Abstract
Background
Definitive chemoradiotherapy (dCRT) for esophageal squamous cell carcinoma (ESCC) is a potentially curative treatment modality, even for patients with unresectable T4 tumors. For patients who fail dCRT, salvage esophagectomy is known to be a high-risk procedure. However, the efficacy and safety of salvage surgery for these patients remain unclear.
Methods
A total of 35 patients who underwent salvage esophagectomy after dCRT for initially unresectable locally advanced T4 ESCC were assessed, and both outcomes and prognostic factors after surgery were investigated.
Results
Among the study population, R0 resection was achieved in 19 patients (54.3%). Postoperatively, 8 patients (22.9%) experienced Clavien–Dindo grade IIIb or higher complications, and 3 patients (8.6%) registered surgery-related mortality. Overall survival rates were 45.7%, 28.6%, and 5.7% at 1, 2, and 5 years, respectively. In Cox regression analysis, residual or relapsed tumor limited to T2 or less was an independent prognostic factor for better survival (P = 0.010). On the other hand, postoperative pneumonia and incomplete resection were negative prognostic factors (P < 0.001 and P = 0.019, respectively). Nodal involvement and extent of lymph node dissection did not impact patient survival.
Conclusions
Although salvage esophagectomy for initially unresectable T4 ESCC is considered a high-risk surgery with poor prognosis, long-term survival may be achieved in patients with ≤ T2 residual tumors. In addition, R0 resection and postoperative pneumonia prevention are crucial to improve patient survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Definitive chemoradiotherapy (dCRT) for esophageal squamous cell carcinoma (ESCC) is a treatment modality that may provide a cure for patients, even for those with unresectable tumors. Ohtsu et al. previously reported that 25% of initial T4 ESCC patients achieved complete response (CR) with dCRT [1]. However, for patients who fail dCRT, salvage esophagectomy is the only modality to offer a chance of long-term survival.
Salvage esophagectomy after dCRT is associated with a high incidence of severe postoperative complications and surgery-related death [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. On the other hand, the efficacy of salvage surgery after dCRT for initially unresectable T4 ESCC patients is not yet clearly established [3, 6, 15]. The Esophageal Cancer Practice Guidelines 2017, edited by the Japan Esophageal Society weakly recommended not to perform salvage surgery for T4 ESCC patients showing residual disease after dCRT [16, 17]. However, in clinical practice, the surgical indication for such cases should be discussed based on the risk–benefit balance, as there is no other curative alternative for these patients.
In this study, the efficacy and safety of salvage esophagectomy after dCRT for initially unresectable T4 ESCC were investigated, and prognostic factors able to identify patients who were candidates for this type of surgery were investigated.
Materials and methods
Patients
From 1988 to 2016, 84 patients underwent salvage esophagectomy for ESCC at the Cancer Institute Hospital of Japanese Foundation for Cancer Research. Among them, 35 patients who had initially unresectable locally advanced T4 ESCC were included in this study. Patients’ medical records were reviewed, and clinicopathologic data were collected. The study protocol for this research project was approved by the Institutional Review Board of the referred institute.
Definition
Patient performance status was described based on the scoring system of the American Society of Anesthesiologists’ Performance Status (ASA-PS). Tumor stage was classified in accordance with the Union for International Cancer Control TNM Classification of Malignant Tumours, eighth edition [18]. Tumor invasion depth and surgical resectability were assessed using esophagogastroduodenoscopy and enhanced computed tomography (CT). Endoscopic ultrasonography, magnetic resonance imaging (MRI), and bronchoscopy were not routinely performed. In patients with possible T4, additional MRI or bronchoscopy were planned. As in the previous studies [19, 20], tracheobronchial invasion was defined by findings of the biopsy or macroscopic view with rigid encasement or indentation by bronchoscopy and CT, and aortic invasion was assumed if CT showed more than 90° contact, with obliteration of the fatty plane between the esophagus and the aorta on CT and MRI. Supraclavicular metastases, which were defined as distant metastases (M1) [18], were not excluded from surgical indications. All postoperative complications were clinically or radiologically diagnosed and classified according to the Clavien–Dindo (CD) grading system [21]. Among those, the incidences of pneumonia (≥ CD grade II), anastomotic leak (AL) (≥ CD grade II), recurrent laryngeal nerve palsy (≥ CD grade I), and severe complications (≥ CD grade IIIb) were investigated.
Treatment
In general, dCRT was given for initially unresectable locally advanced T4 ESCC, and the patient who fails dCRT was scheduled for salvage esophagectomy if curative resection was considered possible. Seven patients were treated with radiation therapy alone, and 28 patients underwent concurrent dCRT. Chemotherapeutic agents used in dCRT included 5-fluorouracil plus cisplatin in 27 patients and cisplatin alone in one patient. Radiation dose ranged from 50 to 70 Gy, with a median dose of 60 Gy. The surgical approach and the extent of lymph node dissection were decided by surgeons based on residual tumor and patient’s general condition. Regional lymph nodes were classified into groups 1–3 according to the Japanese Classification of Esophageal Cancer (11th edition), and the extent of lymph node dissection was described as follows: D0 (no or incomplete dissection of group 1 lymph nodes), D1 (complete dissection of group 1 lymph nodes but no or incomplete dissection of group 2 lymph nodes), and D2 (complete dissection of groups 1 and 2 lymph nodes but no or incomplete dissection of group 3 lymph nodes) [22].
Statistical analysis
All data were presented as median (range) or number (%). In survival analysis, overall and disease-specific survival (OS and DSS) were evaluated using the Kaplan–Meier method, and statistical difference was determined by the log-rank test. Cox proportional-hazards model was used to elucidate the impact of variables on survival, with results presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Significant variables in univariate analysis were included in further multivariate analysis. When the plural significant variables were not found in univariate analysis, further multivariate analysis was performed in a stepwise model including all listed variables. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 23.0; IBM-SPSS, Inc., Armonk, NY, USA).
Results
Patient background and surgical outcomes
Patient characteristics and surgical outcomes are summarized in Table 1. A total of 32 patients (91.4%) were male, and the median age of the study cohort was 61 years. Overall, 34 patients (97.1%) had residual tumors, and one patient had relapsed after CR. The estimated tumor depth immediately prior to salvage surgery was ≥ T3 (including T4a) in 29 patients (82.9%). Postoperatively, pneumonia and AL of CD grade II or higher and recurrent laryngeal nerve palsy of CD grade I or higher were observed in 10 (28.6%), 5 (14.3%), and 5 (14.3%) patients, respectively. Eight patients (22.9%) experienced severe, ≥ CD grade IIIb complications. Surgery-related mortality was observed in three patients (8.6%). One patient died of hemorrhage due to mediastinitis from anastomotic leak, one patient died of hemoptysis due to refractory pneumonia, and one patient died of respiratory failure from refractory pleuritis. R0 resection was accomplished in 19 (54.3%) patients. The detail of R1–R2 resection is shown in Table 2. The most common cause for R1–R2 resection was tracheal and/or bronchial invasion, followed by aortic invasion.
Patient survival and recurrence pattern
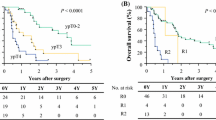
The Kaplan–Meier curves for OS and DSS are shown in Fig. 1. Median OS and DSS of all patients were 8.7 (95% CI 2.2–17.1) and 13.1 (95% CI 3.4–22.8) months, respectively. Both OS and DSS rates were 45.7%, 28.6%, and 5.7% at 1, 2, and 5 years, respectively. Regarding recurrence pattern in 19 patients with R0 resection, the most common pattern of recurrence was distant metastasis in 6, followed by resection margin and/or pleural dissemination in 5 patients. Regional lymph node recurrence was observed in 3 patients. In 6 patients with R1 resection, the most common pattern of recurrence was resection margin and/or pleural dissemination in 5 patients. Distant metastasis and regional lymph node recurrence were observed in each one patient.
Prognostic factors for patient survival
As shown in Table 3, pathological ≥ T3 tumors, postoperative pneumonia, and incomplete resection (R1–R2) were significant variables associated with poor OS and DSS in univariate Cox proportional-hazard analysis. Multivariate Cox analyses revealed that postoperative pneumonia and incomplete resection were independent prognostic factors for poor OS and DSS. Patients who experienced postoperative pneumonia had a significantly worse OS and DSS compared with those who did not (Fig. 2a, P < 0.001 and P = 0.001, respectively). Similarly, a significant OS and DSS benefit was observed in patients who underwent R0 resection compared with those with incomplete resection (Fig. 2b, P = 0.002 and P = 0.001, respectively).
Preoperative clinical factors predicting patient survival
Preoperative clinical factors predicting patient survival were also explored (Table 4). Univariate Cox proportional-hazard analysis revealed that preoperatively estimated ≥ T3 tumors (P = 0.010) were significant predictors of poorer OS, and preoperatively estimated ≥ T3 tumors (P = 0.019) and preoperative nodal involvement (P = 0.042) were significant predictors of poorer DSS. In multivariate Cox analyses, preoperative ≥ T3 tumors were independent prognostic factors for poor OS (P = 0.010). In Kaplan–Meier analysis (Fig. 2c), OS and DSS of patients with preoperatively estimated ≤ T2 tumors were significantly better than those of patients with preoperatively estimated ≥ T3 tumors (P = 0.004 and P = 0.009, respectively).
Significance of lymph node dissection extent among patients with R0 resection
As previously described, nodal involvement was not an independent factor influencing patient’s survival. The significance of lymph node dissection extent among the 19 patients who achieved R0 resection was assessed. OS and DSS were comparable between the seven patients who underwent D0 dissection and the 12 patients who underwent D1–D2 dissection (Fig. 3, P = 0.760 and P = 0.742, respectively).
Discussion
In this study, the clinical outcomes and prognostic factors of salvage esophagectomy after dCRT failure in patients with initially unresectable locally advanced T4 ESCC were analyzed. The prognosis of these patients was found to be unsatisfactory, and postoperative pneumonia and incomplete resection were identified as independent prognostic factors of poor outcomes. However, patients with residual or relapsed ≤ T2 tumors were able to achieve long-term survival with salvage esophagectomy. These results indicate that this high-risk surgery should only be performed in patients who achieved good response to dCRT and with remaining ≤ T2 tumors. In addition, it is essential to perform R0 resection and make every effort to prevent postoperative pneumonia.
In Japan, according to results from the Japan Clinical Oncology Group (JCOG) 0303 study, the standard treatment for initially unresectable locally advanced T4 ESCC patients is dCRT using 5-fluorouracil plus cisplatin in addition to 60 Gy irradiation [16, 17, 23]. dCRT is a potentially curative treatment modality for patients with initially unresectable locally advanced T4 ESCC, and salvage esophagectomy is virtually the only modality to rescue treatment failures. The JCOG 0303 study also showed that 16.9% of patients who received dCRT for initially unresectable locally advanced T4 ESCC underwent salvage surgery for residual or recurrent disease [23].
To date, only a few studies demonstrated the clinical outcomes of salvage surgery after dCRT for initially unresectable T4 ESCC [3, 6, 15]. An Italian study showed a 39.2% R0 resection rate and a 10.2% surgery-related mortality [6]. In addition, prognosis tended to be better in patients with R0 resection than in those with incomplete resection. Two Japanese studies also investigated this subject. Ikeda et al. reported a 92.3% R0 resection rate among 13 patients with incomplete response to dCRT [3]. Recently, Ohkura et al. reported a 42.4% R0 resection rate, 33.3% of postoperative complications (≥ CD grade IIIa), and no surgery-related mortality [15]. The authors also suggested that patients achieving R0 resection had a significantly better survival rate than those achieving only incomplete resection. The common finding among previous studies and the present one is that R0 resection is required to achieve long-term survival.
However, the accurate T4 diagnosis after dCRT could become more difficult, because the changes due to the treatment occurred. We mainly diagnosed the T category according to the CT finding of tumor thickness and the endoscopic finding of macroscopic tumor appearance. Regarding the diagnostic accuracy in this study, 5 of 6 (83.3%) patients with preoperative ≤ T2 tumors had pathological ≤ T2 tumors. Besides, 27 of 29 (93.1%) patients with preoperative ≥ T3 tumors had pathological ≥ T3 tumors. Therefore, the accuracy was 91.4% (32/35). However, we sometimes experience the cases in which the extent and the depth of tumor cannot be well identified in the fibrotic scar after dCRT. Occasionally, CT findings show the presence of significant esophageal wall thickening even when no tumor cells are present. Therefore, we take the extent of abnormal uptake by positron emission tomography–CT into account recently, although the further accumulation of data is required to evaluate the efficacy.
Salvage esophagectomy after dCRT is highly invasive and poses the risk of both postoperative complications and surgery-related death for patients [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Especially, severe and fatal complications which are specific to salvage surgery such as hemorrhagic event and radiotherapy-related complications could occur, as we experienced. Therefore, due to the lack of sufficient evidence of a survival benefit with salvage esophagectomy in this setting, surgical indication should be carefully decided based on the risk–benefit balance for patients.
From this study’s results, we consider that salvage esophagectomy should be performed in patients with a good response to dCRT, with patients with remaining tumors limited to T2 or less being good candidates. Actually, none of these patients failed in R1–R2 resection, whereas R0 resection rate was 44.8% in patients with preoperative ≥ T3 tumors. However, if we excluded such patients from the surgical indication, approximately half of these patients will lose the chance of cure. Therefore, we consider that non-curative surgery to some extent should be allowed in this situation.
To avoid postoperative complications, patient selection by evaluation of their tolerability to esophagectomy is mandatory. In addition, perioperative management to prevent postoperative pneumonia is essential. It has been suggested that recent progress in multidisciplinary perioperative management could reduce postoperative pneumonia [24]. The multidisciplinary care bundle would increase the safety of salvage surgery and could improve long-term outcomes.
The prognostic significance of prophylactic lymph node dissection in salvage surgery after dCRT remains unclear. Recently, Ohkura et al. suggested that standard lymph node dissection, including prophylactic dissection, could be safely performed and lead to improved survival in salvage esophagectomy for initially T4 patients. However, in this study, nodal involvement and the extent of dissection did not influence patient’s survival. We, therefore, consider that prophylactic dissection may be omitted in these patients when R0 resection is achieved.
This study has several limitations that should be addressed. First, it is a small, retrospective, observational study conducted in a single institution. Second, the study period was relatively long, and thus, the treatment strategy for initially unresectable T4 ESCC patients varied during the study period. Third, inter-evaluator variations in T category diagnosis of locally advanced esophageal cancer have been reported [25, 26]. Therefore, accurate clinical diagnosis and further prospective studies assessing a greater number of patients in similar situation are required.
Conclusions
Salvage surgery after dCRT for initially unresectable T4 ESCC patients is a high-risk surgery, with an associated unsatisfactory prognosis. This high-risk surgery should only be performed in good responders to dCRT, with patients with remaining tumors limited to ≤ T2 being good candidates. In addition, R0 resection and postoperative pneumonia prevention are essential to improve patient survival.
References
Ohtsu A, Boku N, Muro K, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–21.
Meunier B, Raoul J, Le Prise E, et al. Salvage esophagectomy after unsuccessful curative chemoradiotherapy for squamous cell cancer of the esophagus. Dig Surg. 1998;15:224–6.
Ikeda K, Ishida K, Sato N, et al. Chemoradiotherapy followed by surgery for thoracic esophageal cancer potentially or actually involving adjacent organs. Dis Esophagus. 2001;14:197–201.
Swisher SG, Wynn P, Putnam JB, et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg. 2002;123:175–83.
Tomimaru Y, Yano M, Takachi K, et al. Factors affecting the prognosis of patients with esophageal cancer undergoing salvage surgery after definitive chemoradiotherapy. J Surg Oncol. 2006;93:422–8.
de Manzoni G, Pedrazzani C, Pasini F, et al. Chemoradiotherapy followed by surgery for squamous cell carcinoma of the thoracic esophagus with clinical evidence of adjacent organ invasion. J Surg Oncol. 2007;95:261–6.
Oki E, Morita M, Kakeji Y, et al. Salvage esophagectomy after definitive chemoradiotherapy for esophageal cancer. Dis Esophagus. 2007;20:301–4.
Smithers BM, Cullinan M, Thomas JM, et al. Outcomes from salvage esophagectomy post definitive chemoradiotherapy compared with resection following preoperative neoadjuvant chemoradiotherapy. Dis Esophagus. 2007;20:471–7.
Miyata H, Yamasaki M, Takiguchi S, et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol. 2009;100:442–6.
Takeuchi H, Saikawa Y, Oyama T, et al. Factors influencing the long-term survival in patients with esophageal cancer who underwent esophagectomy after chemoradiotherapy. World J Surg. 2010;34:277–84.
Markar S, Gronnier C, Duhamel A, et al. Salvage surgery after chemoradiotherapy in the management of esophageal cancer: is it a viable therapeutic option? J Clin Oncol. 2015;33:3866–73.
Watanabe M, Mine S, Nishida K, et al. Salvage esophagectomy after definitive chemoradiotherapy for patients with esophageal squamous cell carcinoma: who really benefits from this high-risk surgery? Ann Surg Oncol. 2015;22:4438–44.
Hayami M, Watanabe M, Ishizuka N, et al. Prognostic impact of postoperative pulmonary complications following salvage esophagectomy after definitive chemoradiotherapy. J Surg Oncol. 2018;117:1251–9.
Kiyozumi Y, Yoshida N, Ishimoto T, et al. Prognostic factors of salvage esophagectomy for residual or recurrent esophageal squamous cell carcinoma after definitive chemoradiotherapy. World J Surg. 2018;42:2887–93.
Ohkura Y, Ueno M, Iizuka T, et al. Prognostic factors and appropriate lymph node dissection in salvage esophagectomy for locally advanced T4 esophageal cancer. Ann Surg Oncol. 2019;26:209–16.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16:25–43.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24.
Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors: International union against cancer. 8th ed. Oxford: Wiley; 2017.
Noguchi T, Moriyama H, Wada S, et al. Resection surgery with neoadjuvant chemoradiotherapy improves outcomes of patients with T4 esophageal carcinoma. Dis Esophagus. 2003;16:94–8.
Fujita H, Sueyoshi S, Tanaka T, et al. Prospective non-randomized trial comparing esophagectomy-followed-by-chemoradiotherapy versus chemoradiotherapy-followed-by-esophagectomy for T4 esophageal cancers. J Surg Oncol. 2005;90:209–19.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1–36.
Shinoda M, Ando N, Kato K, et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci. 2015;106:407–12.
Watanabe M, Okamura A, Toihata T, et al. Recent progress in perioperative management of patients undergoing esophagectomy for esophageal cancer. Esophagus. 2018;15:160–4.
Hamamoto Y, Nojima M, Aoki Y, et al. Inter-evaluator heterogeneity of clinical diagnosis for locally advanced esophageal squamous cell carcinoma. Esophagus. 2017;14:324–32.
Yokota T, Yasuda T, Kato H, et al. Concordance of clinical diagnosis of T classification among physicians for locally advanced unresectable thoracic esophageal cancer. Int J Clin Oncol. 2018;23:73–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All procedures were performed in accordance with the ethical standards of our institutional review board and with the Helsinki Declaration of 1964 and later versions. All authors followed the policy concerning Informed Consent.
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okamura, A., Hayami, M., Kozuki, R. et al. Salvage esophagectomy for initially unresectable locally advanced T4 esophageal squamous cell carcinoma. Esophagus 17, 59–66 (2020). https://doi.org/10.1007/s10388-019-00700-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-019-00700-0