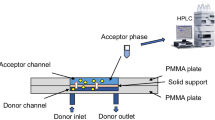
Abstract
Liquid–liquid solvent extraction is frequently used in the purification of active ingredients from plants, and repeated extraction by different solvents with different polarity is often demanded to improve purity. According to this demand, two kinds of laminar flow extraction were designed on the microfluidic chip, i.e. a three-phase laminar chip and a successive laminar flow chip, and compared with a current two-phase chip. P. ginseng was used as a model plant to demonstrate extraction performance, and ether and n-butanol were used as the degrease solvent and extraction solvent, respectively. The results demonstrated that the extraction efficiency of a successive laminar flow chip is higher than that of a three-phase laminar flow chip, and that of both chips are higher than that of a two-phase chip. The chip method provided a new way to extract active ingredients from plants, which should be easily automated and integrated with other units to develop a complex system for sample preparation and ingredient detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The use of medicinal plants (herbs) for medical treatment has a long history throughout the world (Huie 2002). Panax ginseng (P. ginseng), or ginseng, is a key herb of traditional Chinese medicine, and is one of the most valuable functional food worldwide. Recent research has shown that ginseng has pharmacological and therapeutic effects on many aspects including central nervous disease, cancer, and so on (Mi et al. 2012). The most prominent bioactive constituents of ginseng are ginsenosides. Therefore, extraction and purification of ginsenosides from ginseng is an important step for the study of the medicinal value of ginseng.
Unlike modern drugs that invariably comprise a single active species, herbal extracts and/or prescriptions contain multiple active constituents (Mi et al. 2012). Owing to the complexity of herbal extract/prescription, sample preparation became a crucial first step to conduct pharmacological research of active ingredients and the quality evaluation for herbal medicine. Liquid–liquid extraction (LLE) is a conventional sample pre-treatment method for the purification of some active components from crude extracts of herbals. Sometimes, successive extractions by different organic solvents are required to get a stepwise isolation of active components (Süntar et al. 2012; Shi et al. 2007) or to obtain different compounds for subsequent active screening (Vitalini et al. 2016; Yang et al. 2014). For example, one way of isolation of ginsenosides from P. ginseng is to first degrease it by ether and then extract by n-butanol (Shi et al. 2007). Such a method has the disadvantages of the formation of emulsions, difficulty of automation, use of large amounts of organic solvents and samples. Moreover, repeated extractions are often needed, which further increase the analysis time, labor cost, undesirable exposure to toxic solvents and the release of wastes to the environment. In the last decade, the global trend in chemical analysis has shifted towards simplifying, downsizing and greening sample preparation and separation (Plianwong et al. 2012). In these ways, human errors and reagent contamination can potentially be reduced through integration and automation (Arora et al. 2010), and operation time can be shortened (Yang et al. 2015). Many novel miniaturized liquid phase extractions have been developed and became attractive alternatives for some biological sample pretreatments, such as single-drop microextraction (SDME), hollow-fiber microextraction (HF-LPME), directly suspended droplet microextraction (DSDME) and dispersive liquid–liquid microextraction (DLLME)(Plianwong et al. 2012). However, these novel microextraction methods are seldom used in the study of active ingredients of herbal medicines. It is because they cannot satisfy the multi-step extraction requirement, and difficult to be integrated with subsequent separation and analysis.
The microfluidic technology aims to integrate into a single miniaturized system all the analytical steps, including sample pretreatment (Sun et al. 2018), capillary electrophoresis separation (Hu et al. 2017), and cell analysis (Zhang et al. 2017). Compared with conventional LLE, some reports of microfluidic laminar flow-based LLE showed higher extraction efficiency, shorter process time, lower reagent cost, and more environmental friendliness (Hu et al. 2017; Mu et al. 2010). In a microfluidic chip, when two liquids are introduced from two separate inlets into one main microchannel, a stable interface is often observed between the laminar flows of two liquid streams owing to the very small Reynolds number found in microchannels (Zhao et al. 2002). The types of laminar flow in microchips had been developed from single phase (Hatch et al. 2001) to two phases (Hibara et al. 2003), and to three phases (Tetala et al. 2009), and laminar flow extraction had been used in diffusion immunoassay (Hatch et al. 2001), cell culture (Toh et al. 2007), and chemical reaction (Beard et al. 2010; Tokeshi et al. 2002; Hisamoto et al. 2003). The microfluidic LLE had also been used in plant extraction. For instance, Tetala et al. (2009) reported a three-phase microfluidic chip to purify alkaloids from plant extracts, and Mu et al. (2010) employed a three-phase laminar flow chip to provide the double liquid–liquid interfaces to improve the extraction efficiency. Although two or more organic solvents are often required for successive extraction or purification, there is no report using such methods to extract active ingredients from herbs.
In this paper, we aimed to adopt the method of using successive solvent extractions in sample preparation of plant medicine in the microfluidic chip. A three-phase laminar flow chip and a successive two-phase laminar flow chip were designed, for the first time, for the separation of ginsenosides from a crude extract of ginseng. It was demonstrated that both the three-phase laminar flow chip and successive two-phase laminar flow chip can realize the successive extractions by two kinds of solvents. For ginsenosides, the successive flow chip showed higher extraction efficiency than the three-phase chip. An orthogonal array experimental design was employed as a chemometric method for the optimization of the chip extraction conditions. Then, the optimized conditions were applied in the sample preparation of P. ginseng.
2 Materials and methods
2.1 Materials and instruments
The standards of ginsenoside Rg1, Re, Rb1, sanguinarine (SAN), and chelerythrine (CHE) were purchased from Chinese Medical and Biological Products Institute (Beijing, China). Mixed ginsenosides standard stock solutions of Rg1 (0.1 mg mL−1), Re (0.1 mg mL−1), and Rb1 (0.1 mg mL−1), and stock standard stock solutions of CHE (50 μg mL−1) were prepared in methanol. A series of different concentrations were obtained by diluting the mixed standard stock solutions. Acetonitrile and methanol were purchased from Guangzhou Aixin Scientific Instrument Co., Ltd. Ether and n-butanol were purchased from Tianjin Baishi Chemical Co., Ltd. Dichlorodimethylsilane (DCDMS) and 1-hexanol were purchased from Aladdin Industrial Corporation (Shanghai, China). Water was obtained from a Milli-Q water purification system (Millipore, France). All other reagents used were analytically pure grade, and purchased from Damao Chemical Reagent Corporation (Tianjin, China). Observation of laminar flow of liquids in microfluidic chip was performed through an inverted microscope (MI12, Ming Mei Optoelectronic Technology Co., Ltd., Guangzhou, China). The fluids were driven by syringe pumps (SPLab01, Baoding Shen Chen Pump Co., Ltd., Baoding, China). Polytetrafluoroethylene (PTFE) tubes were bought from Alltech (Deerfield, IL, USA).
2.2 Chip fabrication
Photolithographic and wet chemical etching techniques were used for fabricating channels (1.7 mm thick, 32 mm wide and 63 mm long) in the glass slide (Shaoguang Microelectronics Corp., Changsha, China). After drilling holes on the etched glass plate, it was bonded to a blank glass plate permanently using a programmable furnace. As shown in Fig. 1a–c, a two-phase, three-phase, and successive two-phase chips with the same length of extraction channel were showed, respectively. The width of the confluent channel for the extraction of two-phase chip is 200 μm. The width of the confluent channel for extraction of three-phase chip is 300 μm. The width of the confluent channel for extraction of successive two-phase chip is 200 μm, and that of other channels are 100 μm. All channels were 40 μm deep. The lengths of the confluent channels are 2 cm for the three kinds of chips.
Channel arrangements of two laminar flow extraction chips, (a) two-phase chip (b) three-phase chip. (i) Schematic diagram of glass surface modification. The white and gray regions indicate the laminar flows of aqueous solution (e.g. water) and organic solvents (e.g. ether, n-butanol), respectively. (ii) Images of laminar flow of liquids
The surface of the extraction channel was selectively modified by the silanization mechanism in which the channel surface reacted with a water-repellent substance such as a silane, as shown in Fig. 1. For example, during surface modification for the two-phase chip, see Fig. 1a(i), 1-hexanol was introduced to the extraction channel from the inlet B to outlet D at a flow rate of 5 μL min−1 to drive away air in the microfluidic device. Then a silane solution of DCDMS (25% v/v in 1-hexanol) was pumped into the microchannels from inlet A to outlet C at a flow rate of 6 μL min−1. A stable laminar flow was formed, see Fig. 1a(ii), and the flow continued for 20 min. At the end of the modification process, the flow of the silane solution was stopped, and then excess silane in the microchannels was washed away by 1-hexanol to avoid surface modification of unwanted areas. The channels were cleaned by methanol after the modification. Finally, methanol in the channels was removed and the chip was dried. Similarly, the surface of three-phase chip was modified and the laminar flow of the three liquids is shown in Fig. 1b(i) and (ii).
As shown in Fig. 2a, the surface of a successive two-phase chip is modified. After that, the laminar flows of water and ether/n-butanol solution forms a stable laminar flow state in the extraction channel, see Fig. 2b(i), (ii) and (iii).
Glass surface modification and laminar flow of the successive flow chip. a Schematic diagram of surface modification. The white and gray regions indicate the laminar flows of aqueous solution (e.g. water) and organic solvents (e.g. ether, n-butanol), respectively. b Images of laminar flows in (i) inlet diversion, (ii) middle section, (iii) outlet diversion
2.3 Sample preparation before chip treatment
Dried and powdered P. ginseng was passed through a 40 mesh sieve. 2.0 g of P. ginseng powder was accurately weighed. It was extracted in 40 mL of 70% (v/v) ethanol solution twice by sonification (40 kHz, 600 W) for 30 min at each time. This was the 70% ethanol extract. The extracts were also filtered and evaporated to dryness. The residue was then dissolved in 20 mL of water and filtered through a 0.45-μm nylon filter membrane, named as EX I, and stored in 4 °C fridge before use.
2.4 Chip extraction
EXI was introduced into the hydrophilic channel and the organic solvent was introduced into the hydrophobic channels, as shown in Fig. 1. For the two-phase laminar flow chip shown in Fig. 1a, n-butanol was introduced into the hydrophobic channel via inlet A. For the three-phase laminar flow chip shown in Fig. 1b, ether and n-butanol were introduced into the top and bottom hydrophobic channels via inlet A and inlet C, respectively. For the successive two-phase laminar flow chip shown in Fig. 2a, ether was introduced into the upstream hydrophobic channel via inlet A, and n-butanol was introduced into the downstream hydrophobic channel via inlet C. After extraction, the extracted solution was collected into a vial and evaporated to dryness, and then redissolved in same volume of methanol to be analyzed by HPLC. To obtain a stable interface between the laminar flows of aqueous and n-butanol, a ratio of flow rates of aqueous: n-butanol = 2:1 was needed. So the extraction yields of ginsenosides Rg1, Re, and Rb1 were calculated according to the following equation:
where \( R\% \) is the extraction yield, \( C_{E} \) the detected concentration of each ginsenoside in the extracted solution, \( C_{0} \) the original concentration in the aqueous sample EX1, \( v_{E} \) the flow rate of the organic extraction solution, \( v_{0} \) the flow rate of the aqueous sample solution, and \( t \) is the flow time in chip, i.e. the extraction time.
2.5 HPLC analysis
Extracted samples were separated and detected by HPLC. This was carried out on a Shimadzu HPLC system (LC-20A, Shimadzu, Kyoto, Japan), equipped with a LC-20ATVP pump, a UV detector (SPD-M20A), an autoinjector (SIL-20ADVP), and a degasser module (DGU-14A). The chromatographic separations were carried out on ODS-3 column (Inertsil, 250 mm length, 4.6 mm i.d. with particle size of 5 μm). Sample injection volume was 20 μL and the temperature of column was controlled at 35 °C. The binary elution solvents consisted of acetonitrile (A) and water (B) for the analysis of ginsenosides with a gradient elution procedure of 0 ~ 35 min, 19% A, 81% B; 35 ~ 55 min, 19% ~ 29% A, 81% ~ 71% B; 55 ~ 70 min, 29% A, 71% B; 70 ~ 100 min, 29% ~ 40% A, 71% ~ 60% B. The flow rate was kept at 1.0 mL min−1.
2.6 Orthogonal array design
A four-factor (4), three-level (3) orthogonal array design was used to evaluate the effects of the following factors on the successive laminar flow chip efficiency: channel width (A), surface property (B), flow rate (C) and channel length (D). Nine experiments were performed to estimate the best conditions for the extraction of ginsenosides. Such an orthogonal array design is coded as L9(34). The factors and levels tested are reported in Table 1. The data were analyzed using the Orthogonality Experiment Assistant software (Sharetop Software Studio, China).
2.7 Validation of the chromatographic method
A series of standard solutions with ten different concentrations was analyzed by HPLC in triplicates to create the calibration curve. The LOD of ginsenoside Rg1, Re and Rb1 was 3.9,1.3 and 0.6 μg mL−1, respectively, and the LOQ of ginsenoside Rg1, Re, and Rb1 was 13.1,4.5 and 2.2 μg mL−1, respectively. The LOD and LOQ were defined as the concentrations of a compound at which its signal-to-background ratios were detected as 3:1 and 10:1, respectively. The precision of the method (quantified as RSD) was determined by the analysis of six consecutive injections using the same standard mixture solution. The values of RSDs of the peak areas for Rg1, Re, and Rb1 were less than 2.1, 1.8, 2.3%, respectively. In the recovery tests, three different concentrations of Rg1, Re, and Rb1 were spiked in the pre-analyzed samples, and then the spiked samples were analyzed in triplicates. The recoveries ranged from 97.6 to 103.2%, with their RSD values less than 3%.
3 Results and discussion
3.1 Performance of different chip designs
Ginsenosides are the main active constituents in P. ginseng. These constituents can be extracted and purified by many ways; one of the pretreatment methods is LLE. In this method, after the extraction of P. ginseng powder by 70% ethanol, the extract was evaporated to dryness and redissolved in water. The aqueous extract EXI was then successively extracted by ether and water-saturated n-butanol (Shi et al. 2007; Mao et al. 2008). So, in the extraction of ginsenoside from EXI using three different kinds of chips, ether and water-saturated n-butanol were used. The extraction yields by three kinds of chips were compared with the same flow rate of n-butanol at 1 μL min−1. The results are shown in Table 2.
As shown in Table 2, both the three-phase chip and the successive laminar flow chip had shown higher extraction rates than a two-phase chip. Although the confluent channel in the three-phase chip, which has a width wider than that in the two-phase chip, may decrease the amount of molecules diffused from aqueous phase to n-butanol, the extraction efficiency from the three-phase chip is still higher than the two-phase chip with the aid of ether degreasing, so it can be confirmed that ether degreasing was beneficial to the extraction of saponins.
It could be seen that a three-phase chip can realize two functions at the same time, i.e. degreasing and extraction. But the successive laminar flow chip with the same channel length showed a higher extraction rate, which may be caused by more complete degreasing in the successive laminar flow chip. As mentioned in the foregoing paragraph, the width of the confluent channel of the three-phase chip is wider than that of the successive flow chip, so the extraction efficiency of the latter chip is higher. It could be noted that with the use of ether, a higher content of Re can be obtained, while the content of Re was low in the case of the two-phase chip in which no ether was used. As the laminar flow of low speed is not easy to get stable in the three-phase chip, especially at the outlet, as shown in Fig. 3, and a successive laminar flow chip closely resembles conventional LLE, so the successive chip is chosen for further study in this article.
3.2 Condition optimization using P. ginseng crude extract EXI
The extraction efficiency can be influenced by the state of laminar flow and the touch time between the sample phase and the extraction phase. So the factors of the channel surface property (modified and unmodified), the confluent channel width (100–300 μm), and the flow rate (0.5–1.0 μL min−1) were optimized.
First, the factor of the surface property was studied. Although the laminar flow of fluids is a natural phenomenon, the co-current flows of aqueous and organic streams in one channel are not easily to be generated. This is because different phases have diverse interfacial forces to the channel walls, and one of these phases would preferentially wet the boundaries and form discontinuous droplets (Shui et al. 2007). The modification to part of the channel surface is helpful to generate a stable interface of aqueous and organic streams. Therefore, the stability of the laminar flows is improved, and the interface is more accurate, as shown in Fig. 4.
With the flow rate of 0.7 μL min−1 and channel width of 200 μm, the extraction yields obtained by the surface-modified chip and non-modified chip are shown in Table 3. It is determined that higher extraction yields were obtained when the channel surface was selectively modified.
The effects of confluent channel width on the extraction yields of ginsenosides were studied using chips with channel widths of 100 μm and 200 μm, in which the channel surface had been selectively modified. Then, using the selectively modified chip, with the flow rate of 0.7 μL min−1, the influence of the channel width was tested. The extraction yields, shown in Table 4, indicate that higher extraction yields are obtained with an extraction channel of 100 μm width.
At last, with the width of 100 μm and the selectively modified chip, the influence of the flow rate on extraction yields was tested, and are shown in Table 5. This shows the extraction yield of ginsenoside Re is higher when the flow rate is 0.5 μL min−1.
3.3 Orthogonal array experimental design on a successive flow chip
Other than single-factor tests, orthogonal array design was often used for experimental condition optimization (Sun et al. 2010; Yang et al. 2018; Xu et al. 2002). The orthogonal array design can solve the following problems: first, it can save a lot of manpower, material and financial resources and time. Second, it is able to determine the importance of every factor, that is, which factors are more important and which factors are secondary. Third, the optimization scheme can be found quickly, and the cycle of product development and design can be shortened greatly. Fourth, through the analysis of test results, we can further indicate the direction of experiment, overcome blindness of condition optimization and so on. So an orthogonal array design L9(3)4 was employed as a chemometric method for the optimization of the chip extraction conditions.
During sample preparation for plant medicine, successive extraction with different solvents was not only used to purify some active reagents from plant medicine, but was also used for the classification of reagents with different polarities. Obviously, a successive laminar flow chip, as shown in Fig. 1c, most closely simulates the conventional LLE process. So, a successive laminar flow chip was adopted for the orthogonal array design to obtain the optimized chip conditions.
The factors in the experimental design contain confluent channel width (100–300 μm), flow rate (0.5–1.0 μL min−1), and channel surface (modified and unmodified). As the confluent channel length is limited by the glass slides, it was fixed at 2 cm and factor D of the L9(3)4 orthogonal array is set to consist of only one level, as shown in Table 6.
Ginsenoside Rg1 was used as a model compound to evaluate the extraction efficiency. Besides, we want to find another model compound to evaluate the degreasing efficiency. Therefore, chelerythrine was used as a model compound for extraction by a low polarity volatile solvent, such as dichloromethane. Chelerythrine was extracted using a two-phase chip previously (Hu et al. 2017). Therefore, ginsenoside Rg1 and chelerythrine were used as model compounds. The mixture of ginsenoside Rg1 and chelerythrine in water was first extracted by dichloromethane and then by water-saturated n-butanol.
The values of R which was the largest difference in the average recoveries indicate the importance of each factor. It can be seen in Table 7 that factor A, which is a confluent channel width, has the highest value of R in both the extractions of both ginsenoside Rg1 and chelerythrine, and this indicates such a factor plays the most important role in the extraction of both ginsenoside Rg1 and chelerythrine. The importance that influenced extraction rate of both ginsenoside Rg1 and chelerythrine was found to be in the order of channel width > surface property > flow rate according to the R values.
The R values of factor B that affects the extraction of ginsenoside Rg1 and chelerythrine are at the same second place, which indicates that surface modification of the extraction process is very important. Based on the mean value in factor C, it was found that the flow rate had different effects on the ginsenoside Rg1 and chelerythrine, which may be due to the different natures of the extraction solvents on the model compounds. Because dichloromethane is more volatile than n-butanol, which is specially notable when a very low flow rate was adopted. Therefore, when a volatile extraction solvent was used, the use of a slow liquid flow rate can effectively improve the extraction yield; while for non-volatile solvent, a steadier laminar flow is more important than a low flow rate in improving extraction yield. According to the experimental result, the optimum combination of variables for ginsenoside Rg1 extraction was A1B2C1, i.e. channel width 100 μm, selective modified surface, and 0.5 μL min-1 of flow rate.
3.4 Comparison of the laminar flow extraction method with conventional LLE
First, a conventional LLE by a separating funnel was used to test the extraction of Rg1. The recovery of Rg1 from water was 77.5%, and the recovery of chelerythrine from water was 56.7%. Compared with the results in 3.3, it can be inferred that laminar flow extraction may not be good for all kinds of compounds. For chelerythrine, laminar flow extraction is better than conventional LLE, but for ginsenoside Rg1, the result is reversed.
Conventional LLE was also used to extract ginsenosides from P. ginseng. The sample EX1 was mixed with ether and stirred for a while, and the mixture was allowed to separate into two layers. The upper ether layer was discarded, and the lower aqueous layer was further extracted by n-butanol. Then the n-butanol layer was evaporated to dryness. It was redissolved in methanol, and analyzed by HPLC to give 1.88 mg g−1 powder of Rg1, 1.16 mg g−1powder of Re, and 1.11 mg g−1 powder of Rb1. Compared with the laminar flow method, the extraction yield obtained by conventional LLE is higher, but the emulsification problem that occurs during the conventional LLE increased the solvent settling time, and prolonged the whole experiment process (90 min).
4 Conclusion
We have proposed several kinds of laminar flow chip designs to realize LLE. The multi-phase laminar flow chip and successive laminar flow chip can substitute the multi-step extraction employed in conventional LLE using solvents of different polarities. Using the laminar flow chip method, the number of processing steps was reduced and the processing time was shortened. As the chip extraction method is based on the molecular diffusion mechanism, the extraction process is mild. However, the extraction based on laminar flow is not better than conventional LLE for every kind of reagent. Only for some of reagents with high diffusion coefficients, the extraction yield is better in the chip than in the separating funnel.
References
Arora A, Simone G, Salieb-Beugelaar GB, Kim JT, Manz A (2010) Latest developments in micro total analysis systems. Anal Chem 82(12):4830–4847
Beard NP, Edel JB, Demello AJ (2010) Integrated on-chip derivatization and electrophoresis for the rapid analysis of biogenic amines. Electrophoresis 25(14):2363–2373
Hatch A, Kamholz AE, Hawkins KR, Munson MS, Schilling EA, Weigl BH, Yager P (2001) A rapid diffusion immunoassay in a T-sensor. Nat Biotechnol 19(5):461–465
Hibara A, Nonaka M, Hisamoto H, Uchiyama K, Kikutani Y, Tokeshi AM, Kitamori T (2003) Stabilization of liquid interface and control of two-phase confluence and separation in glass microchips by utilizing octadecylsilane modification of microchannels. Anal Chem 74(7):1724–1728
Hisamoto H, Shimizu Y, Uchiyama K, Tokeshi M, Kikutani Y, Hibara A, Kitamori T (2003) Chemicofunctional membrane for integrated chemical processes on a microchip. Anal Chem 75(2):350–354
Hu YZ, Peng HD, Yan YS, Guan SY, Wang SM, Li PCH, Sun Y (2017) Integration of laminar flow extraction and capillary electrophoretic separation in one microfluidic chip for detection of plant alkaloids in blood samples. Anal Chim Acta 985:121–128
Huie CW (2002) A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal Bioanal Chem 373(1–2):23–30
Mao ZL, Li XB, Gong WM, Fan W (2008) Optimization of extraction process of Ginsenoside. Lishizhen Med Materia Med Res 19(11):2762–2763
Mi JN, Zhang M, Ren GX, Zhang HY, Wang YR, Hu P (2012) Enriched separation of protopanaxatriol ginsenosides, malonyl ginsenosides and protopanaxadiol ginsenosides from Panax ginseng using macroporous resins. J Food Eng 113(4):577–588
Mu X, Liang QL, Hu P, Ren KN, Wang YM, Luo GA (2010) Selectively modified microfluidic chip for solvent extraction of Radix Salvia miltiorrhiza using three-phase laminar flow to provide double liquid–liquid interface area. Microfluid Nanofluid 9(2–3):365–373
Plianwong S, Sripattanaporn A, Waewsa-nga K, Buacheen P, Opanasopit P, Ngawhirunpat T, Rojanarata T (2012) Operator care and eco-concerned development of a fast, facile and economical assay for basic nitrogenous drugs based on simplified ion-pair mini-scale extraction using safer solvent combined with drop-based spectrophotometry. Talanta 98:220–225
Shi W, Wang YT, Li J, Zhang HQ, Ding L (2007) Investigation of ginsenosides in different parts and ages of panax ginseng. Food Chem 102(3):664–668
Shui LL, Eijkel JCT, van DBA (2007) Multiphase flow in microfluidic systems-control and 8 applications of droplets and interfaces. Adv Colloid Interface Sci 133(1):35–49
Sun YS, Li W, Wang JH, Bi JJ, Liu ZB, Wang Y, Guo ZD (2010) Optimization of supercritical fluid extraction of saikosaponins from Bupleurum falcatum with orthogonal array design. J Sep Sci 33(8):1161–1166
Sun Y, Yuan JC, Pang JL, Li XN, Wang SM, Zhou YL, Xu F, Li PCH, Jiang SS, Chen H (2018) Millifluidic chip with a modular design used as a sample pretreatment cartridge for flour and flour food products. Talanta 179:719–725
Süntar I, Akkol EK, Keles H, Yesilada E, Sarker SD, Baykal T (2012) Comparative evaluation of traditional prescriptions from Cichorium intybus, L. for wound healing: stepwise isolation of an active component by in vivo bioassay and its mode of activity. J Ethnopharmacol 143(1):299–309
Tetala KK, Swarts JW, Chen B, Janssen AE, van Beek TA (2009) A three-phase microfluidic chip for rapid sample clean-up of alkaloids from plant extracts. Lab Chip 9(14):2085–2092
Toh YC, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, Van ND, Hutmacher DW, Yu H (2007) A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip 7(3):302–309
Tokeshi M, Minagawa T, Uchiyama K, Hibara A, Sato K, Hisamoto H, Kitamori T (2002) Continuous-flow chemical processing on a microchip by combining microunit operations and a multiphase flow network. Anal Chem 74(7):1565–1571
Vitalini S, Madeo M, Tava A, Iriti M, Vallone L, Avato P, Cocuzza CE, Simonetti P, Argentieri MP (2016) Chemical profile, antioxidant and antibacterial activities of achillea Moschatawulfen, an endemic species from the alps. Molecules 21(7):830
Xu ZA, Wang TBO, Li CY, Bao LY, Ma QM, Miao YN (2002) Brief introduction to the orthogonal test design. Sci/Tech Inform Dev Econ 12(5):148–150
Yang LF, Liu HC, Feng FQ, Li DN, Jiang MG (2014) HPLC fingerprint and antitumor activity of different polar parts extracted from crude stems of Derris eriocarpa How. Chin J Pharm Anal 34(12):2112–2118
Yang R, Pagaduan JV, Yu M, Woolley AT (2015) On chip preconcentration and fluorescence labeling of model proteins by use of monolithic columns: device fabrication, optimization, and automation. Anal Bioanal Chem 407(3):737–747
Yang MY, Gu YH, Wu XL, Xi XF, Yang XL, Zhou WF, Zeng HZ, Zhang SB, Lu RH, Gao HX, Li J (2018) Rapid analysis of fungicides in tea infusions using ionic liquid immobilized fabric phase sorptive extraction with the assistance of surfactant fungicides analysis using IL-FPSE assisted with surfactant. Food Chem 239:797–805
Zhang J, Wei XF, Zeng R, Xu F, Li XJ (2017) Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip. Future Sci OA 3(2):FSO187
Zhao B, Viernes NO, Moore JS, Beebe DJ (2002) Control and applications of immiscible liquids in microchannels. J Am Chem Soc 124(19):5284–5285
Acknowledgements
The research was funded by the National Natural Science Foundation of China (No:81001600).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, W., He, Y., Xiao, J. et al. A successive laminar flow extraction for plant medicine preparation by microfluidic chip. Microfluid Nanofluid 23, 61 (2019). https://doi.org/10.1007/s10404-019-2228-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-019-2228-8