Abstract
Gallbladder wall thickening is relatively common in clinical settings, and for appropriate diagnosis, the size, shape, internal structure, surface contour, and vascularity of the gallbladder wall must be evaluated. Morphological evaluation is the most important; however, some gallbladder lesions resemble gallbladder cancer in imaging studies, making differential diagnosis challenging. Vascular evaluation is indispensable for a precise diagnosis in these cases. In this review, we present the current status of vascular evaluation using US and diagnosis using vascular imaging for gallbladder lesions, including those presenting with wall thickening. To date, several ultrasound imaging techniques have been developed to assess vascularity, including Doppler imaging with high sensitivity, use of contrast agents, and microvascular imaging using a novel filter for Doppler imaging. Although conventional color Doppler imaging is rarely used for the diagnosis of gallbladder lesions, the efficacy of contrast-enhanced ultrasound in assessing the vascularity, enhancement pattern, or timing of enhancement/washout has been reported. Presence of multiple irregular microvessels has been speculated to indicate malignancy. However, few reports on microvessels have been published, and further studies are required for the precise diagnosis of gallbladder lesions with microvascular evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gallbladder wall thickening is relatively commonly encountered in clinical settings and during medical examinations. Most gallbladder lesions presenting with wall thickening are detected using ultrasound (US). However, it is challenging to appropriately diagnose them as the wall structure is frequently modified by cholecystitis or adenomyomatosis (ADM). Hence, some gallbladder cancers (GBCs) are revealed in resected specimens after cholecystectomy, which are referred to as incidental cancer [1, 2]. Cholecystitis is occasionally misdiagnosed as invasive GBC, leading to unnecessary extended surgical resection, such as hepatectomy [3,4,5]. Therefore, various aspects of gallbladder wall thickening should be evaluated, including size, shape, internal structure, surface contour, and vascularity prior to making a definitive diagnosis [6].
In this review, we present the current status of vascular evaluation using US and diagnosis using vascular imaging for gallbladder lesions, including those presenting with wall thickening.
Significance of vascular evaluation for the diagnosis of gallbladder lesions presenting wall thickening
Various gallbladder lesions with wall thickening have been reported [7, 8] (Table 1). The fundamental approach to diagnosing these lesions is morphological evaluation, and vascular evaluation is not always necessary. For instance, edematous gallbladder wall thickening caused by diseases of other organs, such as hepatitis, heart failure, or renal failure, shows uniform gallbladder wall thickening; however, the structure of the gallbladder wall layer is retained, which is distinctly different from advanced GBC presenting wall thickening. Gallbladder wall thickening with a retained layer is easily detected on US; therefore, mild cholecystitis or edematous wall thickening of the gallbladder can be diagnosed using US alone, and vascular evaluation is not mandatory.
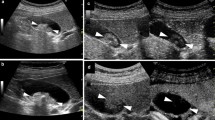
However, some gallbladder lesions present with imaging findings similar to those of GBC, and their differential diagnosis using imaging studies is occasionally challenging. Some inflammatory diseases, such as severe acute cholecystitis, show irregular wall thickening with an obscured structure of the wall layer, which is similar to the findings in invasive GBC. In particular, xanthogranulomatous cholecystitis (XGC), which presents as localized wall thickening on imaging studies, is frequently misdiagnosed as a malignant gallbladder neoplasm [5]. Adenomyomatosis (ADM) is characterized by wall thickening with a dilated Rokitansky-Ashoff sinus [9], and a small cystic structure or comet-like echo is a representative finding of ADM on US [10, 11]. However, these findings have also been observed in nodular invasive GBC with cancerization. Additionally, GBC and ADM may be present concomitantly, and the diagnosis of GBC with ADM in imaging studies is difficult [12] (Fig. 1).
Findings of gallbladder cancer concomitant with adenomyomatosis. a Ultrasound. A protruded lesion was detected on the fundal part of the gallbladder. b Ultrasound. A small cystic structure was observed in the gallbladder lesion, suggesting fundal adenomyomatosis (arrowhead). The mucosal part of the gallbladder lesion was slightly thickened. c Endoscopic ultrasound. A small cystic structure was present in the bottom of the lesion, compatible with adenomyomatosis. However, an elevated lesion protruding into the gallbladder lumen was also seen on the mucosal part of the adenomyomatosis, which is not typical of adenomyomatosis. This case was ultimately diagnosed as gallbladder cancer concomitant with adenomyomatosis
Vascular evaluation provides informative findings in these situations, and hence, should be considered during the differential diagnosis of benign and malignant gallbladder diseases.
Imaging techniques to assess vascularity using ultrasound
Current imaging techniques for visualizing blood flow are summarized in Table 2. The blood flow is generally visualized using US with Doppler effect. Color Doppler imaging (CDI), which has been conventionally used for US vascular imaging, is a vascular imaging technique based on the time between the transmission of ultrasound waves radiated at a certain frequency and the reception of ultrasound reflected by blood cells. CDI allows visualization of the velocity, direction, and signal intensity of blood flow [13]. In addition, power Doppler imaging (PDI) detects blood flow with higher sensitivity than CDI by visualizing the signal intensity of ultrasound alone [14]. However, the transmitted ultrasound waves are also reflected from tissues other than blood cells, which is a cause of artifacts in CDI or PDI. Thus, a filter is used to eliminate these noises (clutter). Clutter is typically low-frequency signals, and CDI utilizes a filter to eliminate signals below a certain frequency. Consequently, low-frequency signals from low-velocity blood flow are ignored, and microvessels with a low flow velocity cannot be visualized using conventional CDI [13].
Several techniques have been developed to improve blood flow detection sensitivity. Advanced dynamic flow (ADF) developed by Canon Medical Systems (Tochigi, Japan) and eFLOW developed by Fujifilm (Tokyo, Japan) improved the local resolution and frame rate of CDI [15, 16] (Fig. 2a). These imaging techniques enable the separate display of fine vessels. B-flow, developed by GE Healthcare (Chicago, IL, USA), detects blood flow by amplifying signals from blood cells [17] (Fig. 2b).
In addition, contrast-enhanced US (CEUS) has been used to evaluate vascularity [18, 19]. Contrast agents for US are composed of microbubbles (2–3 µm); a second-generation hypersonic ultrasound contrast agent (Sonazoid; Dai-ichi Sankyo, Tokyo, Japan) containing perflubutane is now widely used. These microbubbles resonate and collapse upon ultrasound irradiation, generating hyperechoic signals. CEUS visualizes blood flow by detecting hyperechoic signals. The contrast agent for US does not diffuse into extravascular organs, which is a distinctive characteristic from that of computed tomography (CT) and magnetic resonance imaging (MRI). Therefore, CEUS allows the precise evaluation of minute vessels. For instance, microflow imaging (MFI) developed by Canon Medical Systems detects the flow of microvessels without involvement of clutter by observation under a low mechanical index [20] (Fig. 2c). MFI generates microvascular images by tracing and overlapping the hyperechoic signals in each frame. In addition, precise imaging of microvessels is possible using ADF, eFLOW, and B-flow with a contrast agent that provides a distinct contrast between blood flow and extravascular organs (Fig. 2d).
Recently, a novel filter was developed to distinguish tissue motion artifacts from low-velocity blood flow. This filter enables visualization of microvessels. This imaging technique for visualizing microvessels is attracting much attention and is utilized in superb microvascular imaging (SMI), detective flow imaging (DFI), and microvascular imaging (MVI), developed by Canon Medical Systems, Fujifilm, and GE Healthcare, respectively [21,22,23,24] (Fig. 2e, f). SMI has been reported to be useful in various assessments, such as evaluation of liver fibrosis or intrahepatic vascular architecture in chronic hepatitis and differentiation of benign and malignant thyroid, breast, and prostate masses [25,26,27]. DFI is also utilized in endoscopic ultrasound (EUS) processors, and its efficacy in the diagnosis of pancreatobiliary disease using EUS has also been reported [28,29,30].
Evaluation of the vascularity of gallbladder wall thickening
The evaluation of blood flow using CDI or PDI has been mainly applied to differentiate between neoplastic and non-neoplastic gallbladder lesions or to assess the activity of cholecystitis [31, 32]. As for the differential diagnosis between benign and malignant gallbladder lesions, gallbladder wall blood flow (GWBF) has been evaluated [6, 33,34,35,36,37,38]. Li et al. reported that high-velocity arterial blood flow was detected in the cases with GBC while 40% of the cases with benign gallbladder lesions showed a low-velocity blood flow signal [34]. Hirooka et al. reported that GBC showed higher velocity and lower resistive index of GWBF as compared to benign lesions. They also demonstrated through an additional prospective study with 10 and 21 patients with GBC and benign polyp, respectively, that the differentiation between GBC and benign polyps was possible by means of estimation of GWBF velocity when 20 cm/s for velocity and 0.65 for resistive index were set as the cut-off values [35]. Hayakawa et al. also proved the presence of rapid GWBF in 12 patients with GBC as compared to that of 80 patients with no or benign gallbladder lesions, and suggested a GWBF velocity of 30 cm/s as a cut-off value [36]. In addition, Kawashima et al. showed that GWBF velocity was significantly higher in the patients with pancreatobiliary maljunction than those without, and concluded that the measurement of GWBF velocity could be a clue for the diagnosis of pancreatobiliary maljunction [37].
However, the gallbladder lesion should be hypervascular for the evaluation of GWBF using CDI or PDI, which may be a limitation of GWBF evaluation using CDI or PDI [32, 38]. Paulson et al. reported that arterial flow could not be detected in over 60% of the patients regardless of the presence or absence of cholecystitis [38]. Gallbladder lesions with wall thickening, such as inflammatory gallbladder disease and GBC, generally show hypovascularity owing to abundant fibrosis, and GWBF estimation using CDI or PDI for these lesions is not easy.
As for evaluation using microvascular imaging techniques, some studies have been reported (Table 3, Fig. 3). Kin et al. performed SMI in 20 gallbladder lesions, including seven cases presenting with wall thickening, and reported that the quality of microvascular imaging was significantly higher on SMI using a contrast agent as compared to that without, which was due to the vascularity of the gallbladder or the depth between the gallbladder and body surface [39]. They also suggested that the presence of tortuous microvessels or abrupt changes in vessel caliber may indicate malignancy. Osakabe et al. detected multiple tortuous microvessels flowing from the base to the interior of the lesion during GBC examination using DFI [40]. Microvascular evaluation using DFI was also performed using EUS. Yamashita et al. reported that DFI is more sensitive than e-FLOW for the detection of microvessels, and that irregular vessels may be a significant predictor of malignant gallbladder lesions [28]. Miwa et al. examined four patients with gallbladder lesions using EUS and reported that DFI was effective for the precise evaluation of microvessels. They also demonstrated that a single vessel at the base of the lesion might be a sign of non-neoplastic lesions [29]. Considering the results of these studies, morphological evaluation of microvessels may provide important insights for the diagnosis of gallbladder lesions.
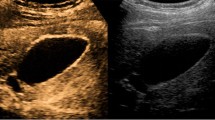
Some reports on the evaluation of gallbladder lesions using CEUS are presented (Fig. 4). Tsuji et al. classified 25 cases of GBCs into six patterns according to their enhancement pattern on CEUS and speculated that branched patterns might be a sign of malignancy [41]. Zhang et al. examined 105 cases of gallbladder lesions using CEUS and demonstrated a 95.2% diagnostic accuracy rate [42]. They considered heterogeneous enhancement with rapid washout as a typical finding in GBC. A prospective observational study conducted by Kumar et al. to evaluate the enhancement patterns of 26 cases with gallbladder lesions presenting wall thickening on CEUS concluded that gallbladder cancer showed early washout of contrast compared to benign wall thickening [43]. Kong et al. observed 49 cases of gallbladder lesions on US with and without enhancement and reported that malignant gallbladder lesions showed more vascularity and required longer to achieve iso-enhancement in comparison with the liver parenchyma than benign lesions [44]. In addition, the diagnostic accuracy of US can be improved in combination with CEUS, which is prominent in cases of gallbladder wall thickening. These studies suggest that analysis of the vascularity, enhancement pattern, or timing of enhancement/washout on CEUS is quite effective for the diagnosis of gallbladder lesions presenting with wall thickening (Table 4).
Findings of gallbladder lesions on contrast-enhanced ultrasound. a Adenomyomatosis. Gallbladder lesions were uniformly enhanced 32 s after injection of contrast agent. A small cystic structure was also found in the lesion (arrow). b Gallbladder cancer. A gallbladder lesion was heterogeneously enhanced, and a perfusion defect was observed 22 s after injection of contrast agent (arrow). c Gallbladder cancer (same case as in b). Contrast agent was partially washed out 53 s after injection of contrast agent (arrow)
Limitations and future perspective of microvascular imaging
Visualization of microvessels is considered a promising technique, but some problems still exist. Visualization of microvessels on US is still under development, and further improvements are required especially for the improvement of local resolution and the reduction of artifacts. Benign or malignant diagnosis based on microvascular findings largely depends on subjective judgement, and the morphological findings of microvessels only serve as a reference at present. There have been few US studies regarding microvessels, and validation of the reported findings has not been fully discussed. However, further studies on the morphology of microvessels will reveal microvascular findings specific to benign and malignant gallbladder lesions.
Conclusion
Although morphological evaluation is fundamental for the diagnosis of gallbladder lesions presenting wall thickening, difficult cases are sometimes encountered. Hemodynamic evaluation is important in such cases. Recent advances in US imaging have made microvascular imaging possible, and detailed hemodynamic evaluations including the enhancement pattern on CEUS and morphological findings of microvessels are expected to aid in differentiating between benign and malignant gallbladder lesions.
Abbreviations
- US:
-
Ultrasound
- GBC:
-
Gallbladder cancer
- ADM:
-
Adenomyomatosis
- XGC:
-
Xanthogranulomatous cholecystitis
- CDI:
-
Color Doppler imaging
- PDI:
-
Power Doppler imaging
- ADF:
-
Advanced dynamic flow
- CEUS:
-
Contrast-enhanced ultrasound
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- MFI:
-
Micro flow imaging
- SMI:
-
Superb microvascular imaging
- DFI:
-
Detective flow imaging
- MVI:
-
Microvascular imaging
- GWBF:
-
Gallbladder wall blood flow
References
Feo CF, Ginesu GC, Fancellu A, et al. Current management of incidental gallbladder cancer: a review. Int J Surg. 2022;98: 106234.
Zaidi MY, Abou-Alfa GK, Ethun CG, et al. Evaluation and management of incidental gallbladder cancer. Chin Clin Oncol. 2019;8:37.
Miyazaki K, Tsutsumi N, Sasatomi E, et al. Benign gallbladder lesions mimicking cancer infiltrating the liver. J Hep Bil Pancr Surg. 1994;1:273–9.
Kulkarni AA, Soni P, Sharma VK, et al. Immunoglobulin G4-related disease mimicking gallbladder cancer with associated choledochal cyst: a case report of a malignant masquerade. JGH Open. 2019;3:536–9.
Khan MR, Begum S. Extended resection for xanthogranulomatous cholecystitis mimicking gallbladder carcinoma: cases and review of diagnostic approach. J Pak Med Assoc. 2019;69:256–60.
Okaniwa S. How can we manage gallbladder lesions by transabdominal ultrasound? Diagnostics (Basel). 2021;11:784.
Miyoshi H, Inui K, Katano Y, et al. B-mode ultrasonographic diagnosis in gallbladder wall thickening. J Med Ultrason. 2021;48:175–86.
van Breda Vriesman AC, Engelbrecht MR, et al. Diffuse gallbladder wall thickening: differential diagnosis. AJR Am J Roentgenol. 2007;188:495–501.
Jutras JA. Hyperplastic cholecystoses. Am J Roentgenol. 1960;83:795–827.
Golse N, Lewin M, Rode A, et al. Gallbladder adenomyomatosis: diagnosis and management. J Visc Surg. 2017;154:345–53.
Okaniwa S, Hirai T, Ogawa M, et al. Manual for abdominal ultrasound in cancer screening and health checkups, revised edition (2021). J Med Ultrason. 2023;50:5–49.
Kin T, Maguchi H, Takahashi K, et al. Clinicopathological features of gallbladder cancer with adenomyomatosis. JJBA. 2014;28:633–40 (Japanese).
Hasegawa H. Fundamentals and applications of Doppler ultrasound. Jpn J Med Ultrasonics. 2016;43:411–5 (Japanese).
Hayashi T, Kawano T, Izumi M, et al. Power doppler imaging—its plinciples and advantages. Neurosonology. 1997;10:51–6.
Wen YL, Kudo M, Maekawa K, et al. Contrast advanced dynamic flow imaging and contrast pulse subtraction imaging: preliminary results in hepatic tumors. J Med Ultrason. 2002;29:195–204.
Das K, Kudo M, Kitano M, et al. Diagnostic value of endoscopic ultrasound-guided directional eFLOW in solid pancreatic lesions. J Med Ultrason. 2013;40:211–8.
Weskott HP. B-flow—a new method for detecting blood flow. Ultraschall Med. 2000;21:59–65 (German).
Sofuni A, Iijima H, Moriyasu F, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40:518–25.
Iijima H. Contrast ultrasound imaging. Kanzo. 2006;47:173–80.
Sofuni A, Moriyasu F, Tsuji S, et al. The usefulness of contrast-enhanced ultrasonography with Sonazoid in pancreatic diseases. Suizo. 2011;26:11–22 (Japanese).
Machado P, Segal S, Lyshchik A, et al. A novel microvascular flow technique: Initial results in thyroids. Ultrasound Q. 2016;32:67–74.
Yamashita Y, Yoshikawa T, Kawaji Y, et al. Novel endoscopic ultrasonography imaging technique for visualizing microcirculation without contrast enhancement in subepithelial lesions: prospective study. Dig Endosc. 2021;33:955–61.
Dall’Asta A, Grisolia G, Volpe N, et al. Prenatal visualisation of the torcular herophili by means of a Doppler technology highly sensitive for low-velocity flow in the expert assessment of the posterior fossa: a prospective study. BJOG. 2021;128:347–52.
Ma Y, Li G, Li J, et al. The diagnostic value of superb microvascular imaging (SMI) in detecting blood flow signals of breast lesions: a preliminary study comparing SMI to color Doppler flow imaging. Medicine (Baltimore). 2015;94: e1502.
Zhu YC, Zhang Y, Deng SH, et al. A prospective study to compare superb microvascular imaging with grayscale ultrasound and color Doppler flow imaging of vascular distribution and morphology in thyroid nodules. Med Sci Monit. 2018;24:9223–31.
Lu R, Meng Y, Zhang Y, et al. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med Imaging. 2017;17:65.
Lee DH, Lee JY, Han JK. Superb microvascular imaging technology for ultrasound examinations: initial experiences for hepatic tumors. Eur J Radiol. 2016;85:2090–5.
Yamashita Y, Ashida R, Tamura T, et al. Novel technique of endoscopic ultrasonography for the differential diagnosis of gallbladder lesions and intraductal papillary mucinous neoplasms: a single-center prospective study. Diagnostics (Basel). 2023;13:2132.
Miwa H, Sugimori K, Maeda S. Vessel images of gallbladder polypoid lesions on detective flow imaging endoscopic ultrasonography. Dig Endosc. 2023;35:e61–2.
Omoto S, Takenaka M, Fukunaga T, et al. Diagnosis of an intraductal papillary neoplasm of the bile duct with fibrovascular stalks using detective flow imaging. Endoscopy. 2023;55:E1012–4.
Uggowitzer M, Kugler C, Schramayer G, et al. Sonography of acute cholecystitis: comparison of color and power Doppler sonography in detecting a hypervascularized gallbladder wall. AJR Am J Roentgenol. 1997;168:707–12.
Komatsuda T, Ishida H, Konno K, et al. Gallbladder carcinoma: color Doppler sonography. Abdom Imaging. 2000;25:194–7.
Ueno N, Tomiyama T, Tano S, et al. Diagnosis of gallbladder carcinoma with color Doppler ultrasonography. Am J Gastroenterol. 1996;91:1647–9.
Li D, Dong BW, Wu YL, et al. Image-directed and color Doppler studies of gallbladder tumors. J Clin Ultrasound. 1994;22:551–5.
Hirooka Y, Naitoh Y, Furukawa T, et al. Differential diagnosis of gall-bladder masses using colour Doppler ultrasonography. J Gastroenterol Hepatol. 1996;11:840–6.
Hayakawa S, Goto H, Hirooka Y, et al. Colour Doppler-guided spectral analysis of gall-bladder wall flow. J Gastroenterol Hepatol. 1998;13:181–5.
Kawashima H, Hirooka Y, Itoh A, et al. Use of color Doppler ultrasonography in the diagnosis of anomalous connection in pancreatobiliary disease. World J Gastroenterol. 2005;11:1018–22.
Paulson EK, Kliewer MA, Hertzberg BS, et al. Diagnosis of acute cholecystitis with color Doppler sonography: significance of arterial flow in thickened gallbladder wall. AJR Am J Roentgenol. 1994;162:1105–8.
Kin T, Nagai K, Hayashi T, et al. Efficacy of superb microvascular imaging of ultrasound for diagnosis of gallbladder lesion. J Hepatobiliary Pancreat Sci. 2020;27:977–83.
Osakabe K, Ichino N, Sugiyama H, et al. Differential diagnosis of gallbladder polyps by detective flow imaging. Jpn J Med Ultrasonics. 2023;50:S237 (Japanese).
Tsuji S, Sofuni A, Moriyasu F, et al. Contrast-enhanced ultrasonography in the diagnosis of gallbladder disease. Hepatogastroenterology. 2012;59:336–40.
Zhang X, Tang S, Huang L, et al. Value of contrast-enhanced ultrasound in the differential diagnosis of gallbladder lesion. World J Gastroenterol. 2018;24:744–51.
Kumar I, Yadav YK, Kumar S, et al. Utility of contrast-enhanced ultrasound in differentiation between benign mural lesions and adenocarcinoma of gallbladder. J Med Ultrasound. 2019;28:143–50.
Kong WT, Shen HY, Qiu YD, et al. Application of contrast enhanced ultrasound in gallbladder lesion: is it helpful to improve the diagnostic capabilities? Med Ultrasonogr. 2018;20:420–6.
Acknowledgements
We thank Editage (http://www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AK has received honoraria from Olympus. The other authors declare no conflicts of interest.
Ethical statements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kin, T., Motoya, M., Hayashi, T. et al. Transabdominal ultrasound evaluation of vascularity of gallbladder lesions: particularly those with wall thickening. J Med Ultrasonics 51, 429–436 (2024). https://doi.org/10.1007/s10396-024-01467-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-024-01467-3