Abstract
Background
The aim of this study was to confirm prognostic factors for salvage esophagectomy for remnant or recurrent esophageal squamous cell carcinoma after definitive chemoradiotherapy.
Study design
We retrospectively analyzed clinicopathological backgrounds of 50 patients who underwent salvage esophagectomy between April 2005 and January 2016. Salvage esophagectomy comprised 40 three-incision esophagectomies, two transhiatal esophagectomies and eight pharyngolaryngoesophagectomies. Independent prognostic factors for overall survival were assessed using Cox regression analysis of the factors.
Results
Salvage esophagectomy remains a highly invasive surgery and correlated with a higher incidence of all morbidities of Clavien–Dindo classification (CDc) ≥II, severe morbidities of CDc ≥ IIIb, any pulmonary morbidities and chylorrhea, compared with those in patients without preoperative definitive chemoradiotherapy. Cox regression analysis suggested that R0 resection (hazard ratio [HR] 6.39; 95% confidence interval [CI] 2.03–9.68, P = 0.002), absence of severe complications (HR 4.97; 95% CI 1.70–14.81, P = 0.004) and early pStage (0–II) (HR 3.42; 95% CI 1.24–10.12, P = 0.018) were independent prognostic factors for salvage esophagectomy.
Conclusions
Salvage esophagectomy remains correlated with a high incidence of postoperative complications. Avoiding non-curative surgery and reducing the incidence of severe postoperative complications are important if patients are to receive prognostic benefit of this highly invasive surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is the sixth leading cause of cancer-related deaths [1]. Despite advances in multimodal therapies, esophageal cancer remains a refractory malignancy.
Chemoradiotherapy (CRT) is one of the primary treatment tools for esophageal cancer and is accepted as a potent therapy for cure. Notably, squamous cell carcinoma, which is the dominant histological type of esophageal cancer in East Asia, has a high radiosensitivity. Even for cT4 stage esophageal cancer, CRT can achieve a complete response (CR) of 17–52% of patients [2,3,4,5,6,7,8,9]. However, a suitable treatment strategy for remnant or recurrent esophageal squamous cell carcinoma (ESCC) after definitive CRT (dCRT) remains unestablished.
Salvage esophagectomy is effective and is frequently considered for these cases. Nevertheless, salvage esophagectomy is also associated with a high incidence of postoperative severe morbidity and surgery-related mortality [10,11,12,13,14,15,16,17,18,19,20,21]. Therefore, identifying the subset of patients who would obtain prognostic benefit from salvage esophagectomy is of considerable importance. In the current study, we clarify the independent prognostic factors for salvage esophagectomy, which may help to determine which patients should undergo or avoid this highly invasive surgery.
Materials and methods
Patients
A CONSORT diagram of the current study is presented in Fig. 1. A total of 277 consecutive patients with ESCC underwent dCRT at the Department of Gastroenterological Surgery, Kumamoto University, between April 2005 and January 2016. Of these patients, 126 (45.5%) achieved a clinical CR, whereas 151 (54.5%) did not. Of those patients with clinical CR, 33 suffered recurrence. In the current study, there was no significant difference between remnant and recurrence cases in median overall survival (OS). Thus, we analyzed remnant and recurrent cases together. Finally, of the 184 patients with residual or recurrent ESCC after dCRT, 50 patients who underwent salvage esophagectomy were eligible. Tumor stage was classified according to the Union Internationale Contre le Cancer TNM staging, version 7 [22]. This study included eight stage IV patients with supraclavicular lymph node metastasis. The Institutional Ethics Committee approved this study (Registry Number 991). Documented comprehensive consent was obtained from all patients.
Chemoradiotherapy
In accordance with a previous report, dCRT was defined in the present study as a CRT of ≥ 50.4 Gy radiation [23]. The methods and anticancer agents of dCRT varied over time. Between April 2005 and June 2008, a low-dose FP regimen, which consisted of low-dose cisplatin (CDDP) and fluorouracil (5-FU), combined with concomitant radiation therapy, was principally administered. Between July 2008 and January 2016, two cycles of the FP regimen or two cycles of the DCF regimen, consisting of CDDP, 5-FU and docetaxel, together with concomitant radiation therapy were principally administered. The low-dose FP regimen consisted of 4 mg/m2/h of CDDP and 200 mg/m2/24 h of 5-FU given by a continuous intravenous (i.v.) infusion on days 1–5 and repeated weekly during radiotherapy. The FP regimen consisted of 80 mg/m2/2 h of CDDP bolus on day 1 and 800 mg/m2/24 h of 5-FU given via a continuous i.v. infusion on days 1 to 5. The DCF regimen consisted of 60 mg/m2 of docetaxel bolus for 1 h on day 1, 350 mg/m2 of 5-FU given via a continuous i.v. infusion and 6 mg/m2 of CDDP given via an i.v. infusion on days 1–5. After 4 weeks of chemotherapy, the next cycle of chemotherapy was initiated. Concomitantly, 50.4–70 Gy of radiation therapy was delivered with megavoltage equipment (6–10 MV) using an opposing portal or multiple field irradiation techniques.
Surgery
Salvage esophagectomy was defined as a surgical procedure for patients with residual or recurrent ESCC after dCRT. There was no difference in occurrence of any postoperative morbidity, severe morbidity or OS between salvage esophagectomy (SLE) and salvage pharyngolaryngoesophagectomy (SLPLE) (Supplementary Figure 1 and Supplementary Table 1). Thus, in the current study, salvage esophagectomy comprised 40 three-incision esophagectomies (from neck, chest and abdomen approach), two transhiatal esophagectomies and eight pharyngolaryngoesophagectomies. All patients had an Eastern Cooperative Oncology Group performance status of 0–2. Lymph node dissection was performed only for lymph nodes that were swollen or suspected to have recurrence. Prophylactic extended lymphadenectomy was not performed. In the perioperative periods, we performed management to prevent morbidities to all patients according to the enhanced recovery after surgery (ERAS) program including preoperative smoking cessation, respiratory rehabilitation [24]. At the start of surgery, methylprednisolone, continuous neutrophil elastase inhibitor and every 3 h antibiotics were also administered to all patients [25]. Minimally invasive esophagectomy by a thoracoscopic technique was not adopted for any of the patients.
Definition of morbidity
Postoperative morbidities were defined in accordance with the risk-adjusted morbidity and mortality for esophagectomy for cancer proposed in the Society of Thoracic Surgeons General Thoracic Surgery Database [26]. The details of each of the morbidities are described in our past reports [27]. In our study, the Clavien–Dindo classification system (CDc) was used to describe morbidity, with a CDc score ≥ II used as a cutoff for the presence of morbidity [28]. A CDc score ≥ IIIb was indicative of a severe morbidity requiring surgical, endoscopic or radiological intervention, under general anesthesia.
Statistical methods
Statistical analyses were performed using JMP (version 10; SAS Institute, Cary, NC, USA). Differences in clinicopathological features were determined using Student’s t test. Survival rates after salvage surgery were calculated by the Kaplan–Meier method, and statistical significance was determined using the log-rank test. Overall survival (OS) was defined as the time from salvage surgery to death from any cause. Regarding OS, univariate analyses were performed, including age, sex, body mass index, performance status, tumor location, cT classification, cN classification, cM classification, pretreatment cStage, combined chemotherapy regimen, efficacy of dCRT, indication for surgery, operative procedure, operative time, bleeding, presence of severe postoperative complications with CDc ≥ IIIb, pStage and surgical curability. When a probability level ≤0.1 was identified, the factor was included in subsequent multivariate analysis. A multivariate analysis, using the Cox proportional hazards model, was adopted to identify independent prognostic factors. A P value of <0.05 was considered an indication of statistical significance.
Results
Short-term outcomes
We initially investigated short-term outcome after esophagectomy according to preoperative treatment (Supplemental Table 2). Salvage esophagectomy was associated with greater blood loss. It also correlated with a higher incidence of any morbidity of CDc ≥ II (58 vs 38%, P < 0.001), severe morbidities of CDc ≥ IIIb (24 vs 12%, P = 0.027), any pulmonary morbidities (34 vs 17%, P = 0.004) and chylorrhea (12 vs 2%, P < 0.001). In this study, there were no surgery-related deaths in the salvage esophagectomy group.
Survival
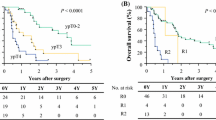
Table 1 presents univariate analyses of median OS after salvage esophagectomy. Figure 2 shows the Kaplan–Meier curves of median OS according to representative significant factors. The OS rate was significantly lower in patients with R1 and R2 resection than with R0 resection (0 vs. 44.4%, P < 0.001). It was also lower in patients with severe postoperative morbidities of CDc ≥ IIIb (0 vs. 46.2%, P = 0.014). In addition, the rate was lower in patients with pStage III–IV than with pStage 0–II (12.1 vs. 56.1%, P < 0.001). Median follow-up time was 406 (22–3317) days. There were no significant differences regarding age (divided by median), sex, primary tumor location, preoperative cT, cN or cM classification, type of chemotherapy regimen, efficiency of dCRT or indication for surgery. Supplementary Figure 2 shows the Kaplan–Meier curves of cancer-specific survival according to the same factors. The cancer-specific survival rate was significantly lower in patients with R1 and R2 resection, and pStage III–IV. However, there were no significant differences in the presence of severe postoperative morbidity. Table 2 presents the results of multivariate analyses of the independent factors related to median OS after salvage esophagectomy. Cox regression analysis indicated that R0 resection (hazard ratio [HR] 6.39; 95% confidence interval [CI] 2.034–19.68, P = 0.002), absence of severe complications (HR 4.97; 95% CI 1.699–14.81, P = 0.004) and early pStage (0–II) (HR 3.42; 95% CI 1.235–10.12, P = 0.018) were independent prognostic factors after salvage esophagectomy.
Discussion
In the current study, we initially reconfirmed that salvage esophagectomy remains correlated with a higher incidence of postoperative morbidities, despite recent advances in postoperative management. In addition, we demonstrated that patients with R1–2 resection, severe morbidity of CDc ≥ IIIb and advanced pStage (III–IV) are associated with significantly poorer survival.
Definitive CRT is clearly a potent therapy for cure in ESCC of various stages. In stage I ESCC, dCRT is considered an equivalent treatment to surgery with a 5-year OS rate of approximately 80% [29]. Definitive CRT for locally advanced ESCC is also effective: According to the latest ESMO guidelines, both dCRT and neoadjuvant chemoradiotherapy (NACRT) are recommended as a first treatment for locally advanced ESCC [30]. Even in T4 stage ESCC, dCRT showed a reasonable CR rate of 17–52% [3,4,5,6,7,8,9]. The NCCN guidelines also classify dCRT as one of the treatment options for stages I–IV esophageal cancer [31]. However, strategy for remnant and recurrent cancer after dCRT remains unestablished so far.
Treatment for remnant and recurrent ESCC after dCRT is an unresolved clinical problem. Although salvage esophagectomy is one of the possible treatment strategies for a cure, postoperative morbidity and mortality are common [16]. In the current study, salvage esophagectomy remained associated with a high incidence of any morbidities, severe morbidities, any pulmonary morbidities and chylorrhea. Thus, we consider it important to clarify which patients would benefit in terms of survival from this highly invasive surgery [32].
We demonstrated here that curative non-R0 resection was an independent factor for a unfavorable prognosis (both OS and CSS) after salvage surgery. Our finding is in agreement with the results of the study by Tomimaru et al. [33], which also identified curative resection as the strongest prognostic factor after salvage esophagectomy. Morita et al. [16] and Watanabe et al. [21] also demonstrated that incomplete resection was an independent unfavorable prognosis factor. These results indicate that salvage surgery for volume reduction or to control symptoms is ineffective. Salvage surgery for borderline resectable ESCC, with a high probability of incomplete resection, should be carefully considered. However, determination of the probability of a curative resection of cancer is frequently difficult, especially when it was initially at the cT4 stage. From the current result, we considered it important to preoperatively foresee a probability of incomplete resection. Univariate analysis of R0 resection suggested that pretreatment clinical advanced stage and poor effectiveness in dCRT (SD/PD) were associated with subsequent non-curative surgery (Supplementary Table 3). Thus, indication of salvage esophagectomy should discreetly be considered against patients with poor effectiveness in dCRT. Watanabe et al. [33] previously reported that cT1–2 tumors, initially resectable tumors, ycT1–2 tumors and relapse after CR were candidate for predictors for R0 resection. We consider further investigation with a larger cohort is necessary to establish predictive factors to estimate R0 resection in salvage esophagectomy, as it is clinically important to elucidate the stratum of patients to avoid this highly invasive surgery.
The incidence of postoperative severe complications of CDc ≥ IIIb was also an independent factor for poor prognosis after salvage surgery. A correlation between postoperative complications and poor prognosis was previously reported in non-salvage surgery for esophageal cancer [34] and other gastrointestinal cancers [35,36,37]. Postoperative complications cause prolonged inflammation and produce a number of inflammatory cytokines, which can induce tumor cell proliferation at micrometastasis [38, 39]. Notably, because salvage surgery for esophageal cancer correlated with frequent severe morbidities, various precautions and careful postoperative monitoring are extremely important. Preoperative smoking cessation [40], respiratory rehabilitation [41], maintaining oral hygiene [42], nutritional support during surgery [27] and perioperative administration of steroids [43] and neutrophil elastase inhibitor [44] could be strong candidates contributing to the reduction in morbidities. Analyses for the risk factors of severe morbidity in the present cases suggested that low serum albumin level and baseline cN factor were the candidates (Supplementary Table 4). In addition, abnormality of pretreatment C-reactive protein and great bleeding during surgery could also be the candidates (data not shown). Thus, patients who met those parameters should be carefully observed after salvage surgery.
Our study had some limitations. Firstly, this is a retrospective study conducted at a single institute and the sample size was insufficient large. Thus, we propose that a multicenter study with a larger cohort should be performed to establish definitive prognostic factors for salvage esophagectomy. Secondly, the period of the present study is rather long, which could give rise to bias with respect to treatment strategy and instruments for diagnosis and surgery. Thirdly, we analyzed the data which combined SLE and SLPLE in present study, although we showed short-term and long-term outcomes of both strategies were similar in the current study. Thus, we should accumulate cases and show each surgical outcome in the future.
In conclusion, salvage esophagectomy remains highly invasive associated with a high incidence of postoperative morbidities. Avoiding surgery in patients with a low probability of curative resection and preventing severe postoperative complications are important for improving the prognosis of salvage esophagectomy for ESCC.
References
Parkin DM, Bray F (2009) Evaluation of data quality in the cancer registry: principles and methods part II. Completeness. Eur J Cancer 45:756–764
Makino T, Doki Y (2011) Treatment of T4 esophageal cancer. Definitive chemo-radiotherapy vs chemo-radiotherapy followed by surgery. Ann Thorac Cardiovasc Surg 17:221–228
Higuchi K, Komori S, Tanabe S et al (2014) Definitive chemoradiation therapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) in advanced esophageal cancer: a phase 2 trial (KDOG 0501-P2). Int J Radiat Oncol Biol Phys 89:872–879
Seto Y, Chin K, Gomi K et al (2007) Treatment of thoracic esophageal carcinoma invading adjacent structures. Cancer Sci 98(937–94):2
Fujita H, Sueyoshi S, Tanaka T et al (2005) Esophagectomy: is it necessary after chemoradiotherapy for a locally advanced T4 esophageal cancer? Prospective nonrandomized trial comparing chemoradiotherapy with surgery versus without surgery. World J Surg 29:25–30. https://doi.org/10.1007/s00268-004-7590-2 (discussion 30–21)
Kaneko K, Ito H, Konishi K et al (2003) Definitive chemoradiotherapy for patients with malignant stricture due to T3 or T4 squamous cell carcinoma of the oesophagus. Br J Cancer 88:18–24
Nishimura Y, Suzuki M, Nakamatsu K et al (2002) Prospective trial of concurrent chemoradiotherapy with protracted infusion of 5-fluorouracil and cisplatin for T4 esophageal cancer with or without fistula. Int J Radiat Oncol Biol Phys 53:134–139
Itoh Y, Fuwa N, Matsumoto A et al (2001) Outcomes of radiotherapy for inoperable locally advanced (T4) esophageal cancer-retrospective analysis. Radiat Med 19:231–235
Miyazaki T, Sohda M, Tanaka N et al (2015) Phase I/II study of docetaxel, cisplatin, and 5-fluorouracil combination chemoradiotherapy in patients with advanced esophageal cancer. Cancer Chemother Pharmacol 75:449–455
Smithers BM, Cullinan M, Thomas JM et al (2007) Outcomes from salvage esophagectomy post definitive chemoradiotherapy compared with resection following preoperative neoadjuvant chemoradiotherapy. Dis Esophagus 20(471–47):7
Nishimura M, Daiko H, Yoshida J et al (2007) Salvage esophagectomy following definitive chemoradiotherapy. Gen Thorac Cardiovasc Surg 55(461–46):4 (discussion 464–465)
Chao YK, Chan SC, Chang HK et al (2009) Salvage surgery after failed chemoradiotherapy in squamous cell carcinoma of the esophagus. Eur J Surg Oncol 35(289–29):4
Miyata H, Yamasaki M, Takiguchi S et al (2009) Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol 100(442–44):6
Tachimori Y, Kanamori N, Uemura N et al (2009) Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 137(49–5):4
Takeuchi H, Saikawa Y, Oyama T et al (2010) Factors influencing the long-term survival in patients with esophageal cancer who underwent esophagectomy after chemoradiotherapy. World J Surg 34(277–28):4. https://doi.org/10.1007/s00268-009-0331-9
Morita M, Kumashiro R, Hisamatsu Y et al (2011) Clinical significance of salvage esophagectomy for remnant or recurrent cancer following definitive chemoradiotherapy. J Gastroenterol 46(1284–129):1
Marks JL, Hofstetter W, Correa AM et al (2012) Salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma. Ann Thorac Surg 94(1126–113):2 (discussion 1132–1123)
Yoo C, Park JH, Yoon DH et al (2012) Salvage esophagectomy for locoregional failure after chemoradiotherapy in patients with advanced esophageal cancer. Ann Thorac Surg 94(1862–186):8
Schieman C, Wigle DA, Deschamps C et al (2013) Salvage resections for recurrent or persistent cancer of the proximal esophagus after chemoradiotherapy. Ann Thorac Surg 95(459–46):3
Markar S, Gronnier C, Duhamel A et al (2015) Salvage surgery after chemoradiotherapy in the management of esophageal cancer: is it a viable therapeutic option? J Clin Oncol 33:3866–3873
Watanabe M, Mine S, Nishida K et al (2015) Salvage esophagectomy after definitive chemoradiotherapy for patients with esophageal squamous cell carcinoma: who really benefits from this high-risk surgery? Ann Surg Oncol 22:4438–4444
Edge SB, Compton CC (2010) The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Ajani JA, Winter K, Komaki R et al (2008) Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol 26:4551–4556
Findlay JM, Gillies RS, Millo J et al (2014) Enhanced recovery for esophagectomy: a systematic review and evidence-based guidelines. Ann Surg 259(413–43):1
Nagai Y, Watanabe M, Baba Y et al (2013) Preventive effect of sivelestat on postoperative respiratory disorders after thoracic esophagectomy. Surg Today 43(361–36):6
Raymond DP, Seder CW, Wright CD et al (2016) Predictors of major morbidity or mortality after resection for esophageal cancer: a society of thoracic surgeons general thoracic surgery database risk adjustment model. Ann Thorac Surg 102(207–21):4
Yoshida N, Baba Y, Shigaki H et al (2016) Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg 40:1910–1917. https://doi.org/10.1007/s00268-016-3549-3
Dindo D, Demartines N (2004) Clavien PA classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Kato H, Sato A, Fukuda H et al (2009) A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan clinical oncology group study (JCOG9708). Jpn J Clin Oncol 39:638–643
Lordick F, Mariette C, Haustermans K et al (2016) Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v50–v57
Esophageal Cancer Treatment (PDQ(R)): Health Professional VersionPDQ Cancer Information Summaries, Bethesda (MD) (2002)
Farinella E, Safar A, Nasser HA et al (2016) Salvage esophagectomy after failure of definitive radiochemotherapy for esophageal cancer. J Surg Oncol 114(833–83):7
Tomimaru Y, Yano M, Takachi K et al (2006) Factors affecting the prognosis of patients with esophageal cancer undergoing salvage surgery after definitive chemoradiotherapy. J Surg Oncol 93(422–42):8
Ott K, Bader FG, Lordick F et al (2009) Surgical factors influence the outcome after Ivor-Lewis esophagectomy with intrathoracic anastomosis for adenocarcinoma of the esophagogastric junction: a consecutive series of 240 patients at an experienced center. Ann Surg Oncol 16:1017–1025
Kubota T, Hiki N, Sano T et al (2014) Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 21(891–89):8
Krarup PM, Nordholm-Carstensen A, Jorgensen LN et al (2014) Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg 259(930–93):8
Tevis SE, Kohlnhofer BM, Stringfield S et al (2013) Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum 56(1339–134):8
D’Journo XB, Michelet P, Marin V et al (2010) An early inflammatory response to oesophagectomy predicts the occurrence of pulmonary complications. Eur J Cardiothorac Surg 37(1144–115):1
Okamura A, Takeuchi H, Matsuda S et al (2015) Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann Surg Oncol 22(3130–313):5
Yoshida N, Baba Y, Hiyoshi Y et al (2016) Duration of Smoking cessation and postoperative morbidity after esophagectomy for esophageal cancer: how long should patients stop smoking before surgery? World J Surg 40:142–147. https://doi.org/10.1007/s00268-015-3236-9
Yamana I, Takeno S, Hashimoto T et al (2015) Randomized controlled study to evaluate the efficacy of a preoperative respiratory rehabilitation program to prevent postoperative pulmonary complications after esophagectomy. Dig Surg 32(331–33):7
Akutsu Y, Matsubara H, Shuto K et al (2010) Pre-operative dental brushing can reduce the risk of postoperative pneumonia in esophageal cancer patients. Surgery 147(497–50):2
Gao Q, Mok HP, Wang WP et al (2014) Effect of perioperative glucocorticoid administration on postoperative complications following esophagectomy: a meta-analysis. Oncol Lett 7(349–35):6
Kawahara Y, Ninomiya I, Fujimura T et al (2010) Prospective randomized controlled study on the effects of perioperative administration of a neutrophil elastase inhibitor to patients undergoing video-assisted thoracoscopic surgery for thoracic esophageal cancer. Dis Esophagus 23(329–33):9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiyozumi, Y., Yoshida, N., Ishimoto, T. et al. Prognostic Factors of Salvage Esophagectomy for Residual or Recurrent Esophageal Squamous Cell Carcinoma After Definitive Chemoradiotherapy. World J Surg 42, 2887–2893 (2018). https://doi.org/10.1007/s00268-018-4536-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4536-7