Abstract
Urinary tract stones are a common clinical condition that affect millions of individuals worldwide. The management of these stones has evolved significantly over the past 70 years, and ultrasound imaging has emerged as a valuable tool for diagnosis, treatment planning, and follow-up. This review aims to provide an overview of the application of ultrasound imaging in the treatment of urinary tract stones, highlighting its advantages, limitations, and current advancements in the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary tract stones (UTS) are crystalline deposits in the kidneys. The trend in prevalence of urolithiasis is increasing, affecting approximately 12% of the global population [1, 2]. The recurrence rate is also high, estimated to be approximately 50% within 10 years [3]. UTS is a clinically significant condition that can cause significant pain and complications and affect kidney function. The intensity of symptoms may vary depending on the stone location, size, and degree of obstruction. Prompt diagnosis, appropriate management, and preventive measures are essential for alleviating symptoms, preventing complications, and reducing the risk of recurrence.
Broadly, the diagnosis of UTS involves imaging modalities, including ultrasound imaging (US), noncontrast computed tomography (NCCT), and abdominal plain radiography (KUB). The sensitivity, specificity, radiation exposure level, and relative cost vary according to the modality (Table 1) [4,5,6,7,8,9]. US plays a significant role in the treatment of UTS, while NCCT has become the imaging modality of choice because of its high diagnostic sensitivity and specificity. However, in recent years, US has been recommended as an imaging modality for pediatric and pregnant patients with suspected UTS because of the long-term risks of ionizing radiation exposure [9,10,11]. Based on the principle of ALARA (As Low As Reasonably Achievable), US will become increasingly important as the first line of investigation for diagnosing UTS.
The clinical use of US for the treatment of kidney stones was first published in 1961 by Schlengel et al., who used intraoperative amplitude (A)-mode US for the localization of kidney stones [12]. Since then, continuous technological innovations in US, such as real-time imaging, have expanded its clinical use. In 1977, Cook et al. first reported the efficacy of intraoperative US using a brightness (B)-mode probe to localize kidney stones [13]. US is widely used for diagnosis, follow-up, percutaneous renal access, shockwave lithotripsy (SWL), ureteroscopic lithotripsy (URSL), and percutaneous nephrolithotomy (PCNL). Recent developments in US technology have focused on improving stone imaging and characterization, as well as enhancing the accuracy and efficiency of stone management. Such technological innovation has the potential to create a paradigm shift in the management of UTS. This review provides an overview of the application of US in the treatment of UTS, highlighting its advantages, limitations, and current advancements in the field.
Ultrasound imaging as a modality for diagnosis of urinary tract stones
Features of ultrasound imaging
Accurate imaging allows for the identification and localization of UTS. Imaging modalities, such as US, NCCT, and KUB, can precisely visualize the presence, size, number, and location of stones within the urinary system. This information is essential for treatment planning and determining the appropriate approach for stone removal, leading to optimal patient outcomes. According to the American Urological Association (AUA) and European Association of Urology (EAU) guidelines, the treatment modality is selected based on the targeted stone size and location [14, 15]. It also assists in estimating the complexity of the procedure, expected surgical time, and potential risks associated with stone removal. The nomogram used to predict PCNL success includes stone burden, location, number, and presence of staghorns [16].
As shown in Table 1, NCCT provides the most accurate diagnosis with high sensitivity and specificity (94–100% and 92–100%, respectively) [4,5,6]. NCCT can determine stone density, inner structure, surrounding anatomy, and stone size and location, all of which affect the treatment modality [17]. However, its major drawback is high-dose radiation exposure. Several studies have demonstrated the risks associated with cumulative radiation exposure owing to repeated CT scans. Ferrandino et al. reported that 20% of patients received doses in excess of the 50 mSv occupational limit established by the International Commission on Radiological Protection for 1 year [18].
US has several advantages. The first is its portability. Advancements in miniaturization and wireless technology have led to the development of portable handheld US devices. These devices offer convenience and flexibility, allowing for US imaging at the point of care, including in resource-limited settings. Point-of-care ultrasound (POCUS) is the standard practice performed by a physician at the bedside and considered to be an effective alternative to NCCT as an initial imaging modality for the diagnosis of an acute stone event in the emergency department [19]. Smith-Bindman et al. conducted a large multicenter, randomized clinical trial (RCT) to evaluate the sensitivity and specificity of POCUS compared with NCCT. According to the study, POCUS was associated with lower radiation exposure than NCCT (10.1 ± 14.1 mSv vs 17.2 ± 13.4 mSv; p < 0.001), with equivalent diagnostic accuracy in the emergency department [20].
The second advantage of US is its noninvasiveness. US is a noninvasive imaging modality that does not involve ionizing radiation, making it a safe option for initial evaluation. Therefore, US is widely used in various patient populations, including children and pregnant women [9,10,11]. In pediatric patients, US is the primary imaging technique that has high sensitivity and specificity (70% and 100%, respectively), with a 96% positive predictive value and 62% negative predictive value [11]. On the other hand, the sensitivity of US to detect UTS during pregnancy is relatively low, ranging from 34 to 80% [21, 22]. A definitive diagnosis may be difficult because of the patient’s body habitus and fetal position. In addition, physiologic changes due to mechanical compression by the enlarged uterus and the effect of progesterone [23] may make it difficult to distinguish between physiologic hydroureter and abnormal findings in the presence of UTS [22]. However, given the serious concern of radiation risk, the AUA and EAU guidelines recommend US as the preferred modality, followed by low-dose CT [14, 15].
The third advantage is its real-time imaging capabilities. US provides real-time imaging for dynamic assessment of stones and their movement within the urinary tract. This feature enables immediate assessment of stone mobility, which may influence treatment options, planning, and monitoring during the examination itself.
Technological innovation of ultrasound in the diagnosis of urinary tract stones
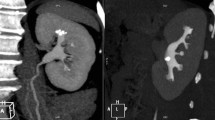
On US, UTS has been identified on grayscale B-mode US as a hyperechoic signal with a hypoechoic shadow called an acoustic shadow (Fig. 1). However, compared to NCCT, inferior sensitivity and limited specificity are critical issues for the detection of UTS (Table 1), because image accuracy depends on the performance of the equipment, patient-related factors (age, visceral fat, and bone), and operator skills. Occasionally, the impaired acoustic shadowing of the UTS may make it difficult to achieve adequate detection. This is caused by the acoustic impedance of the surrounding tissues or an improper balance between the focal distance and transducer power [24]. Moreover, US has limitations in detecting smaller or non-opaque stones, especially those less than a few millimeters in size. Because the kidney has many vasculatures and collecting ducts, B-mode US cannot distinguish UTS from the surrounding organs [25].
To overcome this drawback, technological advances in US equipment have made it necessary to identify UTS. Twinkling Doppler ultrasound (TDU) is a promising alternative imaging modality to NCCT. Twinkling artifacts refer to specific color Doppler and power Doppler signals (Fig. 2). It is commonly observed around highly reflective surfaces such as kidney stones, bones, or certain metallic objects. This occurs because of the interaction of US waves with small reflective structures or rough surfaces, causing a rapid modulation of the Doppler signal and resulting in alternating colors, typically red and blue, or a rapid color change between different shades [26, 27]. Abdel-Gawad et al. conducted a large-scale prospective study to evaluate the efficacy of TDU for stone detection in an acute setting [28]. In this report, the twinkling sign could be observed in 97.1% of patients with ureteral stones, with an associated 97.2% sensitivity and 99% specificity. Similar results could be seen for small stones with high sensitivity (99.12%), specificity (90.91%), and positive predictive values (99.12%) [29]. Although TDU has some issues with low sensitivity (40%) and a high false-positive rate (60%) in patients with unknown urolithiasis [30], with further developments, TDU might provide imaging quality to compete with NCCT in the future.

Source: Reproduced from [32]
Twinkling signal of ureteral stones. a Proximal ureteral stones. b Pelvic ureteral stones. White arrows indicate stones detected using B-mode ultrasonography.
Another issue with US is its poor accuracy in measuring stone size. Sternberg et al. reported that US mismeasured renal stone size with an average overestimation of 2.2 mm for stones < 5 mm (mean, 3.3 mm) [31]. Stone size estimation helps to determine the appropriate treatment modality and predicts the possibility of spontaneous passage. Therefore, stone size strongly affects patient decision-making. Ganesan et al. reported in a retrospective study that due to the overestimation of stone size on US, 22% of patients could be inappropriately counselled for clinical decision-making [32].
The discrepancy in stone measurements may be due to patient and equipment factors. Ray et al. reported that measurement errors were associated with skin-to-stone distance, but not with body mass index or stone location [33]. Another report showed that this discordance in stone measurement might increase secondarily with greater depth and gain in commercial equipment [34, 35]. On the other hand, acoustic shadows are generally unaffected by the system setting and US modality. Dunmire et al. demonstrated that accuracy of stone size could be improved by measuring the width of the posterior acoustic shadow instead of stone width on US (Fig. 3), with the average overestimation of stone size being less than 0.5 mm regardless of depth or imaging method modality [35]. Since absence of a posterior acoustic shadow was reported to be a reliable indicator for stones ≤ 4 or 5 mm [35, 36], this technique might be effective for predicting the possibility of spontaneous passage.
Clinical use of ultrasound imaging for treatment planning
Assessment of hydronephrosis
US is valuable for assessing obstruction and associated hydronephrosis. In adult patients, the degree of hydronephrosis on US is generally diagnosed using the Ellenborgen classification [37] (Fig. 4). In patients with renal colic in the ED, the presence of hydronephrosis on POCUS strongly suggests a large UTS (> 5 mm) [38].
Hydronephrosis is a predictive factor for impacted stones. We previously reported that the independent risk factors for stone adhesion to the ureteral mucosa were severe hydronephrosis (grade 2) and prolonged duration until surgery [39]. Ureteroscopic lithotripsy (URSL) is considered a better option than shock wave lithotripsy (SWL) for the treatment of impacted stones [40, 41]. However, even when using flexible ureteroscopy, URSL for impacted stones showed a lower SFR and more intraoperative complications than those for non-impacted stones [40, 42]. In cases of suspected impacted stones, percutaneous antegrade removal and preoperative percutaneous nephrostomy are considered in the treatment planning [14, 43].
Preoperative assessment of calculi and surrounding organs for percutaneous nephrolithotomy
US can generate images in multiple planes, thereby enabling a comprehensive evaluation of the urinary system. It contributes to the accurate diagnosis of stone size, location, and number, as well as real-time visualization of the kidneys, ureters, bladder, and surrounding structures.
Surgeons must consider the optimal treatment strategy before performing PCNL, including the surgical positions and percutaneous access points. Surgical positions commonly used in PCNL include the prone and oblique positions. However, discussions regarding which position is more suitable for PCNL are ongoing [44, 45]. The difficulty of percutaneous renal access also varies according to position. As the lower kidney may be displaced medially and ventrally in the oblique position owing to gravity [46], even if punctured from the same point, the pass-through line will be different for each surgical position (Fig. 5).
Ultrasound image in the prone (a) and oblique (b) positions; the white dotted arrow indicates the puncture line to the target calyx. In the prone position, the puncture line passes through the cyst (a); on the other hand, it passes through near the cyst and reaches the target calix in the oblique position (b)
The success of PCNL depends on the proper choice of renal calyceal approach. Direct access to the calyx, where most of the calculi are located, is considered ideal. However, visceral organs, including the enlarged liver, spleen, and retrorenal colon, may interfere with the puncture. The superior calyceal approach is anatomically ideal for treating staghorn calculi because of its easy accessibility to many calyces [47, 48]. However, thoracic complications are more common with superior calyceal puncture. Munver et al. [47] reported that the risk of thoracic complications with supra-11th-rib puncture (23.1%) was 16 times higher than with supra-12th-rib puncture (1.4%). Preoperative check-ups using US in a position similar to that used during surgery may be required to prevent visceral and thoracic injuries.
Clinical use of ultrasound in the treatment of kidney stones
Real-time monitoring during shock wave lithotripsy
SWL is a safe and noninvasive treatment for small calculi; however, the success rate after SWL is relatively low compared to that after URSL [49, 50]. One reason for the low success rate of SWL may be that shock waves could be out of focus due to respiratory motion misalignment and stone migration, resulting in a decrease in the crushing effect [51]. Sorensen et al. reported that 40% of shock waves were misfocused on the stone inaccurately, and these shock waves led to damage to the renal parenchyma and adjacent organs [51]. Generally, stone location is monitored using X-ray imaging; however, excessive monitoring of the respiratory tract leads to a risk of excess radiation exposure.
US is used to overcome these drawbacks. US provides real-time monitoring, facilitates precise targeting of the stone, and allows immediate assessment of treatment outcomes (Fig. 6). Some studies have demonstrated the superiority of US detection in clinical practice [52,53,54]. Chen et al. [52] documented that a US-based real-time tracking system increased the accuracy of stone targeting by reducing the number of shock waves. Isogai et al. [53] reported similar results, revealing that the alternative use of US and X-rays led to favorable SWL success for kidney calculi. On the other hand, Besien et al. [54] reported equivalent success rates between US-guided and fluoroscopy-guided SWL, with the clinical benefit of no ionizing radiation in US-guided SWL. US has limited direct therapeutic applications in SWL; however, advances in US technology may change the current trends in stone treatment, shifting away from SWL to URSL.
Ultrasonic propulsion
Shock waves using SWL can efficiently disintegrate calculi; however, the fragments must be passively excreted. As many as 85% of patients had residual fragments on KUB at the time of discharge [55], indicating that fragmented stones could not be expelled immediately. This is especially important for fragments located at the lower pole, where the pelvicalyceal anatomy is unfavorable for spontaneous clearance [56]. Some investigators have reported that the infundibulopelvic angle, width, and length in the lower pole anatomy may influence stone passage after SWL [57, 58].
Ultrasonic propulsion (UP) was developed to overcome this issue and was first reported in 2010 [59]. UP is an application of acoustic tractor-beam technology that uses a short burst of focused US energy to reposition renal calculi [59,60,61,62]. In an in vivo study using eight female pigs and artificial calculi of 2–8 mm in size, transcutaneous UP appeared to be safe and efficient. Moreover, 65% of calculi were successfully relocated from the calyces to renal pelvis, ureteropelvic junction, or ureter without histological evidence of renal parenchymal damage [62]. In humans, investigators have shown that UP can facilitate the spontaneous passage of fragments by pushing low-pole stones toward the pelvis. Harper et al. [60] reported in a Food and Drug Administration-approved feasibility study that 65% of calculi showed some extent of migration, whereas 30% were successfully displaced more than 3 mm toward another location (Fig. 7).
Summary of the results of a human clinical trial of ultrasonic propulsion following lithotripsy. Green represents stone movement. The arrows indicate participants who reported the passage of fragments. The numbers next to each target indicate the number of participants. Circles of different sizes represent target stone sizes. The grids represent the target identified as a single large stone on imaging, but were determined to be a cluster of small stones on ultrasonic propulsion. Adapted from [65]
Asymptomatic residual fragments less than 4 mm are referred to as “clinically insignificant residual fragments (CIRFs)”; however, many reports indicate its significance in contradiction to the term, because residual fragments after SWL have the potential risk of stone growth or recurrence requiring repeat procedures [63, 64]. Despite the technological innovation of the laser system in URSL, residual fragments after laser dusting have a similar risk of reintervention [65]. If small fragments can be passively excluded before growth, the risk of reintervention might decrease. Therefore, this technology may be established and widely used in the future as an adjunct treatment for lithotripsy.
Ultrasonic stone fragmentation: burst wave lithotripsy
Burst wave lithotripsy (BWL) is an emerging transcutaneous technology used to fragment UTS [66,67,68]. BWL utilizes real-time US imaging to precisely target and focus bursts of US energy on the stone. Accurate targeting helps minimize the impact on the surrounding organs and reduces the risk of damage. Furthermore, compared to SWL, BWL involves low-amplitude bursts of US delivered at relatively higher frequencies [66], which might result in a low risk of perioperative complications.
There has been a stepwise progression of development with some human clinical trials conducted to date to evaluate the efficacy of BWL. Maxwell et al. [66] reported in an initial in vitro study that broadly focused US bursts break artificial calculi by producing fractures. Preliminary porcine studies have provided safety evidence for this technology [67]. In a human study, Harper et al. [68] documented that 52% of stones were fragmented partially and 39% were fragmented completely within 10 min without severe complications.
Furthermore, recent studies examined the combined efficacy of UP and BWL in fragmenting UTS [60, 69]. An in vitro study revealed that the integration of UP and BWL technology accelerated stone fragmentation owing to the dispersion of fragments from the targeted stone with UP. In a human clinical study, Hall et al. [69] demonstrated that the combined use of UP and BWL without anesthesia provided an efficient and safe treatment to relieve pain and facilitate stone passage by pushing and breaking ureteral stones. In the emergency department or outpatient clinic, noninvasive immediate lithotripsy and active stone expulsion therapy are ideally required to relieve pain and anxiety, and the clinical application of the BWL plus UP combination may open up an entirely new paradigm for noninvasive stone treatment at the point of care.
Ultrasound-guided renal access during percutaneous nephrolithotomy and endoscopic combined intra-renal surgery
PCNL is a conventional treatment that removes stone fragments through the large percutaneous tract. Endoscopic combined intra-renal surgery (ECIRS) is a hybrid therapy that combines PCNL with retrograde intra-renal surgery. These are standard procedures for large renal calculi that can provide higher stone clearance than SWL and URSL [70]. However, severe complications associated with bleeding occur frequently. A systematic review of approximately 12,000 patients revealed that the incidence of transfusion during PCNL was 7% [71]. As bleeding usually results from injury to major renal vessels, such as the interlobar arteries, during renal puncture and tract creation [72], accurate renal access is required to mitigate bleeding-related complications.
Renal access techniques include fluoroscopy- and US-guided punctures. Fluoroscopy-guided puncture has been the preferred US imaging modality since it was first described by Wichham in 1981 [73]. Fluoroscopic guidance can accurately identify the calyx to be punctured; however, it has several disadvantages, including the inability to clearly identify the surrounding organs in real time, limited visualization of vascularity, lack of depth perception, and radiation exposure. In particular, the high radiation exposure during fluoroscopy has become a great concern for surgeons, medical staff, and patients. Considering the ALARA principle, alternative strategies for reducing radiation exposure from fluoroscopy during PCNL may be required by urologists.
US-guided renal access during PCNL was first described in 1999 by Desai [74] in pediatric cases and has since become more widespread. Whether fluoroscopy or US guidance is more suitable for renal access remains unclear [75, 76]. Agarwal et al. [75] reported that US-guided renal access showed higher accuracy, resulting in fewer attempts for successful renal puncture and a lower duration of radiation exposure than fluoroscopy-guided renal access. On the other hand, Corrales et al. [76] demonstrated in their review article that US and fluoroscopic guidance are equally safe and effective for experienced surgeons. US guidance generally eliminates radiation exposure to the surgeon, providing real-time visualization of the renal and suprarenal tissues lying between the skin and the kidney. However, maintaining clear visualization is sometimes difficult because of excessive bowel gas, patient factors such as a high body mass index, anatomical complexity, and the influence of the surgeon’s skill on image quality. Some reports have revealed that the learning curve until effective US guidance in PCNL might be steep and require 20–60 cases [77, 78]. Basic US knowledge and training in US techniques for stone surgery are essential for ongoing proficiency.
Two types of US transducers, the convex probe (CVP) and micro-CVP, can be used for renal access (Fig. 8a, b). A CVP is designed to provide a wider field of view and deeper penetration, allowing clear visualization in obese patients, while a micro-CVP is smaller and has a flatter curvature than a CVP, allowing for better maneuverability and imaging of shallow areas. Micro-CVP can be applied to the intercostal space in patients undergoing PCNL in the supine position. As surgeons do not have to lose the tip of the needle during renal access, the ultrasound probe is held by the non-dominant hand by pressing firmly against the skin to fix it in place, and the needle is slowly advanced by the dominant hand while maintaining live imaging (Fig. 8c).
Images of a transducer commonly used in PCNL and an example of intraoperative ultrasound used for renal puncture. a Convex probe, b micro-convex probe, c non-dominant hand holding the ultrasound probe and pressing firmly against the skin to fix it in place. The needle was slowly advanced while maintaining live imaging
Wideband Doppler ultrasound
US guidance offers another advantage for assessing blood flow using color Doppler imaging. Generally, a percutaneous puncture ideally heads to the fornix of the target calyx through a relatively avascular zone called Brodel’s bloodless line. However, it is difficult to identify the running renal blood vessels using fluoroscopy or B-mode US, compared with color Doppler US [79]. Lu et al. demonstrated that color Doppler US provides real-time detection and avoids renal blood vessels, resulting in a decreased incidence of bleeding complications [80]. Tzeng et al. conducted an RCT and reported similar results [79]. However, due to the low resolution of images on conventional color or power Doppler US, visualized blood vessels appear as a color overflowing beyond the far wall of a superficial vessel, a phenomenon called “blooming appearance” [81] (Fig. 9a, b). This makes it difficult for surgeons to accurately confirm a bloodless line. However, recent technological innovations such as wideband Doppler modes have overcome this limitation. Wideband Doppler US refers to a high-resolution blood flow display mode that provides clear visualization of peripheral blood vessels by suppressing blooming (Fig. 9c). In a preliminary report of 41 patients undergoing mini-ECIRS, Inoue et al. reported that wideband Doppler US resulted in low incidence of bleeding complications, showing the average hemoglobin drop was 0.54 g/dl [82]. The renal access monitoring twin-view image (Fig. 9d), which shows both wideband Doppler and B-mode US simultaneously on the screen, may represent a promising new technology for use in PCNL and ECIRS.

Source: Reproduced from [84]
Doppler ultrasound images. a Color Doppler mode. b Power Doppler mode. c Wideband Doppler mode. d Twin-view images combined with B-mode ultrasound and wideband Doppler modes.
Screen display of synchronization provided by the real-time virtual sonography system of the reconstructed CT image with the real-time intraoperative US image. a Plain CT and B-mode sonography images. b Reconstructed three-dimensional CT image is synchronized with a B-mode sonogram. c Enhanced CT image synchronized to the B-mode sonogram. d Enhanced CT images obtained in combination with wideband Doppler US mode. Adapted from [85]
Real-time virtual sonography technology
Because US is often regarded as providing less objective information than CT, the quality and interpretation of US findings may be influenced by the surgeon’s experience, skill, and technique. Real-time virtual sonography (RVS) was developed to compensate for this weak point of US. RVS is an advanced imaging technique that synchronizes real-time US with CT or magnetic resonance imaging [83, 84]. It allows the overlay of additional information or images onto US images in real time, enhancing the visualization and interpretation of US examinations. We previously applied this technology for percutaneous renal access during ECIRS (Fig. 10) and reported its efficacy compared to using B-mode US, resulting in fewer puncture attempts (1.6 vs 3.4 times, respectively; p = 0.001) and lower postoperative hemoglobin decrease (0.93 vs 1.39 g/dL, respectively; p = 0.04), while maintaining a similar stone-free rate [85]. This technology has the potential to improve renal access accuracy and become a learning tool for developing skills among novice operators.
Follow-up and surveillance
Symptomatic recurrence of UTS that results in clinical care was reported to be approximately 20% after 5 years after first stone event [86, 87]. On the other hand, D’Costa et al. [87] reported that the recurrence rate was 67% in a 5 year follow-up study, with a more comprehensive definition of recurrence, including symptomatic episodes and asymptomatic radiographic changes. As radiographic changes are associated with symptomatic recurrence in clinical care, appropriate radiographic follow-up should be highlighted when managing stone recurrence. The EAU Guidelines Urolithiasis Panel in 2022 recommended closer imaging at 6 and 12 months and annually thereafter in cases with diagnosed urinary metabolic abnormalities or with CIRFs [88].
The imaging modalities available for follow-up included CT, low-dose CT, US, and KUB. However, image accuracy and the risk of radiation exposure are incompatible (Table 1), and physicians must tailor the type of imaging modality and frequency of imaging to the severity of the risk of stone recurrence. NCCT is the gold standard modality for the follow-up of UTS; however, low-dose CT has become an alternative modality with concerns over radiation exposure. Low-dose CT has been defined as < 3 mSv of ionizing radiation, with an estimated sensitivity of 96.6% and specificity 94.9% [7]. The radiation risk can be reduced with the use of low-dose CT [8, 89, 90]; however, it has been shown to have low sensitivity in obese patients or for ureteral stones < 3 mm [89]. Another alternative to NCCT or low-dose CT for follow up-imaging is the combination of US and KUB, which can achieve good rates of accuracy (95%), sensitivity (96%), and specificity (91%) [91]. Fahmy et al. reported the cumulative radiation exposure during a 2-year follow-up period, in which effective radiation significantly decreased from 29.29 in the first year to 8.04 mSv in the second year of follow-up of UTS due to higher combination use of US [92]. Considering the concerns and increased cost of NCCT, the combination of US and KUB may be a reasonable plan for follow-up imaging, except in cases of symptomatic recurrence. With no consensus guidelines for this clinical protocol in patients with a history of UTS, future research is required to determine the optimal imaging surveillance strategy.
Conclusions
The continued evolution of US technology over the past 70 years has yielded superior images and small CVP, focusing on bursts of energy, and seamless integration with existing percutaneous navigation, resulting in an excellent diagnostic and therapeutic modality.
The optimization of US features such as the twinkling signal and posterior acoustic shadow may provide adequate detection and sizing of UTS. The clinical application of ultrasonic propulsion or BWL may open new avenues for noninvasive stone treatment. Furthermore, considering the ALARA principle, US has grown in importance as an alternative strategy for reducing radiation exposure to NCCT and fluoroscopy. Further technological innovation in US has the potential to create a paradigm shift in the management of UTS.
Data availability
Data sharing is not applicable to this article.
References
Alelign T, Petros B. Kidney stone disease: an update on current concepts. Adv Urol. 2018. https://doi.org/10.1155/2018/3068365.
Sakamoto S, Miyazawa K, Yasui T, et al. Chronological changes in the epidemiological characteristics of upper urinary tract urolithiasis in Japan. Int J Urol. 2018;25:373–8.
Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med. 1989;111:1006–9.
Kluner C, Hein PA, Gralla O, et al. Does ultra-low-dose CT with a radiation dose equivalent to that of KUB suffice to detect renal and ureteral calculi? J Comput Assist Tomogr. 2006;30:44–50.
Caoili EM, Cohan RH, Korobkin M, et al. Urinary tract abnormalities: initial experience with multi-detector row CT urography. Radiology. 2002;222:353–60.
Van Der Molen AJ, Cowan NC, Mueller-Lisse UG, et al. CT urography: definition, indications and techniques. A guideline for clinical practice. Eur Radiol. 2008;18:4–17.
Niemann T, Kollmann T, Bongartz G. Diagnostic performance of low-dose CT for the detection of urolithiasis: a meta-analysis. AJR Am J Roentgenol. 2008;191:396–401.
Zilberman DE, Tsivian M, Lipkin ME, et al. Low dose computerized tomography for detection of urolithiasis-its effectiveness in the setting of the urology clinic. J Urol. 2011;185:910–4.
Fulgham PF, Assimos DG, Pearle MS, et al. Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol. 2013;189:1203–13.
Bhanot R, Hameed ZBM, Shah M, et al. ALARA in Urology: Steps to minimise radiation exposure during all parts of the endourological journey. Curr Urol Rep. 2022;23:255–9.
Tasian GE, Copelovitch L, et al. Evaluation and medical management of kidney stones in children. J Urol. 2014;192:1329–36.
Schlegel JU, Diggdon P, Cuellar J. The use of ultrasound for localizing renal calculi. J Urol. 1961;86:367–9.
Cook JH III, Lytton S. Intraoperative localization of renal calculi during nephrolithotomy by ultrasound scanning. J Urol. 1977;117:543–6.
Turk C, Petrik A, Sarica K, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2016;69:475–82.
Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American urological association/Endourological society guideline. PART I J Urol. 2016;196:1153–60.
Smith A, Averch TD, Shahrour K, et al. A nephrolithometric nomogram to predict treatment success of percutaneous nephrolithotomy. J Urol. 2013;190:149–56.
El-Nahas AR, El-Assmy AM, Mansour O, et al. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution noncontrast computed tomography. Eur Urol. 2007;51:1693–4.
Ferrandino MN, Bagrodia A, Pierre SA, et al. Radiation exposure in the acute and short-term management of urolithiasis at 2 academic centers. J Urol. 2009;181:668–73.
Ripolles T, Agramunt M, Errando J, et al. Suspected ureteral colic: plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2004;14:129–36.
Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100–10.
Stothers L, Lee LM. Renal colic in pregnancy. J Urol. 1992;148:1383–7.
Patel SJ, Reede DL, Katz DS, et al. Imaging the pregnant patient for nonobstetric conditions: algorithms and radiation dose considerations. Radiographics. 2007;27:1705–22.
Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20:209–14.
King WE 3rd, Kimme-Smith C, Winter J. Renal stone shadowing: an investigation of contributing factors. Radiology. 1985;154:191–6.
Wood BG, Urban MW. Detecting kidney stones using twinkling artifacts: survey of kidney stones with varying composition and size. Ultrasound Med Biol. 2020;46:155–66.
Rahmouni A, Bargoin R, Herment A, et al. Color Doppler twinkling artifact in hyperechoic regions. Radiology. 1996. https://doi.org/10.1148/radiology.199.1.8633158.
Winkel RR, Kalhauge A, Fredfeldt KE. The usefulness of ultrasound color Doppler twinkling artefact for detecting urolithiasis compared with low dose nonenhanced computerized tomography. Ultrasound Med Biol. 2012;38:1180–7.
Abdel-Gawad M, Kadasne RD, Elsobky E, et al. A prospective comparative study of color Doppler ultrasound with twinkling and noncontrast computerized tomography for the evaluation of acute renal colic. J Urol. 2016;196:757–62.
Gliga ML, Chirila CN, Podeanu DM, et al. Twinkle, twinkle little stone: an artifact improves the ultrasound performance! Med Ultrason. 2017;19:272–5.
Masch WR, Cohan RH, Ellis JH, et al. Clinical effectiveness of prospectively reported sonographic twinkling artifact for the diagnosis of renal calculus in patients without known urolithiasis. Am J Roentgenol. 2016. https://doi.org/10.2214/AJR.15.14998.
Sternberg KM, Eisner B, Larson T, et al. Ultrasonography significantly overestimates stone size when compared to; ow-dose, noncontrast computed tomography. Urology. 2016;95:67–71.
Ganesan V, De S, Greene D, et al. Accuracy of ultrasonography for renal stone detection and size determination: is it good enough for management decisions? BJU Int. 2017;119:464–9.
Ray AA, Ghiculete D, Pace KT, et al. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010;76:295–300.
Dunmire B, Lee FC, Hsi RS, et al. Tools to improve the accuracy of kidney stone sizing with ultrasound. J Endourol. 2015;29:147–52.
Dunmire B, Harper JD, Cunitz BW, et al. Use of the acoustic shadow width to determine kidney stone size with ultrasound. J Urol. 2016;195:171–7.
Dai JC, Dunmire B, Sternberg KM, et al. Retrospective comparison of measured stone size and posterior acoustic shadow width in clinical ultrasound images. World J Urol. 2018;36:727–32.
Ellenbogen PH, Scheible FW, Talner LB, et al. Sensitivity of gray scale ultrasound in detecting urinary tract obstruction. AJR Am J Roentgenol. 1978;130:731–3.
Wong C, Teitge B, Ross M, et al. The accuracy and prognostic value of point-of-care ultrasound for nephrolithiasis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2018;25:684–498.
Hamamoto S, Okada S, Inoue T, et al. Prospective evaluation and classification of endoscopic findings for ureteral calculi. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-699158-w.
Degirmenci T, Gunlusoy B, Kozacioglu Zm, et al. Outcomes of ureteroscopy for the management of impacted ureteral calculi with different localizations. Urology. 2012;80:811–5.
Yamashita S, Inoue T, Kohjimoto Y, et al. Comprehensive endoscopic management of impacted ureteral stones: literature review and expert opinions. Int J Urol. 2022;29:799–806.
Legemate JD, Wijnstok NJ, Matsuda T, et al. Characteristics and outcomes of ureteroscopic treatment in 2650 patients with impacted ureteral stones. World J Urol. 2017;35:1497.
Taguchi K, Hamamoto S, Osaga S, et al. Comparison of antegrade and retrograde ureterolithotripsy for proximal ureteral stones: a systematic review and meta-analysis. Trans Adnrol Urol. 2021;10:1179–91.
De Sio M, Autorino R, Quarto G, et al. Modified supine versus prone position in percutaneous nephrolithotomy for renal stones treatable with a single percutaneous access: a prospective randomized trial. Eur Urol. 2008;54:196–203.
Yuan D, Liu Y, Rao H, et al. Supine versus prone position in percutaneous nephrolithotomy for kidney calculi: a meta-analysis. J Endourol. 2016;30:754–63.
Hamamoto S, Okada S, Inoue T, et al. Comparison of the safety and efficacy between the prone split-leg and Galdakao-modified supine Valdivia positions during endoscopic combined intrarenal surgery: a multi-institutional analysis. Int J Urol. 2021;28:1129–35.
Munver R, Delvecchio FC, Newman GE, et al. Critical analysis of supracostal access for percutaneous renal surgery. J Urol. 2001;166:1242–6.
Amaresh M, Hegde P, Chawla A, et al. Safety and efficacy of superior calyceal access versus inferior calyceal access for pelvic and/or lower calyceal renal calculi—a prospective observational comparative study. World J Urol. 2021;39:2155–61.
Pearle MS, Lingeman JE, Leveillee R, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2005;173:2005–9.
Drake T, Grivas N, Dabestani S, et al. What are the benefits and harms of ureteroscopy compared with shock-wave lithotripsy in the treatment of upper ureteral stones? A systematic review. Eur Urol. 2017;72:772–86.
Sorensen MD, Bailey MR, Shah AR, et al. Quantitative assessment of shockwave lithotripsy accuracy and the effect of respiratory motion. J Endourol. 2012;26:1070–4.
Chen CJ, Hsu HC, Chung WS, Yu HJ. Clinical experience with ultrasound-based real-time tracking lithotripsy in the single renal stone treatment. J Endourol. 2009;23:1811–5.
Isogai M, Hamamoto S, Kawase K, et al. Efficacy of ultrasound monitoring during extracorporeal shock wave lithotripsy: a multi-institutional propensity score-matched study. Int J Urol. 2022;29:1054–60.
Besien JV, Uvin P, Hermie I, et al. Ultrasonography is not inferior to fluoroscopy to guide extracorporeal shock waves during treatment of renal and upper ureteric calculi: a randomized prospective study. Biomed Res Int. 2017;2017:7802672.
Drach GW, Dretler S, Fair W. Report of the United States cooperative study of extracorporeal shock wave lithotripsy. J Urol. 1986;35:1127–33.
Sumino Y, Mimata H, Tasaki Y, et al. Predictors of lower pole renal stone clearance after extracorporeal shock wave lithotripsy. J Urol. 2002;168:1344–7.
Elbahnasy AM, Clayman RV, Shalhav AL, et al. Lower pole calyceal stone clearance after SWL, percutaneous nephrolithotomy, and flexible URS; impact of radiographic spatial anatomy. J Endourol. 1998;12:113–9.
Sampaio FJ, Aragao AH. Inferior pole collecting system anatomy; its probable role in extracorporeal shock wave lithotripsy. J Urol. 1992;147:322–4.
Shah A, Owen N, Lu W, et al. Novel ultrasound method to reposition kidney stones. Urol Res. 2010;38:491–5.
Harper JD, Cunitz BW, Dunmire B, et al. First in human clinical trial of ultrasonic propulsion of kidney stones. J Urol. 2016;195:956–64.
May PC, Bailey MR, Harper JD. Ultrasonic propulsion of kidney stones. Curr Opin Urol. 2016;26:264–70.
Harper JD, Sorensen MD, Cunitz BW, et al. Focused ultrasound to expel calculi from the kidney: safety and efficacy of a clinical prototype device. J Urol. 2013;190:1090–5.
Candau C, Saussine C, Lang H, et al. Natural history of residual renal stone fragments after ESWL. Eur Urol. 2000;37:18–22.
Sahin C, Tuncer M, Yazici O, et al. Do the residual fragments after shock wave lithotripsy affect the quality of life? Urology. 2014;84:549–54.
Chew BH, Brotherhood HL, Sur RL, et al. Natural history, complications and re-intervention rates of asymptomatic residual stone fragments after ureteroscopy: a report from the EDGE Research Consortium. J Urol. 2016;195:982–6.
Maxwell AD, Cunitz BW, Kreider W, et al. Fragmentation of urinary calculi in vitro by burst wave lithotripsy. J Urol. 2015;193:338–44.
Dai JC, Bailey MR, Sorensen MD, et al. Innovations in ultrasound technology in the management of kidney stones. Urol Clin North Am. 2019;46:273–85.
Harper JD, Lingeman JE, Sweet RM, et al. Fragmentation of stones by burst wave lithotripsy in the first 19 humans. J Urol. 2022;207:1067–76.
Hall MK, Thiel J, Dunmire B, et al. First series using ultrasonic propulsion and burst wave lithotripsy to treat ureteral stones. J Urol. 2022;208:1075–82.
Chung DY, Kang DH, Cho KS, et al. Comparison of stone-free rates following shock wave lithotripsy, percutaneous nephrolithotomy, and retrograde intrarenal surgery for treatment of renal stones: a systematic review and network meta-analysis. PLoS ONE. 2019;14: e0211316.
Seitz C, Desai M, Häcker A, et al. Incidence, prevention, and management of complications following percutaneous nephrolitholapaxy. Eur Urol. 2012;61:146–58.
El-Nahas AR, Shokeir AA, El-Assmy AM, et al. Post-percutaneous nephrolithotomy extensive hemorrhage: a study of risk factors. J Urol. 2007;177:576–9.
Wickham JE, Kellett MJ. Percutaneous nephrolithotomy. Br J Urol. 1981;53:297–9.
Desai M, Ridhorkar V, Patel S, et al. Pediatric percutaneous nephrolithotomy: assessing impact of technical innovations on safety and efficacy. J Endourol. 1999;13:359–64.
Agarwal M, Agrawal MS, Jaiswal A, et al. Safety and efficacy of ultrasonography as an adjunct to fluoroscopy for renal access in percutaneous nephrolithotomy (PCNL). BJU Int. 2011;108:1346–9.
Corrales M, Doizi S, Barghouthy Y, et al. Ultrasound or fluoroscopy for percutaneous nephrolithotomy access, is there really a difference? A review of literature. J Endourol. 2021;35:241–8.
Usawachintachit M, Masic S, Allen IE, et al. Adopting ultrasound guidance for prone percutaneous nephrolithotomy: evaluating the learning curve for the experienced surgeon. J Endourol. 2016;30:856–63.
de la Rosette JJ, Laguna MP, Rassweiler et al. Training in percutaneous nephrolithotomy—a critical review. Eur Urol. 2008;54:994.
Tzeng BC, Wang CJ, Huang SW, et al. Doppler ultrasound-guided percutaneous nephrolithotomy: a prospective randomized study. Urology. 2011;78:535–9.
Lu MH, Pu XY, Gao X, et al. A comparative study of clinical value of single B-mode ultrasound guidance and B-mode combined with color doppler ultrasound guidance in mini-invasive percutaneous nephrolithotomy to decrease hemorrhagic complications. Urology. 2010;76:815–20.
Meola M, Ibeas J, Lasalle G, et al. Basics for performing a high-quality color Doppler sonography of the vascular access. J Vasc Access. 2021;22:18–31.
Inoue T, Kinoshita H, Okada S, et al. Wideband Doppler ultrasound-guided mini-endoscopic combined intrarenal surgery as an effective and safe procedure for management of large renal stones: a preliminary report. Urology. 2016;95:60–6.
Miyagawa T, Ishikawa S, Kimura T, et al. Real-time virtual sonography for navigation during targeted prostate biopsy using magnetic resonance imaging data. Int J Urol. 2010;10:855–60.
Haber GP, Colombo JR, Remer E, et al. Synchronized real-time ultrasonography and three-dimensional computed tomography scan navigation during percutaneous renal cryoablation in a porcine model. J Endourol. 2010;24:333–7.
Hamamoto S, Unno R, Taguchi K, et al. A new navigation system of renal puncture for endoscopic combined intrarenal surgery: real-time virtual sonography-guided renal access. Urology. 2017;109:44–50.
Rule AD, Lieske JC, Li X, et al. The ROKS nomogram for predicting a second symptomatic stone episode. J Am Soc Nephrol. 2014;25:2878–86.
D’Costa MR, Haley WE, Mara KC, et al. Symptomatic and radiographic manifestations of kidney stone recurrence and their prediction by risk factors: a prospective cohort study. J Am Soc Nephrol. 2019;30:1251–60.
Tzelves L, Geraghty R, Lombardo R, et al. Duration of follow-up and timing of discharge from imaging follow-up, in adult patients with urolithiasis after surgical or medical intervention: a systematic review and meta-analysis from the European Association of Urology Guideline Panel on Urolithiasis. Eur Urol Focus. 2023;9:188–98.
Poletti PA, Platon A, Rutschmann OT, et al. Low-dose versus standard-dose CT protocol in patients with clinically suspected renal colic. AJR Am J Roentgenol. 2007;188:927–33.
Moore CL, Carpenter CR, Heilbrun ME, et al. Imaging in suspected renal colic: systematic review of the literature and multispecialty consensus. J Urol. 2019;202:475–83.
Mitterberger M, Pinggera GM, Pallwein L, et al. Plain abdominal radiography with transabdominal native tissue harmonic imaging ultrasonography vs unenhanced computed tomography in renal colic. BJU Int. 2007;100:887–90.
Fahmy NM, Elkoushy MA, Andonian S. Effective radiation exposure in evaluation and follow-up of patients with urolithiasis. Urology. 2012;79:43–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients for inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hamamoto, S., Inoue, T., Okada, S. et al. Application of ultrasound imaging in the treatment of urinary tract stones. J Med Ultrasonics (2023). https://doi.org/10.1007/s10396-023-01343-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10396-023-01343-6