Abstract
Objectives
We discuss in this review, urologists’ expectations of imaging in terms of detection, characterization, pre-planning treatment and follow-up of urinary stones.
Materials and methods
Data acquisition regarding kidney stones and imaging was performed using MEDLINE searches with combinations of the following keywords: urinary stones, CT Urography, low dose CT, MRI urography, renal stones ultrasound, conventional radiography, surgery.
Results
CT has become the gold standard for the evaluation of urinary stones. Scanning provides information regarding stone (composition, size, burden, location), collecting system and renal parenchyma. Those findings are crucial in determining appropriate treatment strategies. Because CT exposes the patient to substantial ionizing radiation, efforts have already been made to decrease the CT radiation dose for CT examination (low dose CT) and optimize image quality. Efforts also are being made to use non ionizing modalities such as ultrasound in combination with radiography particularly for the follow up of renal stones.
Conclusion
CT is the preferred method for the evaluation and treatment planning of urolithiasis. CT radiation dose reduction can be achieved with low dose CT. However, conventional radiography and ultrasound are still recommended in the follow up of renal stones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Imaging in urolithiasis has evolved over the years due to technological advances and a better understanding of the disease process. Because of its high sensitivity and temporal resolution, computed tomography (CT) scanning has supplanted other imaging techniques and has become the gold standard for the evaluation of urinary stone disease. Scanning provides information regarding stone burden, composition, size and location. It also provides information about the collecting system and the renal parenchyma, findings that are crucial in determining appropriate treatment strategies. The major limitation of CT is the increase of radiation, especially for recurrent disease. CT scans are the main sources of radiation exposure as a result of medical imaging. Despite their benefits in diagnosis and follow-up of patients, they are not completely harmless. To resolve this, some strategies to minimize the radiation and optimize image quality have been developed. Efforts also are being made to use nonionizing modalities such as ultrasound in combination with radiography, particularly for the follow-up of renal stones. We discuss in this review, urologists’ expectations of imaging in terms of detection, characterization, pre-planning treatment and follow-up of urinary stones.
Imaging of urolithiasis
Conventional imaging techniques
For decades, the kidney ureter bladder radiograph (KUB) was the initial examination of choice in the evaluation of acute onset of flank pain. The plain abdominal film must be of good quality and carefully examined. Oblique or profile views could be necessary in some circumstances to suppress superimposition (gallstones, costal cartilage, arterial calcifications, Sacrum).
Approximately 90 % of urinary stones are radio-opaque, but radiography has been found to be only approximately 60 % sensitive overall in the detection of urolithiasis [1]. Sensitivity increases to 73 % when KUB is interpreted in conjunction with unenhanced CT [2]. Indeed, small radio-opaque calculi may be obscured by bowel gas and osseous structures [3]. However, KUB is useful for planning fluoroscopically guided SWL and should be used for monitoring calculus burden in patients known to have urolithiasis. The intent in these cases is to significantly reduce a patient’s radiation exposure compared with unenhanced CT.
Ultrasound
US is not dependent on calculus composition and may detect calculi as small as 0.5 mm that manifest as echogenic foci with shadowing within the urinary tract. Ureteral calculi are often located at the ureteropelvic junction but most are located in the distal ureter and US is limited when the urinary bladder is not adequately filled. The direct visualization of ureteral calculi can be difficult because of overlying bowel gas and the relative depth of the ureter within the pelvis. Furthermore, ultrasound visualization may be complicated in obese patients by large amounts of intervening fat. It has been postulated that elevated renal resistive indexes on Doppler sonography may be a useful indicator of acute obstruction and that asymmetric ureteral jets within the bladder on color Doppler can be diagnostic of a distal calculus, but results have been mixed [4]. Renal twinkling artifact commonly associated with nephrolithiasis is relatively insensitive in routine clinical practice with overall sensitivity of 55 % [5].
Although widely available and cost effective, US has limited diagnostic value in the assessment of patients with suspected renal stones. US sensitivity for the detection of calculi is approximately 45 % [6]. The detection rate increase with stone size but the accuracy of stone measurements made with US has been questioned. It was reported that US has a tendency to overestimate the stone size because of ill-defined stone edge detection by US [7]. Measurements by ultrasound versus CT were found to differ by 1.5 ± 0.7 mm in one study [8]. Other recent data support this: In 87 % of cases, ultrasound measurements were found to be greater than CT, and for stones less than 5 mm, the degree of overestimation was almost 2 mm [9]. However, a recent study suggests that US is an effective procedure for the detection and stone size evaluation when done by experience sonographers who are specialized in handling urologic US [10].
Ultrasound can also reveal secondary effects, such as obstruction, superimposed infection, or abscess formation. The sensitivity of sonography improves greatly if secondary signs of obstructive uropathy are included in the diagnostic of renal colic.
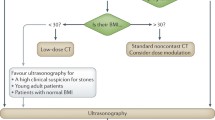
Ultrasound is preferred in pregnant patients and children for whom minimal radiation exposure is desired and is recommended for the follow-up. A recent study suggests a US-inclusive ureteral calculi follow-up protocol would substantially reduce radiation exposure among patients with renal colic, particularly among those with recurrent presentations [11].
MRI
MRI does not visualize calculi. Stones appear as a T1- or T2-weighted sequence signal void. T2-weighted sequences can rapidly reveal the presence of perirenal high-intensity signal, obstruction and level of obstruction and thus provide information for diagnosing the urinary tract abnormality. The diagnosis of ureteral calculi is often relies on detecting secondary signs of obstruction such as ureteral dilatation [12]. For the detection of ureterohydronephrosis, the sensitivity and the specificity were reported to be 90 and 100 % [13]. The sensitivity and specificity or the detection of a specific filling defect such as a calculus was between 64 and 80 % and between 84 and 91 % [13].
Like ultrasound, MRI does not use ionizing radiation and can useful alone or in combination with conventional radiography for evaluation of the pediatric and pregnant patients [14].
Computed tomography (CT)
Due to technological advances, CT, both unenhanced and contrast-enhanced, has quickly become the modality of choice in the diagnosis and follow-up of urolithiasis.
CT can be performed rapidly and is highly sensitive for the detection of stones of all sizes, approaching 100 % in some series. CT can also measure stone attenuation, evaluate secondary effects of obstruction, delineate surgically relevant anatomy, and detect other potential sources of pain such as appendicitis, endometrioma, hemorrhagic cyst, and ovarian torsion.
CT techniques
Unenhanced CT
The CT protocol for evaluation of stone disease is not considered equivalent to routine unenhanced abdominopelvic CT. Intravenous administration of contrast material is not routinely required. Acquisition should include scanning of the entire urinary tract from the upper pole of the kidneys to the base of the urinary bladder. In some cases of follow-up, acquisition can be limited to the region of interest (kidney).
Images can be prospectively acquired at 5-mm collimation, and the data can be reconstructed at a 1–3-mm section thickness. A section thickness >5 mm can lead to frequent missing of small urinary stones and can affect size and attenuation measurements.
Enhanced CT
In some scenarios, such as the incidental detection of tumors or other diseases on unenhanced scans, contrast material is required. Contrast-enhanced CT is also useful in conditions such as ureteral strictures, duplicated systems, or ureteropelvic junction obstruction, in which the delineation of aberrant genitourinary anatomy is necessary for effective treatment.
To reduce radiation exposure, a dual split-bolus protocol with furosemide injection is recommended instead of a three-phase protocol. An unenhanced scan is obtained from the level of the diaphragm to the symphysis pubis, or through the inferior poles of the kidney. Then, IV furosemide is administered in an amount of 20 mg for most patients. Then, a first bolus injection of 60 mL (range 40–80) of contrast medium is given at a flow rate of 2.5 mL/s, followed in 360 s by a second bolus of 60 mL (range 80–40) at the same rate. Nephro-urographic phase scanning is performed 480 s after the first injection, allowing analysis of the collecting system and renal parenchyma in the same acquisition (Figs. 1, 2). This dual-phase protocol is associated with significant reduction in radiation exposure dose with no reduction in image quality.
Reformatted images Coronal reformatted images improve the detection of urinary stones and allow confident differentiation between calculi and other calcifications such as phleboliths. The routine use of reformatted images is essential.
Radiation dose
A significant drawback is that CT utilizes ionizing radiation. The average mean effective dose for a single unenhanced CT for flank plain has been reported to be 8.5 mSv for MDCT, with effective radiation doses for unenhanced CT ranging from 2.8 to 13.1 mSv for men and from 4.5 to 18 mSv for women, all of which are higher than for excretory urography (1.5–2 mSv, depending on the number of films). Patients who have metabolically active disease may require many CT scans during their lifetime, especially young individuals; these scans could result in substantial cumulative radiation exposure [15].
Strategies to minimize radiation dose exposure
Low-radiation dose CT (LDCT)
Recent urolithiasis data have explored the use of low-radiation-dose CT (LDCT) protocols in the hope of maintaining the benefits of full-dose scans while limiting radiation exposure. In LDCT protocols, the tube current (in milliamperes) and tube potential (kilovolt peak) are lower than in standard protocols. A variety of protocols have been described that result in effective radiation dose reductions of up to 95 %, from >10 mSv to as low as 0.5–3.5 mSv.
These studies have shown excellent sensitivity and specificity in the diagnosis of urinary stones, with an accuracy of 93–97 % [16–18]. Low-dose CT is uniformly associated with an increase in image noise, but successes in dose reduction in the setting of renal colic have been aided by the inherent high contrast of renal calculi against the relatively low-density soft tissues surrounding the urinary tract [16–20]. In a recent study, the stone size, and stone radiodensities were similar between the standard CT and LDCT protocols, with a good correlation in their measurements between observers and between modalities. This suggests that LDCT can be reliably used to plan stone therapies [21].
Limitations Low-dose CT techniques appear to reduce the spatial threshold at which calculi become invisible and can potentially degrade image quality in obese patients (BMI > 30) [22]. Although radiation is increased in overweight patients (BMI > 30), it remains lower than that delivered by a standard-dose protocol [23].
Authors have reported reductions in sensitivity and specificity for detecting small calculi (<3 mm) when low-dose CT techniques were employed. Although results vary according to the extent of dose reduction achieved, the majority of studies suggest that confident exclusion of calculi measuring >4 mm in diameter (95–100 % sensitivity) is possible for both low-dose and conventional dose CT [24].
Automatic tube current modulation (ATCM)
Tube current modulation, which was a major development in CT technology in the last decade, can help reduce dose. One of the first major papers that evaluated ATCM as a means of optimizing radiation dose for CT reported dose reductions of 32 % in 87 % of CT examinations using ATCM [25].
Iterative image reconstruction
Reductions in CT dose inherently create an increase in image noise. Iterative reconstruction algorithms represent an exciting development in dose optimization for CT. It allows radiation dose optimization with noise reduction to preserve image quality. Iterative reconstruction algorithms will be particularly useful in low-dose CT of the urinary tract, where image noise is typically high [26], and it will enable effective evaluation of urinary calculi without affecting diagnostic confidence [27].
Limiting scanning range
Examinations can also be anatomically tailored when the location of a calculus is known, for example, the kidney.
Multidetector analyses: factors influencing decisions regarding urologic intervention
Detection
All stones are visible with unenhanced CT, including those that are radiolucent on conventional radiographs, such as uric acid, xanthine, and cystine stones. These stones have an attenuation value >200 HU, which is greater than that of the surrounding soft tissue. The most direct CT sign for urolithiasis is a stone within the ureteral lumen with proximal ureteral dilatation and a normal distal caliber. The only stones that are difficult to visualize with CT are pure matrix stones and stones made of pure indinavir. These stones are likely to be missed with unenhanced CT, and intravenous contrast material may be administered in equivocal circumstances.
The scout radiograph obtained routinely as part of the unenhanced helical CT may also identify the calculus and negate the need for a baseline plain abdominal KUB radiograph. The sensitivity of Ct scout as been found to be between 42 and 52 % [2, 28, 29] depending of the mean size, location and the Hounsfied units (>548 HU) of the stones.
After urologic intervention, residual stones often need to be distinguished from in situ stents or nephrostomy tubes; in such situations, the use of bone window settings is essential.
The number of calculi should be reported. It was shown that non-obstructing renal stones on unenhanced CT may be a source of pain in some patients who present with suspected renal colic [30].
Secondary findings
Hydronephrosis, perinephric edema, periureteral edema, ureterovesical junction edema, and hydroureter are frequently observed in the setting of a ureteral calculus and have been reported to have strong positive predictive value for obstruction. A focal fluid collection may reflect a ruptured calyx with urinoma. Perinephric fat stranding and dilatation of the intrarenal collecting system have a positive predictive value of 98 % and a negative predictive value of 91 % for the detection of ureteral stones [14].
If a calculus is not identified but secondary signs are present, the possibility of a passed calculus or obstruction unrelated to urolithiasis should be considered.
Size
The need to describe a stone in terms of size and shape has become particularly important as noninvasive or minimally invasive treatment modalities have replaced open surgery. Historically, stone size has been defined as the maximum diameter of the stone in any measurable axis. Using unenhanced CT, stone diameter can be measured to the nearest millimeter in a magnified bone window either transversely in the axial plane or coronal plane [31]. Measurement of stone size with CT is used to plan treatment [32, 33]. Measurement of stone size with CT also helps to accurately predict the rate of spontaneous passage of ureteral stones because calculi of up to 5 mm have a 68 % probability of passage, and calculi of 5–10 mm have a 47 % probability of passage [34].
Maximum diameter gives some useful information but can both over- and underestimate stone volume and thus stone burden. The orientation of stones does not conveniently correspond to axial body scanning, making the measurement of maximum diameters relatively artificial. The majority of stones are asymmetrical with a pronounced irregular shape, such as staghorn calculi (Fig. 3); therefore, their volume cannot be calculated using a simple algebraic formula.
Measuring the stone volume eliminates this problem because it takes into account the shape and diameter of the stone [32].
Various authors have used different methods to calculate stone volume [32, 35, 36], including attenuation threshold-based CT methods, which were shown to quantify urinary stone volume accurately and with high precision [33]. Modern CT software can also create a 3D reconstruction using the acquired images. Stone volume calculated using 3D reconstruction is extremely accurate and highly reproducible [35]. Novel semiautomatic segmentation tools can also be used to estimate stone volume. However, those processes require specialized scanning protocols and software and so may not be available as part of a standard acute colic scanning protocol.
The product of three orthogonal measurements, although imperfect, can also be used.
Stone volume is one of the most important factors in determining treatment strategies for management of urolithiasis. Volumetric information may replace axial stone diameter in predicting spontaneous passage of obstructing ureteral stones and for determining whether to recommend ureteroscopy, extracorporeal shock-wave lithotripsy, percutaneous nephrolithotomy, or conservative management.
It was shown that accurate stone volume measured by NCCT is the strongest predictor of stone-free status after ESWL [36].
Location
It is routine to report whether the calculi are located within the upper pole, mid kidney, or lower pole. Ureter is divided into three parts: the proximal one (ureteropelvic junction to the level of the superior margin of the sacrum); the iliac one (from the superior margin to the lower margin of the sacrum); and the pelvic one (from the lower margin of the sacrum to the ureterovesical junction).
Multiple pyramidal calculi, multiple clusters of grape like calcifications at the cortico medullary junction indicate medullary sponge kidney (MSK). MDCT is able in MSK to delineate the collecting tubule dilatations and provide images of the characteristic “brush” and “bouquet of flowers” [37].
Very fine calcifications at the papillary tip or increase of papillae attenuation on high resolution CT images suggest Randall’s plaque. However, distinguish papillary tissue calcifications from papillary stones is a limitation of CT [38, 39].
Stone composition evaluation
Knowledge about the composition of stones may guide decisions about their management. Uric acid calculi can be managed with oral medications that facilitate dissolution, struvite calculi are sensitive to extracorporeal shock-wave lithotripsy, whereas calcium oxalate monohydrate and cystine calculi are relatively resistant to fragmentation with lithotripsy.
Surgical treatments are reserved for stones that do not respond to medical therapy and stones of certain compositions (cystine- or calcium-based stones).
Determination of stone composition can be estimated with CT on the basis of internal structure analysis, attenuation values of the stones and with dual-energy scanning.
Internal structure and shape The stone’s shape can influence whether adequate fragmentation is achieved; stones of irregular aspect, with spikes or cutoff edges, seem to be more fragile. The internal structure can be considered to be either heterogeneous or homogeneous when viewed with bone window settings (Fig. 4). Studies have shown that stones observed to be heterogeneous with CT are more fragile than those that appear homogeneous and require less comminution with SWL. Cystine stones of rough morphology and heterogeneous calcium oxalate monohydrate (COM) stones seem to be more susceptible to SWL. Some authors suggest that the morphologic features of a stone rather than its X-ray attenuation value correlate with the fragility of stones with SWL [40].
Attenuation values Various techniques of CT were utilized for the determination of the Hounsfield unit (HU) values of different types of urinary calculi with the aim of determining the best technique for distinguishing stone compositions.
Attenuation measurements are dependent on the size and accurate placement of the region of interest, and they become more complicated in stones of mixed composition.
CT is fairly accurate in helping predict stone composition in vitro. Bellin et al. [41] reported that CT attenuation and stone density can be used to predict stone composition in vitro with 64–81 % accuracy. The attenuation values usually fall within certain ranges: uric acid, 200–450 HU; struvite, 600–900 HU; cystine, 600–1,100 HU; calcium phosphate, 1,200–1,600 HU; and calcium oxalate monohydrate and brushite, 1,700–2,800 HU.
Several studies tried to predict stone composition using CT density measurements (Hounsfield units) in vivo but were only able to differentiate uric acid from non-uric acid stones.
The association between measurement in Hounsfield units and response to lithotripsy treatment is already known: calcium oxalate stones with smooth surfaces, a diameter >1 cm, and a radiodensity >1,200 HU rarely become fragmented by extracorporeal shock-wave lithotripsy, whereas calcium oxalate stones with a CT density of <1,000 HU can be treated successfully. In contrast, cystine stones with a CT density >1,000 HU are not treated with extracorporeal shock-wave lithotripsy but rather with percutaneous nephrolithotomy or ureterorenoscopy [42].
Dual-energy CT Conventional CT attenuation values (expressed in Hounsfield units) reflect both the density and attenuation coefficient of a substance and therefore may be the same for different materials at a given X-ray tube potential. For this reason, the attenuation values of different subtypes of renal calculi overlap greatly on conventional single-energy CT datasets. Partial-volume effects further complicate the use of attenuation values in single-energy CT for small structures such as renal calculi.
There has been recent interest in the use of dual-energy CT for the characterization of urinary calculi. Dual-energy CT can be performed with either a single or a dual X-ray tube. Dual-source CT allows concurrent scanning in the same anatomic location at two different energies (80 and 140 kVp) and allows the analysis of energy dependent changes in the attenuation of different materials.
Uric acid stones, which are composed predominantly of low-molecular weight elements (oxygen, carbon, and nitrogen), have different X-ray attenuation properties with high- and low-energy CT compared with other types of renal calculi such as calcium oxalate, hydroxyapatite, or cystine stones. These last three types of stones are composed of high-molecular weight elements (phosphorus, calcium, and sulfur) and will therefore have a higher Hounsfield unit value with lower-energy CT. In a study involving a phantom model, Primak et al. [43] demonstrated that dual-energy CT can help distinguish uric acid stones from other types of stones with 92 %-100 % accuracy. Recent works have suggested possibilities for differentiating other stone types, but most of them are in vitro or ex vivo studies [44, 45].
Qu et al. [46] showed a better separation among different stone types when additional tin filtration was used with a five-group stone classification scheme. However, some overlap still exists between particular stone types, including brushite and calcium oxalate stones, and discriminating between those types remains a challenge.
At present, dual-energy imaging is associated with higher doses of ionizing radiation when compared with single-energy imaging [47]. Targeted dual-energy scanning of calculi can be incorporated into a standard non-contrast CT scan in a dose-efficient way as follows: a single low-dose scan of the entire urinary tract can be performed, followed by targeted low-energy scanning of the areas where calculi are located. The use of such imaging strategies has been shown to decrease the effective dose, which nevertheless remains higher compared with low-dose single-energy CT alone.
This characteristic difference in attenuation with dual-energy imaging may potentially allow accurate determination of stone composition.
Treatment planning
Treatment planning provides information about:
Stone characteristics
-
Size, volume
-
Orientation
-
Density, composition
These parameters are analyzed with the low-dose unenhanced scanning limited to the region of the kidney.
Coronal MIP (maximum intensity projection) images are necessary for calculus analysis. Three-dimensional volume rendering (VR) reconstruction of staghorn stones provides a precise picture of their extent and branching within the kidney. These reconstructions are necessary to provide sufficient information for a safe percutaneous approach.
Collecting system anatomy: complex or variant genitourinary anatomy
Dual-phase imaging provides details of the collecting system anatomy, which are important for planning calculus therapy. It also provides information about the kidney parenchyma (size of the kidney, infection, and tumors).
The ramifications of the pelvicalyceal system and calculus are reconstructed and observed in full 3D format for a better representation of the spatial relationships [48]. The majority of stones are identifiable in the presence of contrast because the stones’ density is higher than that of the diluted urine in the dual-phase image. Indeed, after furosemide administration, the contrast density in the collecting system is low (200, 300 HU) and uniform. It allows for better calyceal detail and fewer streak artifacts. We believe that furosemide is essential for achieving this optimal calculus/contrast/parenchyma gradient. There are multiple variants of genitourinary anatomy that may influence the appropriateness and type of urologic intervention. These include both congenital and postsurgical variants:
-
Horseshoe kidney (Fig. 5), pelvic kidney, crossed fused renal ectopia;
-
Transplanted kidney;
-
Calyceal diverticulum (essential to report to the urologist because it may prompt a change in the technique for calculus retrieval);
-
Abnormal infundibular orientation;
-
Solitary kidney (indication for immediate intervention);
-
Cacchi Ricci disease;
-
Duplicated collecting system (Fig. 6).
Relationship between the kidney and surrounding organs
CT permits the precise location of an appropriate calyx for percutaneous access. The relationship of the kidney to the surrounding organs should be reported in the event an intervention becomes necessary. The location of the bowel (retrorenal position, Fig. 7), vessels, or the level of pleural reflections or aberrant vasculature may change the approach of or prevent percutaneous access for calculus removal or treatment.
Post-evaluation treatment
Detection of complications: CT allows the detection of complications such as perirenal hematoma and urinoma and obstruction of the urinary system.
Confirmation of stone-free status: After urologic intervention or medical therapy, it is imperative to perform follow-up imaging to confirm the clearance of the stone/fragments and to assure the absence of obstruction (stones, ureteral stricture).
-
In the conservative management of ureteral stones, follow-up with conventional radiography and ultrasound is recommended. CT may not be the best follow-up option. The choice of imaging modality is made based on the visibility of the stone on the CT scout and the Hounsfield units of the stone. If the stone is visible, and the density is higher than 500 HU, conventional radiography is used for the follow-up. Whenever there are doubts about the visibility of stone on the scout, plain radiographs should be performed because of the higher sensitivity. A good option is to obtain conventional radiographs at the time of the CT and review both of them in conjunction [49]. This increases the accuracy of conventional radiography and allows the expected location of the stones to be known precisely. If the stone is not visible on the CT scout, or if the density is <500 HU on the CT, follow-up by ultrasound is recommended.
-
After urologic intervention:
-
For patients who underwent ureteroscopy with stone fragmentation, follow-up with ultrasound (radiolucent stones) or ultrasound and KUB (radiopaque stones) will document the present of stone fragments and/or hydronephrosis. In case of hydronephrosis on ultrasound and nonopaque stones, a low-dose CT be the preferred method for detecting and identifying the location of residual stones [50].
-
For patients undergoing shock-wave lithotripsy follow-up with renal sonogram with or without KUB (radiopaque vs. radiolucent stones) is recommended [50]. In case of hydronephrosis and radiolucent stones, low-dose CT is indicated.
-
In symptomatic patients with radiopaque stones, follow-up with KUB and ultrasound is sufficient initially. In case of radiolucent stones low-dose CT will be recommended [50].
-
Conclusion
CT is the preferred method for evaluation of urolithiasis because of its performance, availability, and high sensitivity. It plays an important role in disease management from the initial diagnosis in patients with acute flank pain to treatment planning and post-treatment follow-up. CT radiation dose reduction can be achieved with low-dose CT and appropriate measures to optimize image quality, such as iterative image reconstruction. However, conventional radiography and ultrasound are still recommended in the early follow-up of ureteral stones.
References
Levine JA, Neitlich J, Verga M, Dalrymple N, Smith RC (1997) Ureteral calculi in patients with flank pain: correlation of plain radiography with unenhanced helical CT. Radiology 204:27–31
Yap WW, Belfield JC, Bhatnagar P, Kennish S, Wah TM (2012) Evaluation of the sensitivity of scout radiographs on unenhanced helical CT in identifying ureteric calculi: a large UK tertiary referral centre experience. Br J Radiol 85:800–806
Mutgi A, Williams JW, Nettleman M (1991) Renal colic. Utility of the plain abdominal roentgenogram. Arch Intern Med 151:1589–1592
Tublin ME, Bude RO, Platt JF (2003) Review. The resistive index in renal Doppler sonography: where do we stand? AJR 180:885–892
Dillman JR, Kappil M, Weadock WJ et al (2011) Sonographic twinkling artifact for renal calculus detection: correlation with CT. Radiology 259:911–916
Ulusan S, Koc Z, Tokmak N (2007) Accuracy of sonography for detecting renal stone: comparison with CT. J Clin Ultrasound 35:256–261
Viprakasit DP, Sawyer MD, Herrell SD, Miller NL (2012) Limitations of ultrasonography in the evaluation of urolithiasis: a correlation with computed tomography. J Endourol 26:209–213
Fowler KA, Locken JA, Duchesne JH, Williamson MR (2002) US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology 222:109–113
Ray AA, Ghiculete D, Pace KT, Honey RJ (2012) Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology 76:295–300
Kanno T, Kubota M, Sakamoto H et al (2014) The efficacy of ultrasonography for the detection of renal stone. Urology 84:285–288
Moesbergen TC, de Ryke RJ, Dunbar S, Wells JE, Anderson NG (2011) Distal ureteral calculi: US follow-up. Radiology 260:575–580
Karabacakoglu A, Karakose S, Ince O, Cobankara OE, Karalezli G (2004) Diagnostic value of diuretic-enhanced excretory MR urography in patients with obstructive uropathy. Eur J Radiol 52:320–327
Nolte-Ernsting CC, Bucker A, Adam GB et al (1998) Gadolinium-enhanced excretory MR urography after low-dose diuretic injection: comparison with conventional excretory urography. Radiology 209:147–157
White WM, Johnson EB, Zite NB et al (2013) Predictive value of current imaging modalities for the detection of urolithiasis during pregnancy: a multicenter, longitudinal study. J Urol 189:931–934
Fahmy NM, Elkoushy MA, Andonian S (2012) Effective radiation exposure in evaluation and follow-up of patients with urolithiasis. Urology 79:43–47
Katz DS, Venkataramanan N, Napel S, Sommer FG (2003) Can low-dose unenhanced multidetector CT be used for routine evaluation of suspected renal colic? AJR 180:313–315
Mulkens TH, Daineffe S, De Wijngaert R et al (2007) Urinary stone disease: comparison of standard-dose and low-dose with 4D MDCT tube current modulation. AJR 188:553–562
Tack D, Sourtzis S, Delpierre I, de Maertelaer V, Gevenois PA (2003) Low-dose unenhanced multidetector CT of patients with suspected renal colic. AJR 180:305–311
Heneghan JP, McGuire KA, Leder RA, DeLong DM, Yoshizumi T, Nelson RC (2003) Helical CT for nephrolithiasis and ureterolithiasis: comparison of conventional and reduced radiation-dose techniques. Radiology 229:575–580
Liu W, Esler SJ, Kenny BJ, Goh RH, Rainbow AJ, Stevenson GW (2000) Low-dose nonenhanced helical CT of renal colic: assessment of ureteric stone detection and measurement of effective dose equivalent. Radiology 215:51–54
Sohn W, Clayman RV, Lee JY, Cohen A, Mucksavage P (2013) Low-dose and standard computed tomography scans yield equivalent stone measurements. Urology 81:231–234
Poletti PA, Platon A, Rutschmann OT, Schmidlin FR, Iselin CE, Becker CD (2007) Low-dose versus standard-dose CT protocol in patients with clinically suspected renal colic. AJR 188:927–933
Tartari S, Rizzati R, Righi R, Deledda A, Terrani S, Benea G (2010) Low-dose unenhanced CT protocols according to individual body size for evaluating suspected renal colic: cumulative radiation exposures. Radiol Med 115:105–114
Jin DH, Lamberton GR, Broome DR et al (2010) Effect of reduced radiation CT protocols on the detection of renal calculi. Radiology 255:100–107
Kalra MK, Maher MM, Toth TL, Kamath RS, Halpern EF, Saini S (2004) Comparison of Z-axis automatic tube current modulation technique with fixed tube current CT scanning of abdomen and pelvis. Radiology 232:347–353
Silva AC, Lawder HJ, Hara A, Kujak J, Pavlicek W (2010) Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR 194:191–199
Kulkarni NM, Eisner BH, Pinho DF, Joshi MC, Kambadakone AR, Sahani DV (2013) Determination of renal stone composition in phantom and patients using single-source dual-energy computed tomography. J Comput Assist Tomogr 37:37–45
Assi Z, Platt JF, Francis IR, Cohan RH, Korobkin M (2000) Sensitivity of CT scout radiography and abdominal radiography for revealing ureteral calculi on helical CT: implications for radiologic follow-up. AJR 175:333–337
Chu G, Rosenfield AT, Anderson K, Scout L, Smith RC (1999) Sensitivity and value of digital CT scout radiography for detecting ureteral stones in patients with ureterolithiasis diagnosed on unenhanced CT. AJR 173:417–423
Furlan A, Federle MP, Yealy DM, Averch TD, Pealer K (2008) Nonobstructing renal stones on unenhanced CT: a real cause for renal colic? AJR 190:W125–W127
Eisner BH, Kambadakone A, Monga M et al (2009) Computerized tomography magnified bone windows are superior to standard soft tissue windows for accurate measurement of stone size: an in vitro and clinical study. J Urol 181:1710–1715
Bandi G, Meiners RJ, Pickhardt PJ, Nakada SY (2009) Stone measurement by volumetric three-dimensional computed tomography for predicting the outcome after extracorporeal shock wave lithotripsy. BJU Int 103:524–528
Demehri S, Kalra MK, Rybicki FJ et al (2011) Quantification of urinary stone volume: attenuation threshold-based CT method—a technical note. Radiology 258:915–922
Preminger GM, Tiselius HG, Assimos DG et al (2007) 2007 guideline for the management of ureteral calculi. Eur Urol 52:1610–1631
Finch W, Johnston R, Shaida N, Winterbottom A, Wiseman O (2014) Measuring stone volume—three-dimensional software reconstruction or an ellipsoid algebra formula? BJU Int 113:610–614
Yoshida S, Hayashi T, Morozumi M, Osada H, Honda N, Yamada T (2007) Three-dimensional assessment of urinary stone on non-contrast helical computed tomography as the predictor of stonestreet formation after extracorporeal shock wave lithotripsy for stones smaller than 20 mm. Int J Urol 14:665–667
Koraishy FM, Ngo TT, Israel GM, Dahl NK (2014) CT urography for the diagnosis of medullary sponge kidney. Am J Nephrol 39:165–170
Ciudin A, Luque Galvez MP, Salvador Izquierdo R et al (2013) Validation of Randall’s plaque theory using unenhanced abdominal computed tomography. Urology 81:246–249
Miller NL, Humphreys MR, Coe FL et al (2010) Nephrocalcinosis: re-defined in the era of endourology. Urol Res 38:421–427
Williams JC Jr, Kim SC, Zarse CA, McAteer JA, Lingeman JE (2004) Progress in the use of helical CT for imaging urinary calculi. J Endourol 18:937–941
Bellin MF, Renard-Penna R, Conort P et al (2004) Helical CT evaluation of the chemical composition of urinary tract calculi with a discriminant analysis of CT-attenuation values and density. Eur Radiol 14:2134–2140
Weld KJ, Montiglio C, Morris MS, Bush AC, Cespedes RD (2007) Shock wave lithotripsy success for renal stones based on patient and stone computed tomography characteristics. Urology 70:1043–1046 (discussion 1046–1047)
Primak AN, Fletcher JG, Vrtiska TJ et al (2007) Noninvasive differentiation of uric acid versus non-uric acid kidney stones using dual-energy CT. Acad Radiol 14:1441–1447
Boll DT, Patil NA, Paulson EK et al (2009) Renal stone assessment with dual-energy multidetector CT and advanced postprocessing techniques: improved characterization of renal stone composition—pilot study. Radiology 250:813–820
Manglaviti G, Tresoldi S, Guerrer CS et al (2011) In vivo evaluation of the chemical composition of urinary stones using dual-energy CT. AJR 197:W76–W83
Qu M, Ramirez-Giraldo JC, Leng S et al (2011) Dual-energy dual-source CT with additional spectral filtration can improve the differentiation of non-uric acid renal stones: an ex vivo phantom study. AJR 196:1279–1287
Graser A, Johnson TR, Bader M et al (2008) Dual energy CT characterization of urinary calculi: initial in vitro and clinical experience. Invest Radiol 43:112–119
Patel U, Walkden RM, Ghani KR, Anson K (2009) Three-dimensional CT pyelography for planning of percutaneous nephrostolithotomy: accuracy of stone measurement, stone depiction and pelvicalyceal reconstruction. Eur Radiol 19:1280–1288
Smith RC, Coll DM (2000) Helical computed tomography in the diagnosis of ureteric colic. BJU Int 86(Suppl 1):33–41
Fulgham PF, Assimos DG, Pearle MS, Preminger GM (2013) Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol 189:1203–1213
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renard-Penna, R., Martin, A., Conort, P. et al. Kidney stones and imaging: What can your radiologist do for you?. World J Urol 33, 193–202 (2015). https://doi.org/10.1007/s00345-014-1416-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1416-0