Abstract
Purpose
To compare the topographic characteristics of myopic and nonmyopic disc hemorrhage (DH) in primary open-angle glaucoma.
Methods
Patients were assigned to the myopic DH group (spherical equivalent of −1.0 diopters or less) or to the nonmyopic DH group (emmetropia and hyperopia). DH was classified as lamina cribrosa-, cup margin-, disc rim-, or peripapillary-type DH according to its proximal location. The DH types of the two groups were compared using Fisher's exact test. Multivariate logistic regression was used to evaluate the factors associated with myopia.
Results
Thirty-four eyes were assigned to the myopic DH group and 42 eyes to the nonmyopic DH group. A significantly higher proportion (32.4 %) of lamina cribrosa-type DH was found in the myopic DH group than in the nonmyopic DH group (4.8 %; P = 0.008). Eyes with lamina cribrosa-type DH were 12.59 times more likely to be myopic than were eyes with peripapillary-type DH (95 % CI: 1.22–129.53; P = 0.033).
Conclusions
Lamina cribrosa-type DH was significantly more common in myopic eyes than in nonmyopic eyes. This result suggests that the pathogenesis of DH may differ between myopic DH and nonmyopic DH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disc hemorrhage (DH) is a prominent feature of glaucoma and may be a sign of progressive damage of the retinal nerve fiber layer [1, 2], leading to functional deterioration of the visual field [3]. The Collaborative Normal Tension Glaucoma Study and the Early Manifest Glaucoma Trial found that glaucomatous eyes with DH experienced a significantly higher rate of visual field deterioration than did eyes without hemorrhage [4, 5]. Although the pathogenesis of DH remains unclear, DH may indicate stress of the optic nerve in the region of the DH [6].
Myopic individuals are more likely to have tilted, rotated, and larger discs as well as other optic disc abnormalities [7, 8], and myopia has been recognized as a risk factor for the development of open-angle glaucoma [9–12]. A thinner lamina cribrosa in combination with secondary enlargement of the optic disc in high myopia may play a role in the pathogenesis of glaucoma [13–15].
Both myopia and DH are important in the pathogenesis of glaucoma. However, few studies have assessed correlations between myopia and DH. According to our clinical observations, DHs have different characteristics in myopic and nonmyopic eyes; therefore, we speculated that a myopic disc affects the features of DH. The purpose of this study was to compare the topographic characteristics of myopic and nonmyopic DH in primary open-angle glaucoma.
Materials and methods
The study followed the tenets of the Declaration of Helsinki and was approved by the institutional review board/ethnics committee of the Seoul National University Hospital. We retrospectively reviewed the medical records of patients diagnosed as having primary open-angle glaucoma at the glaucoma clinic of the Department of Ophthalmology, Seoul National University Hospital, between October 2005 and November 2011. Initial ophthalmologic examinations included visual acuity and refraction measurements, evaluations of the anterior segment, gonioscopy, and funduscopy by direct ophthalmoscopy. In addition, all patients received a glaucoma evaluation, including disc stereophotography, red-free retinal nerve fiber layer (RNFL) photography, and perimetry. Visual fields were evaluated with the 30-2 program in the Humphrey Visual Field Analyzer (model 750; Carl Zeiss Meditec, Dublin, CA, USA). The patients’ medical histories were reviewed to determine the presence of associated systemic diseases such as diabetes and systemic hypertension.
Patients were considered to have primary open-angle glaucoma if they showed glaucomatous optic disc changes that matched typical glaucomatous visual field defects not attributable to other ocular or systemic pathologies, as well as an open angle on gonioscopy. Glaucomatous optic neuropathy was defined as cup/disc asymmetry of greater than 0.2 between fellow eyes, rim thinning or notching, or a RNFL defect. DH was identified as an isolated blot or a splinter hemorrhage seen on the optic disc or in the adjacent peripapillary retina. Patients with the following alternative causes of hemorrhage were excluded from the study: ischemic optic neuropathy, papillitis, retinal vascular occlusive disease, diabetic retinopathy, or posterior vitreous detachment.
Disc stereophotography
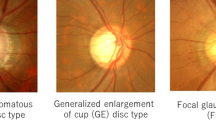
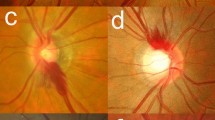
Disc stereophotographs were obtained using a fundus camera (VX-10; Kowa Company, Tokyo, Japan) after pupil dilation with 0.5 % tropicamide and 0.5 % phenylephrine hydrochloride. Eyes with more than one simultaneous DH at different locations were excluded from the study. When both eyes met the inclusion criteria, one eye was randomly selected. The observers described the position of the DH in terms of the proximal and quadrant locations. Eyes with DH were divided into four types according to the proximal location (lamina cribrosa, cup margin, disc rim, and peripapillary types) as well as the quadrant location (inferotemporal, superotemporal, inferonasal, and superonasal; Fig. 1). The proximal and quadrant locations of the DH were evaluated by masked assessment of the disc stereophotographs. Two glaucoma specialists (HSK and JP) evaluated the stereoscopic optic disc photographs, and each grader was masked to the patient’s clinical information and test results. Discrepancies between the two observers were resolved by consensus. The DH patients were divided into two groups according to the spherical equivalent: myopic DH (−1.0 diopter or less) and nonmyopic DH (emmetropia and hyperopia) groups.
Visual field testing
Visual field testing was performed by static automated white-on-white threshold perimetry (SITA Standard 30-2, Humphrey Field Analyzer II; Carl Zeiss Meditec). Glaucomatous visual field loss was defined as the presence of three or more significant (P < 0.05) nonedge contiguous points with at least one point with a probability value of less than 0.01 on the same side of the horizontal meridian in a pattern deviation plot confirmed at a minimum of two consecutive examinations. A visual field was defined as reliable when fixation losses and false-positive and false-negative errors were less than 20 %. The perimetric results within 3 months at the time of the first detected DH were included for evaluation.
Data analysis
All statistical analyses were performed with SPSS version 18.0 (SPSS, Chicago, IL, USA). Categorical variables were compared by using the chi-square or Fisher's exact test. The t test was used to assess differences in age, spherical equivalent, central corneal thickness, mean deviation, and pattern standard deviation. On subgroup analysis, the spherical equivalents were compared using the Kruskal–Wallis test and the Tukey test with ranks (post hoc test). Probability values of less than 0.05 were considered significant. Multivariate logistic regression with forward stepwise selection while controlling for all confounding variables was used to evaluate the factors associated with myopia.
Results
A total of 180 eyes of 180 patients with primary open-angle glaucoma with DH were included in the study. Ninety-six eyes were excluded because of missing data, such as the spherical equivalent or perimetry; five had multiple simultaneous DHs, and three had diabetic retinopathy. Therefore, the remaining 76 eyes from 76 patients with primary open-angle glaucoma with DH were used for further analysis in the study. Thirty-four eyes were assigned to the myopic DH group and 42 eyes to the nonmyopic DH group. All the eyes enrolled were phakic eyes. The basic patient demographics and characteristics are presented in Table 1. The mean age of the patients was 52.0 ± 13.0 years (range, 26–78 years) in the myopic group and 65.7 ± 6.1 years (range, 51–78 years) in the nonmyopic group (P < 0.001). The groups did not differ in gender or systemic comorbidities, i.e., diabetes (P = 0.167, Fisher's exact test) and systemic hypertension (P = 0.955, chi-square test). Most patients in both groups were diagnosed as having normal tension glaucoma (79.4 and 81.0 % of the patients in the myopic and nonmyopic groups, respectively). The myopic and nonmyopic DH groups differed significantly with respect to age (P < 0.001, t test), pattern standard deviation (P = 0.003, t test), and mean deviation (P = 0.052, t test).
Regardless of the group, most of the DHs (79.4 and 61.9 % of the patients in the myopic and nonmyopic groups, respectively) occurred in the inferotemporal sector (Table 2). The proximal location of the DH differed significantly between the two groups (P = 0.008, Fisher's exact test), whereas the quadrant location of the DH showed a borderline difference between the two groups (P = 0.082, Fisher's exact test): lamina cribrosa-type DHs were significantly more common in the myopia group (32.4 %) than in the nonmyopia group (4.8 %). Figure 2 shows the mean spherical equivalent according to the four DH proximal location types. The four groups differed significantly in terms of the spherical equivalent with the Kruskal-Wallis test (P = 0.014). On post hoc analysis, lamina cribrosa-type DH showed a lower spherical equivalent than that of peripapillary-type DH (P = 0.004, Tukey test with ranks; Table 2). Multivariate stepwise logistic regression was carried out to evaluate which variables were associated with myopia. After adjustment for covariates (age, mean deviation, and pattern standard deviation), we found that eyes with lamina cribrosa-type DH were 12.59 times more likely to be myopic than were eyes with peripapillary-type DH (95 % CI: 1.22–129.53; P = 0.033; Table 3).
Discussion
Although the pathogenesis of DH is not entirely understood, various vascular and mechanical theories have been proposed. Some investigators suggest that DH is a sign of an underlying vascular pathology because it is more prevalent in normal tension glaucoma [6, 16]. However, other studies support the theory that the pathogenesis of DH is caused by mechanical injury due to elevated intraocular pressure (IOP) [17–19]. Regardless of the actual pathogenesis, it is evident that the optic nerve is stressed in the DH region.
Why myopia increases the risk of primary open-angle glaucoma is not clearly understood. Fong et al. [20] suggested that myopic eyes with an increased axial length appear to have greater deformability of the lamina cribrosa. The stretching of the globe in myopia results in secondary enlargement of the optic nerve head, stretching and thinning of the lamina cribrosa, and ultimately, higher susceptibility at a given IOP [13, 21, 22]. Furthermore, thinning of the lamina cribrosa decreases the distance between the intraocular and retrobulbar cerebrospinal fluid compartment spaces, causing steeper pressure gradients [23]. The combination of stretching and distortion of the optic nerve fibers resulting from an abrupt change in the scleral curvature may lead to optic nerve fiber damage in highly myopic eyes [24]. These factors might contribute to the lamina cribrosa having a higher vulnerability to glaucomatous damage in a myopic disc than in a nonmyopic disc [25–28].
To the best of our knowledge, this is the first study to compare myopic and nonmyopic DH according to its proximal location. The study was performed retrospectively, and many cases were excluded because of the inclusion and exclusion criteria. Most studies on this subject have dealt only with the quadrant location of DHs, which occur on the temporal side of the disc, most commonly in the inferotemporal and superotemporal locations [29–31]. Our results are consistent with these previous findings. Unlike most earlier studies, however, we classified the proximal location of DH into four types: lamina cribrosa, cup margin, disc rim, and peripapillary types. We found that lamina cribrosa-type DH was significantly more common in myopic patients. This finding suggests that the lamina cribrosa of a myopic disc has a higher vulnerability to glaucomatous damage than that of a nonmyopic disc since the proximal location of the DH could be associated with the location of glaucomatous stress in the optic nerve head. When the temporal tilt of a myopic optic disc is large enough, regional IOP-related strain in the temporal region of the lamina cribrosa of the optic nerve head may lead to the lamina cribrosa type of DH.
In advanced glaucoma, the rim remnants are located mainly in the nasal disc sector [32]. Eyes with DH on the temporal side of the optic disc have a significantly lower IOP and appear to be more likely to develop progressive glaucomatous changes than eyes with DH in the nasal region [17, 33, 34]. These findings suggest that the temporal side of the disc is more vulnerable to glaucomatous injury than the nasal side [35]. In our study, there were no cases of myopic DH at a superonasal location, whereas five eyes (11.9 %) had nonmyopic DH on the superonasal side (P = 0.082). These data suggest that the temporal portion of a myopic disc is relatively more susceptible to glaucomatous damage than the nasal disc side. The regional strain on the temporal side of a tilted myopic optic disc could be responsible for this finding.
In our study, the age and visual field indices (mean deviation and pattern standard deviation) differed significantly between the myopic and nonmyopic groups. No published data have suggested that the topographic features of DH differ according to the age or visual field indices. Multivariate logistic regression was performed to determine which variables were associated with myopia. After controlling for covariates, we found that eyes with lamina cribrosa-type DH were more likely to be myopic than were eyes with peripapillary-type DH. A limitation of our study is the relatively small sample size, particularly with respect to the subgroups, which made it difficult to carry out further subgroup analyses with sufficient statistical power. A further study with a larger number of myopic cases and a subgroup including highly myopic eyes will provide additional clues on the characteristics of DH in highly myopic eyes.
In conclusion, we have documented that the lamina cribrosa type of DH was relatively more common in myopic patients than in nonmyopic patients. This result suggests that the pathogenesis of DH may differ between myopic and nonmyopic DH. We still do not know clearly whether the difference in the proximal location of DH is correlated with myopia (spherical equivalent) or with a myopic-shaped disc type. Further study on the correlation between the DH type and degree of disc tilt or the disc type will be another interesting subject that may partially explain the characteristics and mechanism of DH.
References
Kim SH, Park KH. The relationship between recurrent optic disc hemorrhage and glaucoma progression. Ophthalmology. 2006;113:598–602.
Suh MH, Park KH, Kim H, Kim TW, Kim SW, Kim SY, et al. Glaucoma progression after the first-detected optic disc hemorrhage by optical coherence tomography. J Glaucoma. 2012;21:358–66.
Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000;129:707–14.
Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708.
Bengtsson B, Leske MC, Yang Z, Heijl A. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology. 2008;115:2044–8.
Sonnsjo B, Dokmo Y, Krakau T. Disc haemorrhages, precursors of open angle glaucoma. Prog Retin Eye Res. 2002;21:35–56.
Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–91.
Nakazawa T, Fuse N, Omodaka K, Aizawa N, Kuwahara S, Nishida K. Different types of optic disc shape in patients with advanced open-angle glaucoma. Jpn J Ophthalmol. 2010;54:291–5.
Knapp A. Glaucoma in myopic eyes. Trans Am Ophthalmol Soc. 1925;23:61–70.
Perkins ES, Phelps CD. Open angle glaucoma, ocular hypertension, low-tension glaucoma, and refraction. Arch Ophthalmol. 1982;100:1464–7.
Mastropasqua L, Lobefalo L, Mancini A, Ciancaglini M, Palma S. Prevalence of myopia in open angle glaucoma. Eur J Ophthalmol. 1992;2:33–5.
Kim M, Kim TW, Park KH, Kim JM. Risk factors for primary open-angle glaucoma in South Korea: the Namil study. Jpn J Ophthalmol. 2012;56:324–9.
Jonas JB, Budde WM. Optic nerve damage in highly myopic eyes with chronic open-angle glaucoma. Eur J Ophthalmol. 2005;15:41–7.
Jonas JB, Berenshtein E, Holbach L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci. 2004;45:2660–5.
Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci. 2003;44:5189–95.
Kim YD, Han SB, Park KH, Kim SH, Kim SJ, Seong M, et al. Risk factors associated with optic disc haemorrhage in patients with normal tension glaucoma. Eye (Lond). 2010;24:567–72.
Hendrickx KH, van den Enden A, Rasker MT, Hoyng PF. Cumulative incidence of patients with disc hemorrhages in glaucoma and the effect of therapy. Ophthalmology. 1994;101:1165–72.
Budenz DL, Anderson DR, Feuer WJ, Beiser JA, Schiffman J, Parrish RK 2nd, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113:2137–43.
Bito LZ. Impact of intraocular pressure on venous outflow from the globe: a hypothesis regarding IOP-dependent vascular damage in normal-tension and hypertensive glaucoma. J Glaucoma. 1996;5:127–34.
Fong DS, Epstein DL, Allingham RR. Glaucoma and myopia: are they related? Int Ophthalmol Clin. 1990;30:215–8.
Yasuzumi K, Ohno-Matsui K, Yoshida T, Kojima A, Shimada N, Futagami S, et al. Peripapillary crescent enlargement in highly myopic eyes evaluated by fluorescein and indocyanine green angiography. Br J Ophthalmol. 2003;87:1088–90.
Kim TW, Kim M, Weinreb RN, Woo SJ, Park KH, Hwang JM. Optic disc change with incipient myopia of childhood. Ophthalmology. 2012;119:21–6.
Jonas JB. Role of cerebrospinal fluid pressure in the pathogenesis of glaucoma. Acta Ophthalmol. 2011;89:505–14.
Ohno-Matsui K, Shimada N, Yasuzumi K, Hayashi K, Yoshida T, Kojima A, et al. Long-term development of significant visual field defects in highly myopic eyes. Am J Ophthalmol. 2011;152(256–65):e1.
Begg IS, Drance SM, Sweeney VP. Ischaemic optic neuropathy in chronic simple glaucoma. Br J Ophthalmol. 1971;55:73–90.
Sonnsjo B, Krakau CE. Arguments for a vascular glaucoma etiology. Acta Ophthalmol (Copenh). 1993;71:433–44.
Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–49.
Krakau CE, Bengtsson B, Holmin C. The glaucoma theory updated. Acta Ophthalmol (Copenh). 1983;61:737–41.
Jonas JB, Xu L. Optic disk hemorrhages in glaucoma. Am J Ophthalmol. 1994;118:1–8.
Diehl DL, Quigley HA, Miller NR, Sommer A, Burney EN. Prevalence and significance of optic disc hemorrhage in a longitudinal study of glaucoma. Arch Ophthalmol. 1990;108:545–50.
Yamamoto T, Iwase A, Kawase K, Sawada A, Ishida K. Optic disc hemorrhages detected in a large-scale eye disease screening project. J Glaucoma. 2004;13:356–60.
Jonas JB, Fernandez MC, Sturmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993;100:63–8.
Siegner SW, Netland PA. Optic disc hemorrhages and progression of glaucoma. Ophthalmology. 1996;103:1014–24.
Miyake T, Sawada A, Yamamoto T, Miyake K, Sugiyama K, Kitazawa Y. Incidence of disc hemorrhages in open-angle glaucoma before and after trabeculectomy. J Glaucoma. 2006;15:164–71.
Uhler TA, Piltz-Seymour J. Optic disc hemorrhages in glaucoma and ocular hypertension: implications and recommendations. Curr Opin Ophthalmol. 2008;19:89–94.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kim, H.S., Park, K.H., Jeoung, J.W. et al. Comparison of myopic and nonmyopic disc hemorrhage in primary open-angle glaucoma. Jpn J Ophthalmol 57, 166–171 (2013). https://doi.org/10.1007/s10384-012-0209-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-012-0209-5