers into Staphylococcus epidermidis biofilms visualized by time lapse microscopy. Antimicrob Agents Chemother 49(2):728–732
Ren KN, Liang QL, Mu X, Luo GA, Wang YM (2009) Miniaturized high throughput detection system for capillary array electrophoresis on chip with integrated light emitting diode array as addressed ring-shaped light source. Lab Chip 9(5):733–736
Shen H, Fang Q (2008) Improved microfluidic chip-based sequential-injection trapped-droplet array liquid-liquid extraction system for determination of aluminium. Talanta 77(1):269–272
Shen H, Fang Q, Fang ZL (2006) A microfluidic chip based sequential injection system with trapped droplet liquid-liquid extraction and chemiluminescence detection. Lab Chip 6(10):1387–1389
Shui L, Eijkel JCT, van den Berg A (2007) Multiphase flow in microfluidic systems—control and applications of droplets and interfaces. Adv Colloid Interface Sci 133(1):35–49
Sia SK, Whitesides GM (2003) Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 24(21):3563–3576
Surmeian M, Slyadnev MN, Hisamoto H, Hibara A, Uchiyama K, Kitamori T (2002) Three-layer flow membrane system on a microchip for investigation of molecular transport. Anal Chem 74(9):2014–2020
Swickrath MJ, Shenoy S, Mann JA, Belcher J, Kovar R, Wnek GE (2008) The design and fabrication of autonomous polymer-based surface tension-confined microfluidic platforms. Microfluid Nanofluid 4(6):601–611
Tetala KKR, Swarts JW, Chen B, Janssen AEM, van Beek TA (2009) A three-phase microfluidic chip for rapid sample clean-up of alkaloids from plant extracts. Lab Chip 9(14):2085–2092
Tokeshi M, Minagawa T, Kitamori T (2000) Integration of a microextraction system on a glass chip: ion-pair solvent extraction of Fe(II) with 4,7-diphenyl-1,10-phenanthrolinedisulfonic acid and tri-n-octylmethylammonium chloride. Anal Chem 72(7):1711–1714
Wong I, Ho CM (2009) Surface molecular property modifications for poly(dimethylsiloxane) (PDMS) based microfluidic devices. Microfluid Nanofluid 7(3):291–306
Woolford B, Maynes D, Webb BW (2009) Liquid flow through microchannels with grooved walls under wetting and superhydrophobic conditions. Microfluid Nanofluid 7(1):121–135
Yao B, Luo GA, Feng X, Wang W, Chen LX, Wang YM (2004) A microfluidic device based on gravity and electric force driving for flow cytometry and fluorescence activated cell sorting. Lab Chip 4(6):603–607
Yao B, Yang HH, Liang QL, Luo G, Wang LD, Ren KN, Gao YD, Wang YM, Qiu Y (2006) High-speed, whole-column fluorescence imaging detection for isoelectric focusing on a microchip using an organic light emitting diode as light source. Anal Chem 78(16):5845–5850
Yung CW, Fiering J, Mueller AJ, Ingber DE (2009) Micromagnetic-microfluidic blood cleansing device. Lab Chip 9(9):1171–1177
Zhou LM, Zuo Z, Chow MSS (2005) Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 45(12):1345–1359
Zhou JH, Yan H, Ren KN, Dai W, Wu HK (2009) Convenient method for modifying poly(dimethylsiloxane) with poly(ethylene glycol) in microfluidics. Anal Chem 81(16):6627–6632
Znidarsic-Plazl P, Plazl I (2007) Steroid extraction in a microchannel system—mathematical modelling and experiments. Lab Chip 7(7):883–889
Acknowledgments
This research was supported by National Basic Research Program of China (2007CB714505) and Ministry of Education of China (20080031012). The authors would like to thank Professor Yao Bo (Zhejiang University) for fruitful discussions, Dr. Zhang Min (ECUST) and Qi Xiaocheng (JXUTCM) for helping in herb medicine, and Professor Zhang Xi and Wan Pengbo (Tsinghua University) for measuring contact angle.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mu, X., Liang, Q., Hu, P. et al. Selectively modified microfluidic chip for solvent extraction of Radix Salvia Miltiorrhiza using three-phase laminar flow to provide double liquid–liquid interface area. Microfluid Nanofluid 9, 365–373 (2010). https://doi.org/10.1007/s10404-009-0554-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-009-0554-y