Abstract
Mitral regurgitation (MR) is one of the most frequent indications for valve surgery in developed countries, and echocardiographic assessment is an essential tool to evaluate its etiologies, severity, and therapeutic indications. The mitral valve apparatus is a complex structure composed of several parts: apart from the mitral valve leaflets and annulus, it also includes the chordae tendineae, papillary muscles, and left ventricular (LV) wall. MR can be caused not only by organic changes of the mitral valve leaflets or chordae (primary MR) but also by extreme mitral annular enlargement or mitral leaflet tethering due to displacement and malfunction of papillary muscles and LV wall (secondary MR). In secondary MR with LV dysfunction, a milder degree of MR can be associated with adverse outcomes compared with primary MR. Grading the severity is the first step in evaluation of indication for surgical/transcatheter interventions. As such, there are several techniques to assess the severity of MR using echocardiography. However, none of the techniques is reliable enough by itself, and it is always recommended to integrate multiple methods. In cases where echocardiographic assessment of MR severity is inconclusive, magnetic resonance may be helpful. In addition to the severity, anatomical information, such as localization in primary MR due to mitral valve prolapse and LV size in secondary MR due to LV dilatation/dysfunction, is an important concern in presurgical echocardiography. Transesophageal echocardiography and three-dimensional echocardiography are key techniques for anatomical evaluation including mitral valve and LV volumes. In transcatheter intervention for MR, echocardiography plays a pivotal role as a guide for procedures and endpoints. In this review article, the authors provide a comprehensive summary of current standards of echocardiographic assessment of MR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mitral regurgitation (MR) is one of the most common valvular heart diseases in developed countries and the second most frequent indication for valve surgery in Japan [1, 2]. Chronic MR generally progresses without symptoms for a long time and can cause functional and structural deterioration of the left atrium (LA) and ventricle (LV), which are associated with increased mortality and morbidity [3, 4]. Accordingly, patients with significant chronic MR should undergo routine evaluation regardless of symptoms and physicians must provide optimal interventions at the optimal time.
Echocardiography is noninvasive and the most accessible imaging tool that can be useful for the evaluation of MR or other various conditions and diseases. Even though other cardiac imaging modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT), have been reported to provide more accurate assessment of regurgitant volume and anatomical details [5, 6], echocardiography, along with physical examinations, will remain the first line and routine evaluation tool for valvular heart disease because of its repeatability and accessibility. The strengths of echocardiography in evaluation of MR also include its capability of comprehensive evaluation of anatomical, physiological, and functional aspects in real time, enabling it to be an extension of physical examinations and an intra-procedural guidance. However, inter-and intra-observer variability and moderate reproducibility/accuracy in grading MR severity should be acknowledged. The purpose of this review document is to provide a comprehensive summary of current standards of echocardiographic assessment of MR.
Anatomy of mitral valve
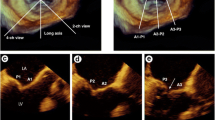
The mitral valve apparatus is a complex structure consisting of several parts: the mitral valve leaflets and annulus, chordae tendineae, papillary muscles, and left ventricular wall. Dysfunction of any of these components can cause MR (Fig. 1a) [2]. Mitral valve leaflets are further classified into anterior and posterior leaflets. The anterior leaflet has double the leaflet length and half the circumferential width of the posterior leaflet, resulting in a similar leaflet area. The posterior leaflet is further divided into three segments by indentations: P1 (lateral scallop), P2 (middle scallop), and P3 (posterior scallop). The corresponding segments of the anterior leaflet are called A1, A2 and A3, respectively. The anterior and posterior leaflets are connected by small redundant tissues called medial and lateral commissures (Fig. 1b). As described below, identifying the localization of the pathology is very important and each component should be carefully assessed in presurgical evaluation of MR.
Mitral valve apparatus. Right panels: A 3D echocardiographic image of the entire mitral apparatus. Left upper panel A specimen of the mitral valve apparatus, Left bottom panel echocardiographic surgeon’s view of mitral valve leaflet. APM anterior papillary muscle, PPM posterior papillary muscle, LV left ventricle
General classification of MR
Primary and secondary MR
Chronic MR is generally classified into primary (organic) and secondary MR (functional) (Fig. 2). In primary MR, the most common cause is mitral valve prolapse [7, 8] in which the leaflet body deviates above the mitral annulus toward the LA in systole (Fig. 3a) [2, 3]. Etiologies of mitral valve prolapse can be further subclassified into fibroelastic deficiency and Barlow’s disease [2, 9]. While fibroelastic deficiency is generally characterized by localized involvement of the leaflet with healthy adjacent segments and is commonly seen in elderly patients, Barlow’s disease is a disease entity with diffuse excess tissue including large and myxomatous leaflet, diffusely elongated chordae, and enlarged annulus, although there is an overlap between these two [10, 11]. Flail is a term that is usually used as a specific pattern of mitral valve prolapse in which the leaflet body and also the tip are flipped toward the LA (Fig. 3b) [3, 4]. It is often caused by chordae rupture and is usually associated with severe MR. Other etiologies of primary MR include rheumatic valve diseases, infective endocarditis, congenital anomaly of the mitral valve, and calcification of the mitral valve apparatus.
Secondary MR is dominantly caused by LV dilatation and dysfunction. Papillary muscles are displaced to a lateral and apical location in the dilated LV, tethering the chordae and leaflets toward it. In systole, tethered leaflets cannot close at the mitral annular level, resulting in insufficient coaptation [12, 13]. One of the most common scenarios is LV dilatation due to myocardial infarction, which is seen in about 5% of patients who undergo primary catheter intervention for acute myocardial infarction [14], and such secondary MR caused by ischemic etiology is called ischemic MR. Any other diseases that cause LV dilatation and dysfunction can be related to secondary MR. Furthermore, recent studies reported MR caused by extreme dilatation of the LA and related mechanisms, which is now called atrial functional MR and is mostly observed in patients with atrial fibrillation (AFib) [15, 16]. Although the detailed mechanisms of atrial functional MR are yet to be established, it is generally characterized by an extremely enlarged LA and mitral annulus, absence of LV dysfunction or dilatation, and a normal mitral valve leaflet [17]. This type of MR can be considered a subtype of secondary MR, and other etiologies such as systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy can also be included in secondary MR by definition, although the term “secondary MR” generally implies secondary MR due to LV dilatation and dysfunction.
It is well known that primary and secondary MR have different significance on outcome, and the effectiveness of therapeutic interventions is also different. While moderate or less primary MR is usually considered benign, even mild secondary MR is associated with an increase in adverse cardiac events [6]. Thus, guidelines recommend different management strategies for primary and secondary MR [3, 18, 19]. However, it should be acknowledged that the evidence is mostly based on mitral valve prolapse for primary MR and MR due to LV dilatation and dysfunction for secondary MR. Thus, physicians should not apply the same strategy to all etiologies of primary (or secondary) MR.
Carpentier classification of MR based on leaflet motion
The Carpentier classification is another classification of MR based on leaflet motion (Fig. 4). Type I refers to MR with normal leaflet motion, which includes annular dilatation and leaflet perforation. Some congenital anomalies such as cleft mitral valve are also considered Type I. Type II leaflet motion is defined by excessive deviation toward the LA that is similar to mitral valve prolapse. Type III refers to restrictive leaflet motion where leaflets do not close at the level of the mitral annulus. Type III is further divided into Type IIIa, which is restricted by the leaflet itself due to rheumatic or other post-inflammatory disorders, and Type IIIb, where the leaflet is tethered by displaced papillary muscle and/or shortened chordae. This classification is independent of classification into primary/secondary MR, i.e., Type I can be either primary (leaflet perforation) or secondary (annular dilatation). As the Carpentier classification is more directly associated with surgical planning than primary/secondary MR, it can help facilitate communication between sonographer/cardiologist and surgeons.
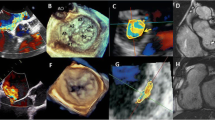
Carpentier classification of MR. The upper panels show transesophageal echocardiographic images of the mitral valve in each type of mitral regurgitation, and the lower panels are the color Doppler images corresponding to the upper panels. Type I refers to MR with normal leaflet motion. Type II leaflet motion is defined by excessive deviation toward the left atrium. Type IIIa MR is caused by restriction by the leaflet itself. In Type IIIb, the leaflet is tethered by displaced papillary muscle and/or shortened chordae
Acute MR
Acute MR occurs in several situations, such as acute impairment of LV function in acute myocardial infarction or acute myocarditis, papillary muscle or chordae rupture, and infective endocarditis. Although acute MR is much less common than chronic MR, it usually relates to severer clinical presentation and frequent pulmonary edema due to less cardiac compensation to the sudden increase in volume overload [20,21,22]. It generally causes acute elevation of LA pressure, tachycardia, and sometimes hypotension due to decreased LV stroke volume. Increased LA pressure shortens the duration of MR, resulting in less visible color Doppler MR jet. Accordingly, acute MR is often underestimated by echocardiography. Other echocardiographic findings independent of color Doppler, such as systolic flow reversal in the pulmonary vein, anatomic findings of severe MR such as flail, and hyperdynamic LV, are helpful [4].
Echocardiographic assessment of MR severity
Accurate and reproducible assessment of the severity of MR is crucial for its appropriate management as it is directly associated with the prognosis [23, 24]. Table 1 summarizes the strengths and limitations of the approaches for grading MR severity, and Table 2 shows the cutoff for each grade. Most current guidelines recommend treatment strategies based on MR severity [3, 18, 19]. Echocardiography, owing to its wide availability and completely noninvasive nature, is the most important and repeatable tool for MR grading. However, every single echocardiographic approach has limitations and, thus, a comprehensive approach with a good understanding of those limitations is always recommended.
Color Doppler jet area
Color Doppler jet area is one of the most commonly used approaches for grading MR severity in routine clinical practice. Using the apical 4-chamber view, with tilting of the plane to capture the largest jet, MR jet area and LA area should be measured on the same plane, and the ratio of MR jet area to LA area was evaluated (Fig. 5a). This approach is commonly used as a first screening of the presence of MR. On the other hand, this is not essentially a flow image but rather an image of the spatial distribution of velocities within the image plane. This approach is easy and visually intuitive, but it is imprecise for grading MR severity compared with other approaches [4, 25]. In cases of acute MR, eccentric jet, and significantly enlarged LA, in particular, the MR jet area tends to be underestimated. Accordingly, when moderate or more MR is suspected, other approaches should be employed to grade MR severity together in conjunction with this.
Vena contracta width
Vena contracta width is a relatively easy and objective method that provides semiquantitative grading of MR severity. Vena contracta width is the width at the narrowest part of the MR jet (Fig. 5b). It is measured on the parasternal long axis view as other views, e.g., the 2-chamber view, can cause significant over/underestimation of the severity. Vena contracta < 0.3 cm and ≥ 0.7 cm is relatively specific to mild and severe MR, respectively, whereas there is an overlap of mild, moderate, and severe MR in vena contracta of 0.3–0.7 cm. Vena contracta is also reported to be accurate in evaluating eccentric jet [26]. Furthermore, a previous study demonstrated that vena contracta width reflected orifice size and was resistant with changes in the flow [27]. However, it is significantly influenced by the orifice shape, i.e., it underestimates MR when the orifice is elliptical and it cannot be applied to multiple jets.
Proximal isovelocity surface area method
The proximal isovelocity surface area (PISA) method is one of the most commonly used quantitative approaches for MR grading. Previous studies have reported that MR quantification using the PISA method is associated with the prognosis [6, 23, 28]. Using an apical view, the size of the proximal flow convergence radius is measured with the color Doppler baseline shifted in the direction of the jet (= change the Nyquist limit: often to 30–40 cm/s). Then, MR in continuous wave Doppler is traced to obtain the time velocity integral (TVI) and maximum velocity (Fig. 5c). The effective regurgitant orifice area (EROA) and regurgitant volume (RV) can both be calculated using these measurements. The biggest pitfall is in measuring the size of the proximal flow convergence radius, which is sometimes difficult, mainly due to unclear location of the orifice. As small errors in the size of the radius result in a big difference in the estimated value, it should be measured carefully using a zoomed image with accurate detection of orifice location. For an eccentric jet or elliptical orifice, this approach can underestimate MR severity as well as vena contracta.
Volumetric method
In the volumetric method, MR volume is derived as the difference between the stroke volume (SV) calculated at the LV outflow tract and the volume of LV inflow [29,30,31]. Alternatively, MR volume can be obtained as the difference between the SV calculated at LV outflow and the SV measured by tracing the LV endocardium (2D disc method or ideally 3D method). In the former method, the LV inflow volume is calculated using TVI of mitral inflow measured at the mitral annular level and mitral annular diameters. This approach is resistant to multiple jets, elliptical orifice shape, eccentric jet, and duration of MR (non-holosystolic MR), in contrast to other quantitative/semiquantitative approaches. However, there are many steps in the calculation process and small errors in each measurement can accentuate error in the final results [4]. Therefore, it requires certain training, and reproducibility remains a major concern.
Other supporting findings
Other than the quantitative/semiquantitative approaches, several simple approaches are sometimes helpful for differentiating severe MR (Table 2) [4]. For example, if clear proximal convergence flow is observed, the severity of MR should be more than moderate. Flail leaflet, with a clear gap between the diseased and adjacent leaflet tip, is mostly related to severe MR. Reversal Doppler wave pattern of pulmonary vein flow is an additional metric indicating severe MR. Also, it is sometimes useful to check the continuous wave Doppler pattern and the shape of MR. In severe MR, the pattern is dense, and the shape can become triangular since the flow velocity drops fast due to a rapid increase in LA pressure. The mitral inflow pattern is usually E wave dominant with a high peak velocity in severe MR (> 1.2 m/s). Lastly, hyperdynamic LV motion without any other cause may indicate compensation effort against severe MR. These metrics can help to make a quick assessment of the severity and can also help when other quantitative metrics are inconsistent.
MRI has recently been reported as a more accurate tool for quantification of MR [32, 33]. In cases where echocardiographic assessment of MR severity is inconclusive, magnetic resonance may be helpful.
3D Echocardiography
3D echocardiography, especially with transesophageal echocardiography, has already become a routine tool for presurgical assessment of MR. Compared with 2D echocardiography, which requires some experience to visualize the 3D morphology, 3D echocardiography provides much more intuitive images, improving the accuracy of localization of the mitral valve pathology even by novice readers (Fig. 6) [34], and making it easier to communicate with surgeons and other medical staff who are not experts in echocardiography.
Furthermore, 3D echocardiography allows not only visual assessment but also quantitative approaches that may be more accurate than 2D methodologies. For example, 3D quantitative assessment including prolapse volume and its ratio to height allows accurate differentiation of fibroelastic deficiency from Barlow’s disease, which requires more complex repair [9, 35]. Recently, such quantitative approaches have become easier and faster because of advances in technologies [36,37,38].
Vena contracta area is an area of vena contracta measured by 3D color Doppler [39]. Although the principle of vena contracta area is the same as vena contracta width, i.e., assessment of the narrowest part of MR flow, vena contracta area can be applied to any form of MR orifice because of its 3D nature. Similarly, 3D PISA methods can assess the morphology and size of PISA without shape assumption, with a previous study reporting its superiority over 2D PISA [40]. Currently, these 3D color Doppler-based techniques require manual processing of multiplanar reconstruction and have limited evidence in clinical settings, although they seem to be promising in assessment of MR severity.
Echocardiography for guiding therapeutic planning
Key echocardiographic findings in primary MR
In primary MR, the primary indication for surgery is “severe MR + symptoms”. If a patient is symptomatic with severe MR, intervention should only be considered as far as the patient is suitable for the surgery. Risk of surgical treatment should be contemplated by a multidisciplinary heart team based on a multifactorial assessment. The echocardiologist/sonographer must, at the least, consider LV function including LVEF. Both ACC/AHA and ESC guidelines consider LVEF < 30% as high risk [3, 18, 19, 41]. For asymptomatic patients with severe MR, reduced LVEF, enlarged LV end-systolic diameter, elevated pulmonary artery pressure, and new onset of atrial fibrillation are associated with poor prognosis and, therefore, a marker of indication of surgery [42,43,44]. Recently, impaired global longitudinal strain has been reported as an earlier marker of LV deterioration associated with increased adverse events [45]. Exercise stress echocardiography may be helpful to reveal exercise-induced change in MR severity, pulmonary artery pressure, and symptoms [46,47,48,49]. A simplified flowchart is provided in Fig. 7.
Simplified treatment flow diagram for primary MR. Signs of cardiac deterioration include left ventricular ejection fraction ≤ 60%, left ventricular end-systolic diameter ≥ 40 (or 45) mm, systolic pulmonary artery pressure > 50 mmHg, and new onset of atrial fibrillation. Impaired global longitudinal strain may also be considered a sign of cardiac deterioration
Repairability of the valve is one of the most important factors that should be evaluated using echocardiography. In mitral valve prolapse, the most repairable type is posterior leaflet prolapse involving a relatively small area, which is usually repaired by a simple technique such as triangular resection. Anterior leaflet prolapse generally requires a more complex surgical technique such as artificial chord and is considered more difficult to repair [19]. Furthermore, widespread prolapse with diffuse excess tissue and significantly enlarged leaflets and annulus, such as Barlow’s disease, requires even more complex repair [9]. However, as techniques used for each pathology and success rates of repair vary significantly between institutes, it is important for the echocardiologist/sonographer to be familiar not only with the general techniques of mitral valve repair but also with the institutional characteristics such as preferred techniques. 3D echocardiography is very useful for detecting accurate localization of the prolapse lesion and for communicating with surgeons, as discussed above.
Percutaneous mitral valve repair using MitraClip is a new option in mitral valve repair; however, it should be acknowledged that the use of MitraClip is currently limited only to those patients with high risk of mortality and morbidity for open heart surgery. This is primarily because it provides only one technique used in surgical mitral valve repair, i.e., Alfieri stich. Since the MitraClip procedure is dominantly guided by echocardiography, i.e., operators cannot directly look at the valve, pre- and intra-procedural echocardiography should be performed meticulously. Anatomical features of ideal candidates for MitraClip include: (1) MR originating from the mid portion of the valve (2) lack of severe calcification/rheumatic change on leaflet tips (3) mitral valve area > 4 cm2 (4) posterior leaflet length ≥ 10 mm (5) flail width < 15 mm and gap < 10 mm, and (6) coaptation depth (tenting height) < 11 mm and coaptation length > 2 mm [50]. As echocardiography is practically the only imaging modality that provides hemodynamic, functional, and tissue information throughout the procedure, appropriate imaging and determination of endpoints are essential. In particular, 3D transesophageal echocardiographic imaging, especially an en-face view (surgeon’s view) of the mitral valve, plays a pivotal role in showing delivery catheters, wires, devices, and cardiac structures in a single view and in relation to each other. Supplemented by the simultaneous bi-plane function, 3D echocardiography helps guide proper positioning of the transseptal puncture (superior and posterior position: 4–5 cm above the mitral annulus in primary MR and approximately 3.5 cm in secondary MR [50]) angle between the clip and the mitral valve, and the position that the device grasps the mitral valve. Since the objective of MitraClip is usually not to eliminate MR but rather to reduce the amount of MR, and the use of too many clips may cause mitral stenosis, the definition of endpoints varies from patient to patient. Recently published guidelines recommend: (1) evaluation by color Doppler image and integration with other methods, (2) check for changes in patterns of pulmonary vein flow and mitral inflow, (3) when possible, vena contracta area, and (4) check the transmitral gradient [51].
Treatment strategy for secondary MR
Indication for interventions in secondary MR is more complicated than in primary MR as the therapeutic effect of interventions is less clear [18, 19, 52, 53]. Even though there is mounting evidence of an association between the presence of significant secondary MR and increased adverse events, there has been no established evidence for surgical interventions. Accordingly, both AHA/ACC and ESC guidelines currently do not have class I or IIa indication criteria for isolated surgery for secondary MR [3, 18, 19, 41]. In clinical practice, surgical intervention for secondary MR is often considered in symptomatic patients who are refractory to medical therapy and have no other therapeutic options. Frequent recurrence of MR and high peri-surgical comorbidity/mortality remain big concerns [54], yet many repair techniques have been invented for secondary MR [52, 53, 55, 56]. These techniques have not been standardized, and echocardiographic findings associated with successful repair are also yet to be established. When planning such surgeries, discussion with surgeons would be helpful to define important echocardiographic points.
The results of the COAPT trial, therefore, had a big impact on the therapeutic strategy for secondary MR [57]. This randomized control trial published in the end of 2018, where symptomatic patients with severe secondary MR were randomized to MitraClip + medical therapy or optimal medical therapy alone, showed a 40% decrease in hazard for death by MitraClip. However, it may be too early to unconditionally accept this result. Importantly, the MITRA-FR study, an RCT with a similar design, showed completely opposite results including no difference between MitraClip and the control group [58]. Currently, the success of COAPT trial is mainly considered because of the patient inclusion criteria. Compared with MITRA-FR, COAPT included patients with less dilated LV despite severer MR. Pibarot et al. proposed the following criteria for patients who benefit from MitraClip: (1) ≥ moderate-to-severe secondary MR defined as EROA 30 mm2 and/or regurgitant volume > 45 ml (2) LVEF between 20% and 50% and LV end-systolic diameter < 70 mm, and (3) persistent heart failure symptoms (NYHA ≥ II) despite optimal (maximally tolerated) GDMT with cardiac resynchronization and coronary revascularization, if appropriate [59].
Conclusions
Echocardiography is the first and the most important imaging tool in evaluation of MR, although there are pitfalls and limitations. Appropriate use of echocardiography while keeping these limitations in mind is essential and will become even more important in the era of transcatheter valve treatment.
References
Shimizu H, Endo S, Natsugoe S, et al. Thoracic and cardiovascular surgery in japan in 2016: annual report by the japanese association for thoracic surgery. Gen Thorac Cardiovasc Surg. 2019;67:377–411.
Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. 2010;31:1958–66.
O’Gara PT, Grayburn PA, Badhwar V, et al. 2017 acc expert consensus decision pathway on the management of mitral regurgitation: a report of the american college of cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70:2421–49.
Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30:303–71.
Nishino S, Watanabe N, Kimura T, et al. Acute versus chronic ischemic mitral regurgitation: an echocardiographic study of anatomy and physiology. Circ Cardiovasc Imaging. 2018;11:e007028.
Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative doppler assessment. Circulation. 2001;103:1759–64.
Hepner AD, Ahmadi-Kashani M, Movahed MR. The prevalence of mitral valve prolapse in patients undergoing echocardiography for clinical reason. Int J Cardiol. 2007;123:55–7.
Theal M, Sleik K, Anand S, et al. Prevalence of mitral valve prolapse in ethnic groups. Can J Cardiol. 2004;20:511–5.
Kagiyama N, Toki M, Hayashida A, et al. Prolapse volume to prolapse height ratio for differentiating barlow’s disease from fibroelastic deficiency. Circ J. 2017;81:1730–5.
Barlow JB, Pocock WA. The significance of late systolic murmurs and mid-late systolic clicks. Md State Med J. 1963;12:76–7.
Carpentier A, Chauvaud S, Fabiani JN, et al. Reconstructive surgery of mitral valve incompetence: ten-year appraisal. J Thorac Cardiovasc Surg. 1980;79:338–48.
Otsuji Y, Handschumacher MD, Schwammenthal E, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008.
Otsuji Y, Handschumacher MD, Liel-Cohen N, et al. Mechanism of ischemic mitral regurgitation with segmental left ventricular dysfunction: three-dimensional echocardiographic studies in models of acute and chronic progressive regurgitation. J Am Coll Cardiol. 2001;37:641–8.
Nishino S, Watanabe N, Kimura T, et al. The course of ischemic mitral regurgitation in acute myocardial infarction after primary percutaneous coronary intervention: from emergency room to long-term follow-up. Circ Cardiovasc Imaging. 2016;9:e004841.
Gertz ZM, Raina A, Saghy L, et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011;58:1474–81.
Kagiyama N, Hayashida A, Toki M, et al. Insufficient leaflet remodeling in patients with atrial fibrillation: association with the severity of mitral regurgitation. Circ Cardiovasc Imaging. 2017;10:e005451.
Kagiyama N, Mondillo S, Yoshida K, et al. Subtypes of atrial functional mitral regurgitation: imaging insights into their mechanisms and therapeutic implications. J Am Coll Cardiol Img. 2019. https://doi.org/10.1016/j.jcmg.2019.01.040.
Baumgartner H, Falk V, Bax JJ, et al. 2017 esc/eacts guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, et al. 2014 aha/acc guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:e521–643.
Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the shock trial registry. J Am Coll Cardiol. 2000;36:1104–9.
Smyllie JH, Sutherland GR, Geuskens R, et al. Doppler color flow mapping in the diagnosis of ventricular septal rupture and acute mitral regurgitation after myocardial infarction. J Am Coll Cardiol. 1990;15:1449–55.
Roberts WC, Braunwald E, Morrow AG. Acute severe mitral regurgitation secondary to ruptured chordae tendineae: clinical, hemodynamic, and pathologic considerations. Circulation. 1966;33:58–70.
Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–83.
Devereux RB, Kramer-Fox R, Shear MK, et al. Diagnosis and classification of severity of mitral valve prolapse: methodologic, biologic, and prognostic considerations. Am Heart J. 1987;113:1265–80.
Lancellotti P, Moura L, Pierard LA, Reviewers: D, Sicari R, Vahanian A, Roelandt JRTC., et al. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur Heart J Cardiovasc Imaging. 2010;11:307–32.
Hall Shelley A, Brickner ME, Willett DuWayne L, et al. Assessment of mitral regurgitation severity by doppler color flow mapping of the vena contracta. Circulation. 1997;95:636–42.
Baumgartner H, Schima H, Kühn P. Value and limitations of proximal jet dimensions for the quantitation of valvular regurgitation: an in vitro study using doppler flow imaging. J Am Soc Echocardiogr. 1991;4:57–66.
Enriquez-Sarano M, Seward JB, Bailey KR, et al. Effective regurgitant orifice area: a noninvasive doppler development of an old hemodynamic concept. J Am Coll Cardiol. 1994;23:443–51.
Enriquez-Sarano M, Bailey KR, Seward JB. Quantitative doppler assessment of valvular regurgitation. Circulation. 1993;87:841–8.
Blumlein S, Bouchard A, Schiller NB, et al. Quantitation of mitral regurgitation by doppler echocardiography. Circulation. 1986;74:306–14.
Dujardin Karl S, Enriquez-Sarano M, Bailey Kent R, et al. Grading of mitral regurgitation by quantitative doppler echocardiography. Circulation. 1997;96:3409–15.
Uretsky S, Gillam L, Lang R, et al. Discordance between echocardiography and mri in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol. 2015;65:1078–88.
Uretsky S, Aldaia L, Marcoff L, et al. The effect of systolic variation of mitral regurgitation on discordance between noninvasive imaging modalities. J Am Coll Cardiol Img. 2019. https://doi.org/10.1016/j.jcmg.2019.02.014.
Tsang W, Weinert L, Sugeng L, et al. The value of three-dimensional echocardiography derived mitral valve parametric maps and the role of experience in the diagnosis of pathology. J Am Soc Echocardiogr. 2011;24:860–7.
Chandra S, Salgo IS, Sugeng L, Weinert L, Tsang W, Takeuchi M, Spencer KT, O’Connor A, Cardinale M, Settlemier S, Mor-Avi V, Lang RM. Characterization of degenerative mitral valve disease using morphologic analysis of real-time three-dimensional echocardiographic images: objective insight into complexity and planning of mitral valve repair. Circ Cardiovasc Imaging. 2011;4:24–32.
Tsang W, Salgo IS, Medvedofsky D, et al. Transthoracic 3d echocardiographic left heart chamber quantification using an automated adaptive analytics algorithm. JACC Cardiovasc Imaging. 2016;9:769–82.
Kagiyama N, Toki M, Hara M, et al. Efficacy and accuracy of novel automated mitral valve quantification: three-dimensional transesophageal echocardiographic study. Echocardiography. 2016;33:756–63.
Toki M, Kagiyama N, Maeda M, et al. Novel automated left heart chamber quantification software is useful for both beginners and experts: three-dimensional transthoracic echocardiographic study. J Am Soc Echocardiogr. 2017;30:B46.
Goebel B, Heck R, Hamadanchi A, et al. Vena contracta area for severity grading in functional and degenerative mitral regurgitation: a transoesophageal 3d colour doppler analysis in 500 patients. Eur Heart J Cardiovasc Imaging. 2018;19:639–46.
Schmidt FP, Gniewosz T, Jabs A, et al. Usefulness of 3d-pisa as compared to guideline endorsed parameters for mitral regurgitation quantification. Int J Cardiovasc Imaging. 2014;30:1501–8.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 aha/acc focused update of the 2014 aha/acc guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–89.
Enriquez-Sarano M, Tajik AJ, Schaff HV, et al. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation. 1994;90:830–7.
Badhwar V, Peterson ED, Jacobs JP, et al. Longitudinal outcome of isolated mitral repair in older patients: Results from 14,604 procedures performed from 1991 to 2007. Ann Thorac Surg. 2012;94:1870–7 discussion 1877-9.
Le Tourneau T, Richardson M, Juthier F, et al. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart. 2010;96:1311–7.
Mentias A, Naji P, Gillinov AM, et al. Strain echocardiography and functional capacity in asymptomatic primary mitral regurgitation with preserved ejection fraction. J Am Coll Cardiol. 2016;68:1974–86.
Lancellotti P, Stainier PY, Lebois F, et al. Effect of dynamic left ventricular dyssynchrony on dynamic mitral regurgitation in patients with heart failure due to coronary artery disease. Am J Cardiol. 2005;96:1304–7.
Lancellotti P, Lebois F, Simon M, et al. Prognostic importance of quantitative exercise doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:I377–82.
Lancellotti P, Troisfontaines P, Toussaint AC, et al. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation. 2003;108:1713–7.
Magne J, Lancellotti P, Pierard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation. 2010;122:33–41.
Wunderlich NC, Siegel RJ. Peri-interventional echo assessment for the mitraclip procedure. Eur Heart J Cardiovasc Imaging. 2013;14:935–49.
Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the american society of echocardiography developed in collaboration with the society for cardiovascular angiography and interventions, Japanese society of echocardiography, and society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2019;32:431–75.
Komeda M, Koyama Y, Fukaya S, et al. Papillary heads “optimization” in repairing functional mitral regurgitation. J Thorac Cardiovasc Surg. 2012;144:1262–4.
Matsui Y, Shingu Y, Wakasa S, et al. Papillary muscle tugging approximation for functional mitral regurgitation. Ann Thorac Surg. 2019;107:e427–9.
Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2013;370:23–32.
Shingu Y, Yamada S, Ooka T, et al. Papillary muscle suspension concomitant with approximation for functional mitral regurgitation. Circ J. 2009;73:2061–7.
Arai H, Itoh F, Someya T, et al. New surgical procedure for ischemic/functional mitral regurgitation: mitral complex remodeling. Ann Thorac Surg. 2008;85:1820–2.
Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–18.
Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–306.
Pibarot P, Delgado V, Bax JJ. Mitra-fr vs. Coapt: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovascular Imaging. 2019;20:620–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical statements
There are no ethical issues to disclose (the article is not original research).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kagiyama, N., Shrestha, S. Echocardiographic assessment of mitral regurgitation. J Med Ultrasonics 47, 59–70 (2020). https://doi.org/10.1007/s10396-019-00971-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-019-00971-1