Abstract
Endoscopic ultrasound (EUS) has recently played an increasing role in the diagnosis of gallbladder diseases. This review aims to summarize the role of EUS in the diagnosis of gallbladder lesions. EUS provides high-resolution images that can improve the diagnosis of gallbladder polypoid lesions and microlithiasis, in addition to evaluating gallbladder thickness and staging of gallbladder carcinoma. Contrast-enhancing agents may be useful for the differential diagnosis of gallbladder lesions, but the evidence of their effectiveness is still limited and further studies are required in this area to establish its usefulness. Endoscopic ultrasound combined with fine needle aspiration has played an increasing role in providing histological diagnosis of gallbladder tumors in addition to gallbladder thickening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diseases of the gallbladder are relatively common, with the most common pathology, cholelithiasis, affecting 10–15 % of the population [1]. Other conditions such as gallbladder polyp are found in around 5 % of the world population, while gallbladder cancer has an incidence of approximately 2 per 100,000 population worldwide [1] to 7 in 100,000 population in Japan [2]. Although not a common gallbladder pathology, the prognosis of gallbladder carcinoma is often dismal as a result of late diagnosis [1], with only 10 % of patients being candidates for curative resection at the initial presentation [3].
Endoscopic ultrasound (EUS) plays an increasing role in the diagnosis of pancreatobiliary diseases, with advantages over the other modalities including proximity of the ultrasound probe to the region of interest, real-time imaging of the lesions, evaluation of the vascularity using contrast-enhanced study, and the ability for tissue acquisition and therapeutic interventions. The role of EUS in the diagnosis of gallbladder lesions has been demonstrated in many studies, and this review aims to summarize the role of EUS in the diagnosis of gallbladder pathologies. To achieve this, a search was conducted of English language human studies listed in the PubMed database that were published between 1996 and 2015. The following keywords were used in combination with EUS: gallbladder, gallbladder polyp, gallbladder thickening, adenomyomatosis, gallbladder carcinoma, microlithiasis, contrast-enhanced ultrasound of gallbladder, staging gallbladder carcinoma, and gallbladder EUS-FNA. References of subsequently identified articles were also examined for potentially relevant studies.
Transabdominal versus endoscopic ultrasound in the detection of gallbladder lesions
Transabdominal ultrasound (TUS) is the primary screening modality for hepatobiliary diseases. Despite its excellent safety profile and wide availability, TUS has a sensitivity of only 66 % but 100 % specificity in distinguishing between gallbladder polyps and calculi [4]. The sensitivity of TUS in the detection of polypoid lesions of the gallbladder ranges from 36 to 99 % depending on the presence of gallstones [5, 6]. TUS still has some difficulty in identifying particularly small gallbladder lesions and microlithiasis [7].
EUS is considered superior to TUS for imaging of the biliary system because of its ability to achieve closer proximity and obtain higher resolution images using higher ultrasound frequencies than conventional ultrasonography (5–12 versus 2–5 MHz). The benefit of EUS was demonstrated in the diagnosis of small (<2 cm) polypoid lesions [8–11], which increased the diagnostic sensitivity to up to 91.7 % and specificity to up to 87.7 % when compared with TUS (sensitivity of 54.2 % and specificity of 53.8 %) [9]. In a separate study, EUS showed higher specificity and comparable sensitivity to TUS in diagnosing neoplastic lesions [11]. The difference in sensitivity between TUS and EUS could be overcome using high-resolution transabdominal ultrasound with a high frequency (2.5–7 MHz). This showed similar diagnostic efficacy to EUS, computed tomography (CT), or magnetic resonance imaging (MRI) [12, 13]. However, there have been only a few studies that have used high-resolution ultrasound, all of which originated from the same institute.
The benefit of using EUS over TUS has been best demonstrated in the diagnosis of microlithiasis. Using EUS, gallbladder sludge or microlithiasis could be detected in 52.4–94.2 % of cases that had a negative result on TUS [14–16]. EUS demonstrated a 92.6–100 % sensitivity and 55.6–91 % specificity for the diagnosis of gallbladder microlithiasis [7, 14]. In most of these cases, the gallstones were located at the gallbladder infundibulum [16], which is difficult to effectively visualize with TUS.
Differential diagnosis of gallbladder polypoid lesions and gallbladder thickening
The gallbladder wall is composed of four layers: mucosa, lamina propria, muscularis propria, and serosa. Polypoid lesions of the gallbladder are defined as elevated mucosal lesions of the gallbladder, which include both neoplastic and non-neoplastic lesions [17, 18]. Gallbladder wall thickening is defined as the gallbladder wall measuring more than 3 mm, and can be the result of various processes [19]. Gallbladder wall thickening in adenomyomatosis involves hyperplasia of mucosa and muscularis propria (Fig. 1), while cholesterol polyps involve the lamina propria with sparing of the mucosal layer (Fig. 2) [18]. As these lesions are frequently incidental findings during abdominal examinations, the differential diagnosis between benign lesions such as cholesterol polyps and the early stage of gallbladder carcinoma is crucial.
EUS can visualize the layered structure of the gallbladder and provide high-resolution images using high ultrasound frequencies. Akatsu et al. [20] described the usefulness of EUS in the differential diagnosis of gallbladder lesions. In their study, the presence of hyperechoic spots and multiple microcysts was an important indicator of non-neoplastic lesions. In contrast, the presence of hypoechoic foci strongly predicted neoplastic lesions in a separate retrospective study [21]. In another study, a granular contour and spotty internal echo pattern in pedunculated polypoid lesions indicated benign pathology, which sometimes disappeared during the follow-up study [22]. In the absence of these findings, therefore, neoplastic lesions should be suspected [11].
Some studies demonstrated that EUS had some limitations in differentiating between malignant and nonmalignant polyps less than 1 cm in size [10]. In one retrospective review, the accuracy of EUS was lower in the lesions larger than 2 cm in size. This may be because the larger lesions were adenomyomatosis, xanthogranulomatous cholecystitis, or tumefactive biliary sludge, which were similar in appearance to the gallbladder carcinoma [23]. In this study, the presence of gallbladder stones affected the diagnostic accuracy, but the difference was not statistically significant.
To aid diagnosis, EUS scoring systems have been proposed to differentiate between benign and malignant gallbladder polyps. Choi et al. [24] have proposed a EUS scoring system for differential diagnosis of gallbladder lesions between 5 and 15 mm in size based on the layer structure, echo patterns, margin of polyp, presence of stalk, and number of polyps (Table 1). Using a cutoff score of 6, the sensitivity was 81 % and the specificity was 86 % in the differential diagnosis of neoplastic and non-neoplastic polyps. Another scoring system was proposed by Sadamoto et al. [25], in which the maximum diameter and internal echo pattern were calculated. Using a cutoff score of over 12, the sensitivity and specificity were 77.8 and 82.7 %, respectively. Both scoring systems, however, have been applied in only one study, which reported sensitivity of 67 and 85 % and specificity of 65 and 65 %, respectively, using Choi and Sadamoto’s scoring systems [21].
Contrast-enhanced EUS has been used to improve diagnostic accuracy based on the different levels of vascularity and blood flow that are found across different pathologic processes. Many studies using contrast-enhanced EUS imaging, however, have focused on pancreatic lesions, and there have been limited studies of contrast-enhanced EUS in the differential diagnosis of gallbladder polyps or gallbladder thickening [26–28].
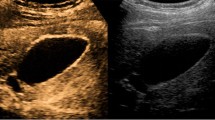
Various descriptions of the different contrast-enhanced patterns between benign and malignant polyps have been proposed, utilizing both TUS and EUS. In a report by Hirooka using sonicated albumin, enhancement was observed in adenocarcinomas, but not in adenosquamous carcinomas and cholesterol polyps [26]. The later studies were based on the second-generation contrast agents such as SonoVue® and Sonazoid®. In a multicenter trial using transabdominal ultrasound with SonoVue®, malignant lesions exhibited branched or linear intralesional vessels and inhomogeneous enhancement with rapid contrast washout before 35–36.5 s when compared with benign lesions [29, 30]. In this study, the contrast benefited most in the differentiation of sludge from cancer. It also helped in identification of the lesion characteristics, assessment of tumor extension and vascularization, and evaluation of liver involvement. This rapidly enhanced washout pattern in the contrast study was confirmed in other studies [31], with a diffuse and eccentric pattern reported [31]. Gallbladder wall discontinuity was also an important sign of malignancy in many studies [29–31]. Based on these principles, contrast-enhanced EUS of the gallbladder should have shown a similar result. Contrast-enhanced EUS finding of gallbladder sludge is shown in Fig. 3.
In a EUS study by Choi, the perfusion patterns were classified as diffuse enhancement, perfusion defect, and no enhancement. The vessels were categorized as regular spotty vessel, irregular vessel, or no vessels [27]. In this study, irregular vessel pattern and perfusion defect indicated malignant lesions. The sensitivity and specificity of contrast-enhanced harmonic imaging were 93.5 and 93.2 %, respectively, versus 90.0 and 91.1 % for conventional EUS. Another study by Park classified the perfusion pattern into homogeneously enhanced, as in gallbladder adenoma, and heterogeneously enhanced, as in cholesterol polyp, and used them together with the size of the lesion [28]. The sensitivity and specificity of the differential diagnosis between gallbladder adenoma and cholesterol polyps in this study were 75.0 and 66.6 %, respectively.
Despite many studies of TUS in the differential diagnosis of gallbladder wall thickening, there have been only limited studies of the role of EUS [32, 33]. Using EUS, gallbladder thickening with loss of the multiple-layer pattern indicated gallbladder carcinoma, while these layers were preserved in benign gallbladder wall thickening [34]. In this study, the ability of EUS to demonstrate the gallbladder wall layers was superior to that of TUS, CT, or MRI. In the study by Kim, gallbladder thickening >10 mm and hypoechoic internal echogenicity were predictive factors for neoplastic gallbladder thickening. The role of contrast-enhanced studies in the differential diagnosis of benign and malignant gallbladder thickening has been reported using TUS [32]. Malignant gallbladder thickening demonstrated branched or linear intralesional vessels, inhomogeneous gallbladder wall enhancement, quicker time to hypo-enhancement, and discontinuous inner or outer layer, and involvement of adjacent liver. Another study by Imazu [33] using contrast-enhanced EUS in differential diagnosis of gallbladder wall thickening demonstrated inhomogeneous enhancement as a strong predictive factor of malignant gallbladder wall thickening. Recently, a retrospective study using contrast-enhanced harmonic EUS compared to B-mode imaging in the diagnosis of gallbladder lesion was reported [35]. In this study, non-enhanced lesions and homogeneously enhanced lesions were classified as benign, while the brindled enhanced lesions were classified as malignant, with the sensitivity, specificity, and accuracy being 100, 94.4, and 95.8 %, respectively, for the prediction of malignancy. In our opinion, with limited data, EUS provided information that might support the differential diagnosis of benign and malignant gallbladder lesions. On the other hand, contrast-enhanced EUS may also be useful, but the benefit of this method over contrast-enhanced TUS is still unclear.
Staging of gallbladder carcinoma
EUS has been used in the staging of gallbladder carcinoma as it is able to clearly demonstrate the multilayer of the gallbladder wall structure and aids evaluation of regional lymph node involvement [36–38]. Gallbladder carcinomas are classified into four types, as shown in Table 2. The accuracy of EUS classification and T staging was reported to be 100, 75.6, 85.3 and 92.7 % according to a report by Sadamoto [37]. EUS images of a Tis lesion and T2 lesion are shown in Figs. 4 and 5, respectively.
EUS images of gallbladder carcinoma. The conventional EUS image (a) demonstrated a pedunculated lesion with a preserved outer hyperechoic layer. The color Doppler image of the lesion (b) showed color signal at the base of the lesion. The contrast-enhanced harmonic image 20 s after the injection of Sonazoid® (c) showed inhomogeneous hypervascularity with a perfusion defect (arrow). The pathology result of surgical resection showed a well-differentiated tubular adenocarcinoma with Tis stage
EUS images of gallbladder carcinoma. The conventional EUS image (a) showed a sessile lesion with a narrowed outer hyperechoic layer. The contrast-enhanced harmonic image after the injection of Sonazoid® (b) showed a hypervascular lesion with discontinuation of the outer layer (arrowhead). The pathology result showed subserosal invasion of gallbladder carcinoma (T2)
EUS fine needle aspiration (EUS-FNA) or fine needle biopsy (EUS-FNB) for the diagnosis of gallbladder lesions
The role of EUS-FNA and EUS-FNB in tissue sampling for various lesions has been established. However, its role in the diagnosis of gallbladder lesions has not been elucidated. Despite the fact that the utility of EUS-FNA in gallbladder lesions has been reported for more than a decade [39], studies based on this method have been limited, with most being case series. The accuracy of EUS-FNA in gallbladder lesions ranged from 80 to 100 % [39–41], which was superior to endoscopic transpapillary gallbladder aspiration with cytology [42] or endoscopic retrograde cholangiography-guided sampling [43]. No immediate complication or tumor seeding was reported. In contrast to gallbladder EUS-FNA in the setting of suspected microlithiasis [44], which has significant risk of bile peritonitis, there has been no report of bile peritonitis in EUS-FNA in gallbladder disease. This might have been due to the fibrotic wall of the diseased gallbladder preventing bile spillage [40], as well as the FNA technique, which targeted the gallbladder wall and was not directly inserted into the gallbladder.
Apart from diagnosis of gallbladder adenocarcinoma, there have been several case reports of EUS –FNA in other uncommon lesions of the gallbladder, such as neuroendocrine tumor [45], xanthomatous cholecystitis [41], squamous cell carcinoma [46], and plasmacytoma [47].
In summary, EUS has played an increasing role in the diagnosis and treatment of gallbladder diseases. EUS provides higher resolution images of gallbladder lesions using the higher frequency and closer proximity to the gallbladder when compared to conventional TUS. Studies of contrast-enhanced EUS are limited, but the evidence from contrast-enhanced TUS showed improvement of the differential diagnosis of gallbladder carcinoma. In addition, EUS-FNA could provide histologic diagnosis both in gallbladder tumors and gallbladder thickening.
References
Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172–87.
Kanthan R, Senger JL, Ahmed S, et al. Gallbladder cancer in the twenty first century. J Oncol. 2015;2015:967472.
Zhu AX, Hong TS, Hezel AF, et al. Current management of gallbladder carcinoma. Oncologist. 2010;15:168–81.
Chattopadhyay D, Lochan R, Balupuri S, et al. Outcome of gall bladder polypoidal lesions detected by transabdominal ultrasound scanning: a 9 year experience. World J Gastroenterol. 2005;11:2171–3.
Yang HL, Sun YG, Wang Z. Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg. 1992;79:227–9.
Terzi C, Sokmen S, Seckin S, et al. Polypoid lesions of the gallbladder: report of 100 cases with special reference to operative indications. Surgery. 2000;127:622–7.
Dahan P, Andant C, Levy P, et al. Prospective evaluation of endoscopic ultrasonography and microscopic examination of duodenal bile in the diagnosis of cholecystolithiasis in 45 patients with normal conventional ultrasonography. Gut. 1996;38:277–81.
Sugiyama M, Xie XY, Atomi Y, et al. Differential diagnosis of small polypoid lesions of the gallbladder: the value of endoscopic ultrasonography. Ann Surg. 1999;229:498–504.
Azuma T, Yoshikawa T, Araida T, et al. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am J Surg. 2001;181:65–70.
Cheon YK, Cho WY, Lee TH, et al. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J Gastroenterol. 2009;15:2361–6.
Sugiyama M, Atomi Y, Yamato T. Endoscopic ultrasonography for differential diagnosis of polypoid gall bladder lesions: analysis in surgical and follow up series. Gut. 2000;46:250–4.
Jang JY, Kim SW, Lee SE, et al. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg. 2009;250:943–9.
Bang SH, Lee JY, Woo H, et al. Differentiating between adenomyomatosis and gallbladder cancer: revisiting a comparative study of high-resolution ultrasound, multidetector CT, and MR imaging. Korean J Radiol. 2014;15:226–34.
Ardengh JC, Malheiros CA, Rahal F, et al. Microlithiasis of the gallbladder: role of endoscopic ultrasonography in patients with idiopathic acute pancreatitis. Rev Assoc Med Bras. 2010;56:27–31.
Mirbagheri SA, Mohamadnejad M, Nasiri J, et al. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg. 2005;9:961–4.
Thorboll J, Vilmann P, Jacobsen B, et al. Endoscopic ultrasonography in detection of cholelithiasis in patients with biliary pain and negative transabdominal ultrasonography. Scand J Gastroenterol. 2004;39:267–9.
Ito H, Hann LE, D’Angelica M, et al. Polypoid lesions of the gallbladder: diagnosis and followup. J Am Coll Surg. 2009;208:570–5.
Mellnick VM, Menias CO, Sandrasegaran K, et al. Polypoid lesions of the gallbladder: disease spectrum with pathologic correlation. Radiographics. 2015;35:387–99.
van Breda Vriesman AC, Engelbrecht MR, Smithuis RH, et al. Diffuse gallbladder wall thickening: differential diagnosis. AJR Am J Roentgenol. 2007;188:495–501.
Akatsu T, Aiura K, Shimazu M, et al. Can endoscopic ultrasonography differentiate nonneoplastic from neoplastic gallbladder polyps? Dig Dis Sci. 2006;51:416–21.
Cho JH, Park JY, Kim YJ, et al. Hypoechoic foci on EUS are simple and strong predictive factors for neoplastic gallbladder polyps. Gastrointest Endosc. 2009;69:1244–50.
Kimura K, Fujita N, Noda Y, et al. Differential diagnosis of large-sized pedunculated polypoid lesions of the gallbladder by endoscopic ultrasonography: a prospective study. J Gastroenterol. 2001;36:619–22.
Muguruma N, Okamura S, Ichikawa S, et al. Endoscopic sonography in the diagnosis of gallbladder wall lesions in patients with gallstones. J Clin Ultrasound. 2001;29:395–400.
Choi WB, Lee SK, Kim MH, et al. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest Endosc. 2000;52:372–9.
Sadamoto Y, Oda S, Tanaka M, et al. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy. 2002;34:959–65.
Hirooka Y, Naitoh Y, Goto H, et al. Contrast-enhanced endoscopic ultrasonography in gallbladder diseases. Gastrointest Endosc. 1998;48:406–10.
Choi JH, Seo DW, Park do H, et al. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest Endosc. 2013;78:484–93.
Park CH, Chung MJ, Oh TG, et al. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414–21.
Liu LN, Xu HX, Lu MD, et al. Contrast-enhanced ultrasound in the diagnosis of gallbladder diseases: a multi-center experience. PLoS One. 2012;7:e48371.
Xie XH, Xu HX, Xie XY, et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol. 2010;20:239–48.
Yuan HX, Cao JY, Kong WT, et al. Contrast-enhanced ultrasound in diagnosis of gallbladder adenoma. Hepatobiliary Pancreat Dis Int. 2015;14:201–7.
Kim HJ, Park JH, Park DI, et al. Clinical usefulness of endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2012;57:508–15.
Imazu H, Mori N, Kanazawa K, et al. Contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2014;59:1909–16.
Mizuguchi M, Kudo S, Fukahori T, et al. Endoscopic ultrasonography for demonstrating loss of multiple-layer pattern of the thickened gallbladder wall in the preoperative diagnosis of gallbladder cancer. Eur Radiol. 1997;7:1323–7.
Sugimoto M, Takagi T, Konno N, et al. The efficacy of contrast-enhanced harmonic endoscopic ultrasonography in diagnosing gallbladder cancer. Sci Rep. 2016;6:25848.
Mitake M, Nakazawa S, Naitoh Y, et al. Endoscopic ultrasonography in diagnosis of the extent of gallbladder carcinoma. Gastrointest Endosc. 1990;36:562–6.
Sadamoto Y, Kubo H, Harada N, et al. Preoperative diagnosis and staging of gallbladder carcinoma by EUS. Gastrointest Endosc. 2003;58:536–41.
Fujita N, Noda Y, Kobayashi G, et al. Diagnosis of the depth of invasion of gallbladder carcinoma by EUS. Gastrointest Endosc. 1999;50:659–63.
Jacobson BC, Pitman MB, Brugge WR. EUS-guided FNA for the diagnosis of gallbladder masses. Gastrointest Endosc. 2003;57:251–4.
Varadarajulu S, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration in the evaluation of gallbladder masses. Endoscopy. 2005;37:751–4.
Hijioka S, Mekky MA, Bhatia V, et al. Can EUS-guided FNA distinguish between gallbladder cancer and xanthogranulomatous cholecystitis? Gastrointest Endosc. 2010;72:622–7.
Ogura T, Kurisu Y, Masuda D, et al. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for thick-walled gallbladders? Dig Dis Sci. 2014;59:1917–24.
Hijioka S, Hara K, Mizuno N, et al. Diagnostic yield of endoscopic retrograde cholangiography and of EUS-guided fine needle aspiration sampling in gallbladder carcinomas. J Hepatobiliary Pancreat Sci. 2012;19:650–5.
Jacobson BC, Waxman I, Parmar K, et al. Endoscopic ultrasound-guided gallbladder bile aspiration in idiopathic pancreatitis carries a significant risk of bile peritonitis. Pancreatology. 2002;2:26–9.
Samad A, Kaplan A, Arain M, et al. Endoscopic ultrasound-guided fine-needle aspiration diagnosis of large cell neuroendocrine carcinoma of the gallbladder and common bile duct: report of a case. Diagn Cytopathol. 2013;41:1091–5.
Chambers MR, Hasan MK, Hebert-Magee S. Pearls before bile: primary squamous cell carcinoma of the gallbladder diagnosed on-site by endoscopic ultrasound-guided fine-needle aspiration. Dig Endosc. 2016;28:105.
St Romain P, Desai S, Bean S, et al. Extramedullary plasmacytoma of the gallbladder diagnosed by endoscopic ultrasound fine needle aspiration (EUS-FNA). J Gastrointest Oncol. 2015;6:E7–9.
Acknowledgments
The authors would like to thanks Dr. Brett Ibbeson for the English proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflicts of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
About this article
Cite this article
Chantarojanasiri, T., Hirooka, Y., Kawashima, H. et al. The role of endoscopic ultrasound in the diagnosis of gallbladder diseases. J Med Ultrasonics 44, 63–70 (2017). https://doi.org/10.1007/s10396-016-0742-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-016-0742-9