Abstract
Tree growth is a multifaceted process influenced by various factors at different spatial and temporal scales, including intrinsic tree traits and environmental conditions. Climate factors have a significant impact on tree growth dynamics, while geological controls can also play a crucial role. However, our understanding of the interplay between these factors concerning tree growth is currently limited. This study focuses on Norway spruce (Picea abies [L.] Karst.), one of the economically most important coniferous tree species in Europe, to investigate the interplay of growth, climate, and environment at the forest and corresponding treeline sites in the High Tatra Mountains of Slovakia. Specifically, we developed chronologies of tree-ring width (TRW) and late-wood density (MXD) for different tree size classes across two limestone and granitic sites. Growth rates of Norway spruce trees have been increasing in forests since the 1930s and from the 1950s at treelines. Growth rates were consistently higher on limestone bedrock compared to granitic bedrock conditions. Variability of radial growth is primarily driven by climate at both geological settings with trees on granitic bedrock displaying more pronounced responses to climatic variables. We observed weakening (non-stationarity) in climate signals over time and across all size classes in both geological settings. The magnitude of these effects is small, but varies across size classes, with larger trees generally displaying stronger climate sensitivities compared to smaller ones. Therefore, our findings accentuate the potential implications of geological settings, climate, and environmental factors on the absolute growth and growth dynamics of Norway spruce, highlighting the need for further research to fully understand and manage forest ecosystems in mountainous regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tree growth is a complex dynamic process influenced by multiple interacting factors (intrinsic and extrinsic) acting at different spatial and temporal scales. Intrinsic factors include genetic traits (Wang et al. 2022), species characteristics (Pretzsch 2021) and physiological processes (Avanzi et al. 2019) determining the tree’s growth potential and ability to capture and allocate resources efficiently. Extrinsic factors encompass large-scale environmental factors such as climate and landscape characteristics and small scale factors such as soil nutrients and water availability for growth, development, and competition (Robertson 1992; Cienciala et al. 2016; Chakraborty et al. 2017; Bayat et al. 2021). Additionally, disturbances such as fire, wind, or insect outbreaks can have both negative and positive impacts on tree growth (Splechtna et al. 2005; Szewczyk et al. 2011). Understanding the complex interplay between abiotic and biotic factors and their impact on the structure and resilience of forest communities across space and time is a major goal of ecological research.
Climate factors, including temperature and precipitation, have profound effects on tree growth, altering the dynamics and productivity of individual trees and stands in forest ecosystems (Fei et al. 2017). Although the relationship between climate and tree growth is often non-stationary (Wilmking et al. 2020), rising temperatures and changing precipitation patterns associated with climate change can extend growing seasons, promoting increased photosynthesis, and potentially enhancing growth rates (Gray and Brady 2016; Weiskopf et al. 2020). However, excessive high temperatures and prolonged heatwaves can also cause drought conditions and water stress, reducing tree growth and increasing mortality (Esperon-Rodriguez et al. 2021; Marchin et al. 2022). Changes in precipitation patterns affect tree growth with droughts limiting water availability and floods causing soil erosion and nutrient loss (Kreuzwieser and Gessler 2010). The interaction of climatic factors can also influence competition among tree species and can thus shape forest succession dynamics (Oboite and Comeau 2019). Changes in climate may result in vegetation shifts (Harsch et al. 2009) leading to significant changes in species composition (Bugmann 1997) and biomass production (Speed et al. 2015) including tree growth decline (Matskovsky et al. 2021).
The influence of bedrock on tree growth, especially when combined with climatic drivers, is complex, multifaceted, and often uncertain (Vestin et al. 2013; Richter and Billings 2015a). Different types of bedrock can have distinct properties that influence soil development and fertility, affecting the ability of plants, including trees, to establish and thrive (Hahm et al. 2014; Jiang et al. 2020). For example, limestone bedrock tends to contribute to alkaline soils with high nutrient content, while granite bedrock may result in more acidic and nutrient poor soils (Morford et al. 2011). Trees in areas dominated by soluble rock substrate are often more vulnerable to drought conditions (Schwinning 2008. In contrast, trees in areas with granitic bedrock may have access to deeper soil moisture as these bedrock types can create an impenetrable barrier of residuals to water loss making them potentially more resilient to drought conditions leading to increase overall productivity (Ivits et al. 2014a). Similarly, bedrock significantly influences numerous ecological processes (Mage and Porder 2013; Gerdol et al. 2016; Delgado-Baquerizo et al. 2020), and its impact is likely universal. Nevertheless, most research on tree growth has traditionally emphasized climate and topsoil attributes, including soil composition, nutrient availability, pH levels, water retention, drainage, and mineral content (Jones and Graham 1993; Vestin et al. 2013; Lévesque et al. 2016; van der Maaten-Theunissen et al. 2016; Gerdol et al. 2016; Rehschuh et al. 2017). Conversely, the significance of deeper belowground elements remains less comprehensively explored (Rose et al. 2003; Richter and Billings 2015b; Jiang et al. 2020).
Norway spruce, (Picea abies [L.] Karst.) is one of the most economically valuable forest species native to mountain habitats in central and eastern Europe. Its distribution range covers a natural range from the montane zone up to treeline (San-Miguel-Ayanz et al. 2016). The impact of climate change on spruce has been discussed extensively and Norway spruce seems one of the most vulnerable tree species in Europe regarding drought (Lebourgeois et al. 2010; Hentschel et al. 2014; Conedera et al. 2017). While spruce trees at higher elevations typically exhibit slower growth rates compared to those at lower elevations (Körner and Riedl 2012), recent studies suggest a positive growth trend of Norway spruce at higher elevations due to increasing temperatures (Pretzsch et al. 2014; Cienciala et al. 2018; Marchand et al. 2022). In contrast, there is observed decline in growth trends especially at lower elevations, which are in line with the effects of ongoing climate changes (Savva et al. 2006; Lévesque et al. 2013; Zang et al. 2014). However, little is known about the complex interplay between bedrock, climate and tree growth (but also see (Petitcolas et al. 1997). Thus, our study aims to help disentangle this complexity and investigate the growth pattern, climatic response and its stationarity of Norway spruce at typical treeline and at corresponding forest sites at two distinct geological settings in the High Tatras of Slovakia.
Methods and methodology
Study area
Site description and climate
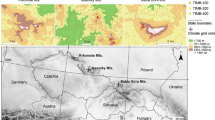
We selected two sites in the High Tatra National Park, on the border between Poland and Slovakia in eastern Central Europe (Fig. 1).
Geologically, the Tatra Mountains belong to the Carpathian uplift. The interior region is mostly comprised of granite, while the exterior regions consist mainly of calcite, dolomite, claystone, and shale, displaying a variety of different bedrocks and potential environmental heterogeneity (Gawęda et al. 2013). Mountain areas in Europe have been impacted by diverse human activities for thousands of years (Valsecchi et al. 2010). The natural environment of the Tatra Mountains today is often protected to some extent but has been subject to major anthropogenic influence over the past centuries and decades (Piscová et al. 2023), exhibiting varying intensity across space and time. As evidenced by historical materials and documented sediments in glacial lakes within cirques and over-deepened basins (Kłapyta and Kołaczek 2009; Valsecchi et al. 2010; Rączkowska 2019), human influence resulted in both intentional and unintentional alterations, particularly in lower lying landscapes and across different altitudinal zones. Nevertheless, humans are seen to have made minor alterations to the high-mountain environment of the Tatras (Rączkowska 2019). High-elevation forests are dominated by tree species such as Picea abies, Abies alba, Pinus sylvestris, Pinus cembra, Larix decidua, and Pinus mugo (Mitchell 1976). The climate is described as temperate Central European with strong continental influences. July is the wettest month, while February is the driest. The annual average temperature ranges from 5.3 °C at 850 m a.s.l. to 1.7 °C at 1750 m a.s.l., while annual precipitation ranges from 800 mm to 1350 mm, with the maximum occurring in summer months (Niedzwiedz 1992).
Site selection and sampling
We selected two distinct geological settings located approximately 20–30 km apart: Mieguszowiecka Valley (49.15631°N, 20.07659°E), which features granitic bedrock, and Javorova Valley (49.2365°N, 20.20147°E), known for its limestone bedrock (Fig. 1).Both sites were located on south-east facing slopes, with slopes slightly inclined at 10–15° in granitic settings and 20–25° in limestone settings. The sites were selected for their rather similar climate conditions (Fig. S1) and stand properties with respect to tree size and stand density. Although treeline and forest plots were not separated by a steep altitude gradient, significant differences in stand structure existed. In our selected study areas at the treeline and within the forest, there were no documented instances of logging, forest fires, or other biotic disruptions, such as grazing, but grazing has been a driver of landscape dynamics in the larger region in the past (Solár and Solár 2020).
In total, we sampled over 160 Norway spruce trees from each geological setting in 2021 and 2022. In the treeline plots all Norway spruce trees with a diameter at breast height (DBH) > 10 cm were sampled. In the forest plot we selected a representative subplot, where we collected samples from 100 trees of > 10 cm DBH. Two increment cores were taken per tree at breast height perpendicular to each other to reduce a possible bias due to irregular/eccentric growth. From each sampled tree, the diameter at breast height (DBH), tree height, and position (GPS) coordinates were recorded. After that we stratified the samples according to diameter size classes (i.e. DBH) (Meyer and Bräker 2001; Nehrbass-Ahles et al. 2014). Tree core samples were divided into three DBH classes: 11–30 cm, 31–50 cm, 51–70 cm at the forest plots and one DBH class (11–30 cm) at the treeline plots from both geological settings for further analysis.
Sample preparation and data acquisition
The samples were processed and analysed following a standard dendrochronological procedure as prescribed by (Fritts 2014) and (Speer 2012). First, the two core samples from individual trees were divided into core A and B for two different measurements (i.e. TRW and MXD) and left for air drying. Core A was later boiled for 24 h in a Soxhlet extractor filled with 96% Ethanol to remove resins from the increment cores (Schweingruber et al. 1978). The resins extracted cores were glued to wooden sample holders and were sanded and polished using a sanding machine with different grades of sandpaper (grid size of 120–600) in order to make annual rings visible. The samples were then scanned using a conventional scanner (Epson Expression 12000XL). TRW was measured on the scanned image with 0.001 mm precision using the software CooRecorder (version 9.3; Cybis Elektronik and Data AB, Sweden) and all radii were cross-dated using CDendro (version 9.3.1; Cybis Elektronik and Data AB, Sweden).
The B cores were glued to a wooden holder in order to cut out laths of 1.24 ± 0.1 mm thickness using a twin-bladed circular saw. The lath samples were then resins extracted following the same procedures as described above. After drying, the laths were X-rayed under controlled conditions (20 °C and 50% relative humidity) using an ITRAX MultiScanner (Cox Analytical Systems) with an exposure time of 25 ms, an intensity of 30 kV/50 mA, and in steps size of 20 μm. A standard calibration plexi ladder was used to calibrate grey-level light intensity to wood density in g/cm³ for each X-ray scan. MXD was measured on the obtained grey-scale images using the CooRecorder (version 9.3; Cybis Elektronik and Data AB, Sweden). The mean thickness of each extracted sample was measured with electronic callipers and later used for calibration of the respective sample to account for possible deviations in thickness among the individual laths. All measured TRW and MXD series were visually crossdated in CDendro (version 9.3.1; Cybis Elektronik and Data AB, Sweden). The visually crossdated TRW and MXD series were further verified and standardized using the dplR-package (Bunn 2008) in R version 4.0.1 (R Core Team 2020).
Standardization and chronology development
Tree-ring series contain long-term trends related to biological effects as well as to climate (Speer 2012). For the climate-growth correlations, we used a 31-year cubic smoothing spline with a 50% frequency cut-off to remove non climatic trends, such as age and stand effects (Cook et al. 1990). Final chronologies (TRW and MXD) for each site and DBH class were computed using the bi-weight robust mean function to mitigate the influence of statistical outliers (Cook 1985). Chronology quality was then checked using the mean inter-series correlation (R-bar), expressed population signal (EPS) and Gleichlaeufigkeit (glk) for both geological settings, DBH classes and both proxies (Wigley et al. 1984) (Tables S1 and S2).
Statistical analyses
Growth rates analysis
We compared differences in absolute growth between granitic and limestone bedrock conditions in terms of Ring-width (RW), age, Basal Area Increment (BAI) and MXD using Mann-Whitney U-tests, accounting for the non-normal distribution of data, as indicated by the Shapiro-Wilk normality test (Fig. S2). The Regional Curve (RC) method was used to examine the radial growth of Norway spruce along cambial age between and within the sites according to different DBH classes (Mitchell 1976; Esper et al. 2003). In this method we compute the regional curve by taking the average of ring-width measurements for a given biological age fitting a 61 year cubic smoothing spline with a 50% variance response (Esper et al. 2003; Bunn et al. 2004).
Growth–climate relationships
To explore the relationship between tree growth and climate, obtaining climate data from the closest meteorological stations is preferable (Speer 2012; Fritts 2014). However, climate data from the closest meteorological stations (Hala Gąsienicowa, Strbskie Pleso, Skalnate Pleso) lack the necessary length and completeness for a robust statistical analysis. Therefore, we extracted the climate research unit time series data sets (CRU TS 4. 05) from the nearest grids region at 0.5° resolution, downloaded using KNMI climate explorer (Harris et al. 2020) (http://climexp.knmi.nl/). Monthly resolved temperature means and precipitation sums were used for the response analysis. To study the influence of droughts on tree growth, we used SPEI (standardized precipitation and evapotranspiration index), integrated over 6 months (SPEI06) (Vicente-Serrano et al. 2010; Beguería et al. 2014). The SPEI calculated from March to August (SPEI6) provides the most accurate representation of drought impact on the growth of N. spruce in areas characterized by mild oceanic climates (Bhuyan et al. 2017). Additionally, this timeframe aligns with the duration of cambial activity in N. spruce within Central Europe, making it the selected period for further analysis (Rossi et al. 2008). Monthly water balance was calculated by subtracting the potential evapotranspiration (PET) from precipitation. PET was calculated using the Thornthwaite equation(Thornthwaite 1948) in the R-package SPEI (Beguería et al. 2014).We used the temperature means from the year 1901 to 2021. Using the ‘Segment’ packages in R(Muggeo et al. 2014) the year 1990 was identified as the breakpoint (Fig. S3). To check for stationarity(Wilmking et al. 2020) i.e. change of climate sensitivity over time, we therefore split the time series into two equally long time periods from 1960 to 1990 and from 1990 to 2020 based on the break point analysis. Bootstrapped correlation coefficients were calculated between tree-ring standard chronologies and climate variables for the period of (1960–1990) and (1990–2020) using the ‘treeclim’ R package (Zang and Biondi 2015). A climatic window of 16 months starting in previous growth year June to September of the current growth year was used to compute correlations between the monthly climatic variables and both TRW and MXD chronologies. The temporal stability of the climatic response /stationarity(Wilmking et al. 2020) was also tested by carrying out a moving correlation analysis between the chronology and climate proxies using a 30-year window with a one-year window offset in the R package ‘treeclim’ (Zang and Biondi 2015).
Growth model construction and statistical procedures
To investigate the best set of explanatory variables (climate variables, different bedrock conditions, DBH classes, and altitude) that affect the growth (TRW) and maximum latewood density (MXD) of Norway spruce, as well as the interactions between these variables, we fitted linear mixed-effects models using the ‘lme4’ (Bates et al. 2008) and ‘model-based’ (Lüdecke et al. 2021) packages over the two sub-periods (1960–1990 and 1990–2020).
In the first steps:
-
(a)
We identified the primary drivers (i.e. variables that are most influential on TRW and MXD). The proposed models included Tree IDs as a random effect and climate variables of current year (Temperature, Precipitation, and SPEI06), elevation, DBH, and bedrock types as fixed effects. Analysis of the “lag effect” between tree growth and climatic variables suggested current-year’s climate have the highest influence on tree growth. Therefore, we started with one-parameter models with climate variables and then tested the significance of adding other explanatory variables. The key indicator for accepting significant covariates within the model was the p-value (< 0.05), and the variance inflation factor (VIF) < 5, indicating an acceptable level of collinearity between explanatory variables. In this way, we successively added and deleted independent variables to identify the optimal models with only significant covariates.
-
(b)
Second, to explore the interactions between the variables (climate, DBH class, elevation, and bedrock condition), our proposed models included interactions of climate variables, DBH size class, elevation, and different bedrock conditions as dependent variables. Similarly, we used individual RWI and MXD series as our dependent variables and Tree IDs as a random effect. Although the criteria imposed for accepting all the climate variables within the same models were significance at p < 0.05 and variance inflation factor (VIF) < 5, we selected only one climate variable at a time to run the models. We considered only those three months showing the highest significant responses (p < 0.05) in our climate-growth correlation analyses in each of the climate variables for further analysis. After fitting the initial model, we performed a stepwise model selection, which iteratively added or removed predictors based on their statistical significance (p < 0.05) to find a more parsimonious model that still fits the data well.
The best-fitted models were considered those showing the lowest Akaike Information Criterion (AIC; (Akaike 1974) values and complementary statistics, including coefficients of determination for marginal and conditional estimates (Lefcheck 2016). Similarly, we used maximum likelihood (ML) estimation to compare models with different fixed effects, as recommended by (Zuur et al. 2009), whereas the restricted maximum likelihood method (REML) was used to parameterize the final model as followed by (Cienciala et al. 2016).
Besides growth model construction and analysis as described above, we estimated the marginal effects (slopes) of a predictor variable at different levels of other categorical variables to understand how the relationship between the predictor and the response variable changes across these different levels. We used the R package ‘model-based’ to estimate the marginal effects (slopes) of the climate variables at different levels of other factors (bedrock condition, elevation, and DBH).
Results
Chronology statistics
Site characteristics and chronology (tree-ring width (TRW), maximum latewood density (MXD)) statistics of Picea abies are provided in Tables S1 and S2. The average radial growth rates ranged from 0.88 mm/yr to 1.87 mm/yr, with the higher annual radial growth rates found at limestone sites. When comparing the growth rates between the forest and treeline plots, the minimum rates (0.88 and 0.99 mm/yr) were observed at the treeline sites of both geological settings. Similarly, the maximum latewood density ranged from 0.85 g/cm³ to 0.99 g/cm³, with the higher densities found at limestone sites. When comparing the maximum latewood density (MXD) values between the forest and treeline plots, the treeline sites displayed the highest latewood density (0.97 and 0.99 g/cm³) in both geological settings. Overall, radial growth rates increased from high to low elevations, while the maximum latewood density decreased in both geological settings (Table S2). But no persistent trends in the chronology statistics (MS, Rbar, AR1 and EPS) were apparent along the elevation, DBH size classes and bedrock conditions (Tables S1 and S2).
Growth rates using Regional curves (RC)
The regional curves at the two geological settings showed the expected age-related reduction of ring width (Fig. 2). In general, over the first 80 years of tree growth, the sites with limestone bedrock conditions had higher growth in comparison to the sites with granitic bedrock conditions in forest plots. While for the next 130 years, the tree growth is same (Fig. 2a). Similarly, in the treeline, the growth rate is higher in limestone site over the first 60 years (Fig. 2a). However, growth rates differ between the DBH classes in both forest plots (Fig. 2b and c). The larger size classes show generally higher growth than the smaller size classes at a similar age across both geological settings. When comparing the treeline to the forest across the common size class, the growth rates were quite similar, with a tendency for the forest plot to grow slightly better than the treeline plot (Fig. 2b and c).
Growth-climate relationships
Climate growth correlations between (TRW and MXD) with climatic variables indicated that tree growth (in treeline and forest) in both geological settings showed positive (negative) correlations with temperature (moisture availability) during growing season months, although the magnitude and intensity of correlation coefficients varied among the proxies (Figs. 3, 4, 5 and 6) (Fig. S4 to Fig. S9).
Correlation between different dbh size classes tree-ring width chronologies from forest plots and monthly (temperature, precipitation and SPEI06: standardized precipitation- evapotranspiration index over six months) climate variables for Picea abies (L.) H. Karst for (1960–1990) and (1990–2020). Symbol * indicates a significant correlation at 95% or above confidence level. Similarly, Jun to Dec indicates the months of the preceding growth year: June–December, while JAN-SEP represents the month of the current growth year: January to September respectively
Correlation between tree-ring width chronology from treeline plots and monthly (temperature, precipitation and SPEI06: standardized precipitation- evapotranspiration index over six months) climate variables for Picea abies (L.) H. Karst for (1960–1990) and (1990–2020). Symbol * indicates the correlation significant at 95% or above confidence level. Similarly, Jun to Dec indicates the months of the preceding growth year: June–December, while JAN-SEP represents the month of the current growth year: January to September respectively
In all DBH size classes and both proxies, growth in forest plots from both geological sites is more strongly correlated with climate parameters during the first time period (1960–1990) compared to the second period (1990–2020) (Figs. 3, 4 and 5, and 6). TRW shifts its climate response (temperature) to later summer months from 1960 to 1990 to 1990–2020 in both geological settings in all DBH size classes and loses its sensitivity to SPEI and precipitation in bigger size classes (Fig. 3). MXD seems to lose its sensitivity to spring and summer temperatures except in granitic settings, with a more pronounced impact of SPEI06 and a mixed response to precipitation across the time periods (Fig. 5).
Correlation between different dbh size classes MXD chronologies from forest plots and monthly (temperature, precipitation and SPEI06: standardized precipitation- evapotranspiration index over six months) climate variables for Picea abies (L.) H. Karst for (1960–1990) and (1990–2020). Symbol * indicates the correlation significant at 95% or above confidence level. Similarly, Jun to Dec indicates the months of the preceding growth year: June–December, while JAN-SEP represents the month of the current growth year: January to September respectively
Correlation between MXD chronology from treeline plots and monthly (temperature, precipitation and SPEI06: standardized precipitation- evapotranspiration index over six months) climate variables for Picea abies (L.) H. Karst for (1960–1990) and (1990–2020). Symbol * indicates the correlation significant at 95% or above confidence level. Similarly, Jun to Dec indicates the months of the preceding growth year: June–December, while JAN-SEP represents the month of the current growth year: January to September respectively
Climate-growth relationships of Picea abies from the treeline sites at the two geological settings, for both proxies (TRW and MXD), varied strongly over time, with sensitivity mainly lost in the second time period (Figs. 4 and 6). TRW shifts its climate response to later summer months from 1960 to 1990 to 1990–2020 only in granitic settings and loses its sensitivity to SPEI and precipitation (Fig. 5), similar to the forest sites.
Tree growth model: factors affecting tree growth
The following variables were influential on tree ring width (TRW) and maximum latewood density (MXD), which are summarized below in (Table S3 and Table S4). At the individual tree level, random intercept linear mixed-effect models revealed tree growth is sensitive to climatic variables, with varying effects over time and between sites (Tables S3 and S4).
In both proxies (TRW and MXD) and both geological sites, a significant positive effect of temperature showed an important growth-limiting factor followed by precipitation (negative) in both sub-periods (1960–1990) and (1990–2020). Additionally, the Standardized Precipitation-Evapotranspiration Index (SPEI06) consistently shows a significant negative effect on RWIs and MXDs across the studied models (summary Tables S3 and S4).
Elevation and DBH exert varied effects on tree growth across different models and periods. Elevation has a significant negative effect on RWIs and MXDs at both sites during the 1960–1990 period. However, this effect is not consistently observed in the second period (1990–2020). Similarly, DBH shows a significant negative effect in some models, especially from limestone sites (summary Tables S3 and S4).
The explained variance of all best-fit models ranged between 4.4 and 53%, with marginal R2-values being consistently higher for the first time period (1960–1990) compared to the second period (1990–2020) in both bedrock conditions, which implies that variables better explain the observed growth variation in time period (1960–1990) similar to the results of the correlation analysis (summary Tables S3 and S4).
Effect of climate variables across different combinations of bedrock, elevation and size
Based on our interaction models between individual (RWI and MXD) series as our dependent variables and climate variables, DBH size class, elevation, and different bedrock conditions as fixed effects, the estimated marginal effects (slopes) of climate variables categorized by bedrock type, plot, and DBH size class, across two time periods are shown in Tables 1 and 2 and are generally small.
Temperature (Tmjj) generally promotes growth, while precipitation (Pamj) and drought conditions (SPEI06jas) inhibit tree growth (TRW) and (MXD) in both limestone and granitic bedrock conditions across all DBH size classes and both periods (Tables S1 and S2).
Over time, there is a decreasing positive effect of temperature (Tmjj) on both proxies (TRW and MXD) across both limestone and granitic bedrock sites, while precipitation’s (Pamj) negative influence remains relatively stable. Additionally, the standardized precipitation-evapotranspiration index (SPEI06jas) consistently exerts a negative effect on tree growth, with minor fluctuations (Tables S1 and S2).
The effects of climate variables vary by DBH size class, with smaller trees (11–30) cm often showing weak sensitivities for both TRW and MXD, while the higher size classes (31–50) cm and (51–70) cm demonstrate stronger climate effects based on the coefficient values of temperature, precipitation and SPEI06 across different bedrock types and elevation (Tables S1 and S2).
Overall, the positive effects of temperature and the negative effects of precipitation and SPEI06 on both TRW and MXD are generally stronger in all size trees in granitic bedrock compared to trees on limestone bedrock across both periods (1960–1990 and 1990–2020) (Tables S1 and S2).
Discussion
Growth rates and different bedrock condition
After 1950, notable increments in Norway spruce radial growth were observed in both limestone and granitic bedrock conditions. Generally, spruce growth increases with rising temperatures, particularly at higher elevations (Cienciala et al. 2018; Schurman et al. 2019; Pretzsch 2021; Bosela et al. 2021) and decreases especially at lower elevations due to drought stress or competition (Lévesque et al. 2013; Zang et al. 2014; Seidl et al. 2017). Also pollution reduction can lead to growth increases. (Hauck et al. 2012) for example observed a rapid growth increase in Norway spruce in the Harz Mountains in Germany since the 1990s, and they relate it to the dramatic reduction in SO2 concentrations.
However, the observed variations in Norway spruce growth rates between limestone and granitic bedrock conditions in our study over different time periods may be attributed to complex interactions between geological, ecological, and historical factors. Specifically, the recent 90-year period (1930–2020) in forest plots and the 65-year period (1955–2020) in treeline plots reveal a distinct pattern, with trees from limestone bedrock conditions exhibiting higher absolute growth rates. This may result from the beneficial effect of climate warming or the legacy effects of historical land-use practices gradually diminishing over time, with the inherent soil properties of limestone-derived soils becoming more prominent drivers of tree growth (Melo et al. 2013; Fritts 2014; Monger et al. 2015). Limestone soils typically possess better drainage, aeration, and nutrient availability, creating a more conducive environment for root development and nutrient uptake, thus promoting enhanced tree growth (Morford et al. 2011; Vestin et al. 2013; Hahm et al. 2014; Zhang et al. 2015; Rempe and Dietrich 2018b; Jiang et al. 2020; Pastore et al. 2022). However, it’s also important to note that specific tree species, their genetic makeup, local climate, age, competition, and other environmental factors can also influence tree growth, leading to exceptions or variations within these general patterns (Baker et al. 2003; Büntgen et al. 2007; Zielonka and Malcher 2009; Bowman et al. 2013; Filotas et al. 2014; Gourlet-Fleury et al. 2023).
On the other hand, the similarities in growth rates between the two geological settings in forest plots over the earlier 120 years (1810–1930) may be potentially attributed to historical land-use practices. Research indicates that human activities, including mining, grazing, and wood and oil extraction, have significantly impacted the natural environment of the Tatra Mountains since medieval times (Kłapyta and Kołaczek 2009; Valsecchi et al. 2010; Rączkowska 2019; Piscová et al. 2023). Especially, grazing in the Belianske Tatras reached its peak in the 1800s but ceased by the mid-1900s (Zelina 1965; Bohuš 1994), it likely had significant impacts on vegetation dynamics, potentially altering soil properties, nutrient cycling, and competition dynamics, leading to comparable growth rates between limestone and granitic settings during this time period (Frank et al. 2003; van der Wal et al. 2004; Mitchell 2005; Wei et al. 2011; Ramirez et al. 2018; Bernes et al. 2018; Yu et al. 2019; Solár and Solár 2020).
Growth rates and size classes
Detecting long-term tree growth trends by means of dendroecological analyses poses a number of challenges (Bowman et al. 2013; Peters et al. 2015; Duchesne et al. 2019; Trouillier et al. 2020). Trees which grow slow, tend to live longer (Bigler and Veblen 2009) and therefore are overrepresented in a sample (“slow-grower survivorship bias”). This then can lead to a “false-positive growth trend” of the chronology when combining older slow growing trees and younger fast growing trees, as young slow-growing trees are likely to be too small to be considered for sampling (“big tree-selection bias”) (Brienen et al. 2012). We therefore used size class categories and sampled small and large trees following (Nehrbass-Ahles et al. 2014; Jochner et al. 2017). In our study, growth rates of trees differ between different size classes, with bigger trees growing radially much more than smaller trees (Fig. 2b and c). This is not what could be expected based on the ‘slow-growth survivorship bias’ (Büntgen et al. 2005; Black et al. 2008; Bigler and Veblen 2009; Bowman et al. 2013; Alexander et al. 2018), which would lead to an overestimation of growth of younger trees, since the older fast growing trees are lost to a large degree from a contemporary sample. Narrow rings in smaller trees in comparison to larger trees in our study sites might be due to 1) smaller and presumably younger trees being more susceptible to environmental stresses like drought and temperature extremes (van Mantgem et al. 2009). 2) bigger trees have extensive root systems, are often dominant which allows them to access more resources like water, nutrients and sunlight (Pretzsch et al. 2014; Jochner et al. 2017). 3) Climatic or environmental conditions at earlier time periods were more conducive to tree growth setting the now larger trees on a different growth trajectory (Melo et al. 2013; Solár and Solár 2020). The comparison between the forest and higher elevation treeline plots showed that spruce trees from treeline are growing, as expected, slower than those from the forest, which was also found by (Körner 1998) indicating small scale variability towards the edge of the distribution of this species. In general, climatic limitations (Körner 1998) and limited access to resources in harsh treeline environments hinder tree growth (Ellison et al. 2019; Hagedorn et al. 2019).
Temporal stability of climate sensitivity
When comparing climate-growth relationship over two distinct intervals, 1960–1990 and 1990–2020, we observed a notable temporal instability (non-stationarity) in these relationships over time, particularly a stronger correlation of climatic indices with tree-ring width (TRW) and maximum latewood density (MXD) chronologies during the early period (Figs. 3, 4 and 5, and 6), (Tables 1 and 2), supported by moving windows correlation analysis (Fig. S4, S5, S6, S7, S8).
The positive correlation with spring and summer month temperatures weakened, while the sensitivity to precipitation and the drought index (SPEI06) was mainly lost in the second time period in both treeline and forest sites. MXD chronologies show the sensitivity of tree growth to moisture availability more pronounced during the second period. Previous studies on the temporal variability of climatic responses of trees from other European spruce sites have also shown similar non-stationarity in climate-growth responses over time (Hasenauer et al. 1999; Wilson and Elling 2004; Büntgen et al. 2006).We also noted that the radial growth responses to October temperatures in the year prior to ring formation became insignificant over the second period (1990–2020) at both sites, possibly due to a warming climate where forest trees are no longer limited by the amount of resources stored at the end of the growing season (Treml et al. 2012; Ponocná et al. 2016). Several studies have examined the change in the relationship between tree growth and climate, yielding variable results. For instance, (Briffa et al. 1998a, b; Solberg et al. 2002; Wilson and Elling 2004; Hauck et al. 2012; Bošela et al. 2014) have reported alterations in tree ring width and density responses to temperature, suggesting potential influences from non-climatic factors, such as human-induced local emissions like SO2 or a combination of SO2 and NOx. In contrast, reduction in tree growth sensitivity to climate over the last few decades could be climatic (Brázdil et al. 2002; Wilson and Elling 2004; Friedrichs et al. 2009; Hauck et al. 2012). Similarly, the choice of detrending method (Esper and Frank 2009), the effects of tree age and variations in microsite conditions (Zhang and Wilmking 2010; Altman et al. 2017) could also be the causes of potential non-significant tree growth correlations. Additionally, the non-stationarity in these correlations may be attributed to the natural ontogenetic dynamics of individual trees as they adapt to various disturbances, competition, and changing environmental conditions (Savva et al. 2002; Smith 2008; Carrer Marco 2011; Zhang et al. 2018).
Climate growth relationship and DBH size class effects
It is commonly believed that large trees are more sensitive to year-to-year climate variations and are more suitable for dendrochronological purposes (Carrer Marco and Urbinati 2004). Consequently, tree-ring studies typically prioritize trees with larger diameters to minimize the influence of competition and to simplify cross-dating, as there are fewer missing rings (Fritts 2014). In this study, climate growth correlations between (TRW and MXD) with climatic variables showed that trees of all diameter classes generally responded to climatic variables (temperature, precipitation and SPEI06) in similar patterns at both geological settings. However, the strength varied among individual size classes (Figs. 3, 4 and 5, and 6), with smaller trees (11–30 cm) often showing weaker sensitivities for both TRW and MXD, while the larger size classes (31–50) cm and (51–70) cm demonstrate stronger climate effects based on the coefficients values of temperature, precipitation and SPEI06 across different bedrock types in both time periods (Tables 1 and 2).(Chhin et al. 2008) found no differences in growth–climate relationships between the diameter classes in Picea abies, but(Meyer and Bräker 2001) suggested the difference to be more related to elevational differences (subalpine versus lower montane). The overall uniformity in the response to climate variables could be attributed to similarities in genetic composition and microclimatic conditions of trees reaching the co-dominant/dominant canopy layer, and potentially the sharing of carbohydrates among trees across various diameter size classes (Fraser et al. 2005; Chhin et al. 2008). Our findings do not fully align with other studies where climate sensitivity remained constant (Esper et al. 2008; Vieira et al. 2009, 2009; Mérian and Lebourgeois 2011), or either increased or decreased with age/size (Carrer Marco and Urbinati 2004; Luis et al. 2009; Zang et al. 2012; Konter et al. 2016; Trouillier et al. 2019; Campbell et al. 2021; Schmied et al. 2022). The variability in climate sensitivity observed across studies highlights the complexity of how tree species respond to climatic factors and the reasons for these divergent conclusions which remain somewhat unclear. Differences in the relationship between growth and climate across different diameter groups can largely be attributed to factors like resource availability, competitive interactions, and physiological processes within trees (Mencuccini et al. 2005). However, most of the previously reported studies are based on comparisons among trees of different ages, while eco-physiological research suggests that these signals are more closely related to tree size rather than age (Peñuelas 2005; Luis et al. 2009). Therefore, this overlap in size and age-related effects due to environmental factors potentially contributed to the divergent findings (Luis et al. 2009).
Bedrock condition and climate sensitivity
The overall pattern of monthly climate growth responses of trees growing in two different bedrock conditions (limestone and granitic) were similar (Figs. 3, 4, 5 and 6), with only slight differences in strength (Fig. S10 and S11). We found the positive effects of temperature and the negative effects of precipitation and SPE106 on both TRW and MXD in our models for both geological conditions and the early and late sub-periods (1960–1990 and 1990–2020) respectively (Table S1 and S2) and (Table S3 and S4). Overall, trees growing on granitic bedrock demonstrate somewhat greater climate sensitivity, with more pronounced responses to both positive (temperature) and negative (precipitation and SPEI06jas) climatic factors (Table S3 and S4). Even though climate sensitivity of growth might not depend on the specific site but is mainly driven by climate conditions (Oberhuber et al. 1998; Boden et al. 2014), differences among geological settings in drought tolerance could be expected because moisture conditions vary within the species’ distribution area and water availability is often the limiting factor for growth (Mäkinen et al. 2002; Andreassen et al. 2006). This differential sensitivity in our two geological sites even when climate is similar might be attributed to the distinct physical and chemical properties of the bedrock (Morford et al. 2011; Hahm et al. 2014; Rempe and Dietrich 2018a; Dai et al. 2018; Jiang et al. 2020). Granitic bedrock, being more acidic and less nutrient-rich than limestone, likely induces greater water stress and nutrient limitations in trees, making them more responsive to climatic variations. Limestone bedrock, characterized by higher pH and nutrient availability, provides a buffering effect, mitigating the impact of climatic stressors on tree growth (Hahm et al. 2014; Zhang et al. 2015; Jiang et al. 2020; Xu et al. 2021). Generally, areas with soluble rock substrates experience significant fluctuations in plant water availability due to intermittent rainfall and droughts, which impact vegetation growth potential (Schwinning 2008; Di Long et al. 2014). Conversely, resistant bedrock types improve water accumulation by acting as an impenetrable barrier, reducing water stress and enhancing plant productivity during extended droughts (Jones and Graham 1993; Wang et al. 2004; Ivits et al. 2014b). But, the negative effects of precipitation (Pamj) and standardized precipitation-evapotranspiration index (SPEI06jas) on RWI and MXD at our granitic site, in both periods suggests that trees on the granitic site are more vulnerable to excessive moisture and related stressors, likely due to poorer water retention capacity and nutrient availability in granitic substrates. In comparison, the limestone site shows a weaker negative response to these variables, indicating that the higher water retention and nutrient-rich limestone soils provide a buffering effect against precipitation-related stress and provide a more stable growing environment (Jiang et al. 2020).
Bedrock plays a crucial role in various ecological processes (Mage and Porder 2013; Gerdol et al. 2016; Delgado-Baquerizo et al. 2020), with its influence likely extending globally. However, most studies on tree growth have traditionally focused on climate and topsoil characteristics such as soil composition, nutrient availability, pH levels, water retention, drainage, and mineral content (Jones and Graham 1993; Vestin et al. 2013; Lévesque et al. 2016; van der Maaten-Theunissen et al. 2016; Gerdol et al. 2016; Rehschuh et al. 2017). In contrast, the importance of deeper belowground components is not well understood (Rose et al. 2003; Richter and Billings 2015b; Jiang et al. 2020). Despite the significant impact of bedrock type on climate sensitivity observed in this study, its broader influence remains underexplored. Further research integrating comprehensive analyses of all relevant variables affecting growth and climate sensitivity, including interactions with soil characteristics, deeper belowground components and water conditions, is essential to establish robust conclusions.
Conclusions
In conclusion, this study sheds light on the complex interplay between tree growth, climate, and geological factors, focusing on Norway spruce in the High Tatra Mountains of Slovakia. The findings indicate that growth rates of Norway spruce have been increasing over time, with limestone bedrock conditions consistently associated with higher growth rates compared to granitic bedrock conditions. Despite varying growth rates, our findings consistently show that temperature positively influences tree growth, whereas precipitation and SPEI06 have negative effects across both bedrock types and time periods. Trees on granitic bedrock exhibit heightened climate sensitivity compared to those on limestone, displaying more pronounced responses to climatic variables. This potentially suggests increased water stress and nutrient limitations in granitic bedrock conditions. Additionally, the magnitude of these effects varies across DBH size classes, with larger trees generally displaying stronger climate sensitivities compared to smaller ones. We observed significant temporal variability (non-stationarity) in the climate-growth relationships across the study periods (1960–1990 and 1990–2020). Specifically, there was a stronger correlation between climatic indices and TRW/MXD chronologies during the earlier period (1960–1990), followed by a decrease in sensitivity during the later period (1990–2020). This shift highlights the dynamic nature of climate influences on tree growth, likely influenced by changing climatic conditions over time. Nevertheless, the impact of bedrock remains somewhat underexplored and no one factor can change without affecting all the others. Hence, our findings highlight the necessity of integrating lithology, climate variables, tree size, environmental factors, and temporal dynamics to comprehensively understand and manage forest ecosystems, particularly in mountainous areas. Further investigation is needed to unravel the intricate interplay among these elements and their enduring consequences for forest dynamics and resilience.
Data availability
Data will be made available on request.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723
Alexander MR, Rollinson CR, Babst F et al (2018) Relative influences of multiple sources of uncertainty on cumulative and incremental tree-ring-derived aboveground biomass estimates. Trees 32:265–276
Altman J, Fibich P, Santruckova H et al (2017) Environmental factors exert strong control over the climate-growth relationships of Picea abies in Central Europe. Sci Total Environ 609:506–516
Andreassen K, Solberg S, Tveito OE et al (2006) Regional differences in climatic responses of Norway spruce (Picea abies L. Karst) growth in Norway. For Ecol Manag 222:211–221
Avanzi C, Piermattei A, Piotti A et al (2019) Disentangling the effects of spatial proximity and genetic similarity on individual growth performances in Norway spruce natural populations. Sci Total Environ 650:493–504
Baker TR, Swaine MD, Burslem DF (2003) Variation in tropical forest growth rates: combined effects of functional group composition and resource availability. Perspectives in Plant Ecology. Evol Syst 6:21–36
Bates D, Mächler M, Bolker B et al (2008) Fitting linear mixed-effects models using the lme4 package in R
Bayat M, Bettinger P, Heidari S et al (2021) A combination of biotic and abiotic factors and Diversity Determine Productivity in Natural Deciduous forests. Forests 12:1450
Beguería S, Vicente-Serrano SM, Reig F et al (2014) Standardized precipitation evapotranspiration index (SPEI) revisited: parameter fitting, evapotranspiration models, tools, datasets and drought monitoring. Int J Climatol 34:3001–3023
Bernes C, Macura B, Jonsson BG et al (2018) Manipulating ungulate herbivory in temperate and boreal forests: effects on vegetation and invertebrates. A systematic review. Environ Evid 7
Bhuyan U, Zang C, Menzel A (2017) Different responses of multispecies tree ring growth to various drought indices across Europe. Dendrochronologia 44:1–8
Bigler C, Veblen TT (2009) Increased early growth rates decrease longevities of conifers in subalpine forests. Oikos 118:1130–1138
Black BA, Colbert JJ, Pederson N (2008) Relationships between radial growth rates and lifespan within north American tree species. Écoscience 15:349–357
Boden S, Kahle H-P, Wilpert K von et al (2014) Resilience of Norway spruce (Picea abies (L.) Karst) growth to changing climatic conditions in Southwest Germany. For Ecol Manag 315:12–21
Bohuš I (1994) Use of natural resources. Využívanie prírodných zdrojov
Bosela M, Tumajer J, Cienciala E et al (2021) Climate warming induced synchronous growth decline in Norway spruce populations across biogeographical gradients since 2000. The Science of the total environment 752:141794
Bošela M, Petráš R, Sitková Z et al (2014) Possible causes of the recent rapid increase in the radial increment of silver fir in the western carpathians. Environ Pollution (Barking Essex: 1987) 184:211–221
Bowman DM, Brienen RJ, Gloor E et al (2013) Detecting trends in tree growth: not so simple. Trends Plant Sci 18:11–17
Brázdil R, Stepankova P, Kyncl T et al (2002) Fir tree-ring reconstruction of March-July precipitation in southern Moravia (Czech Republic), 1376–1996. Climate Res 20:223–239
Brienen RJ, Gloor E, Zuidema PA (2012) Detecting evidence for CO 2 fertilization from tree ring studies: The potential role of sampling biases. Global Biogeochem. Cycles 26:n/a-n/a
Briffa KR, Schweingruber FH, Jones PD et al (1998a) Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature 391:678–682
Briffa KR, Schweingruber FH, Jones PD et al (1998b) Trees tell of past climates: but are they speaking less clearly today? Ser B Biol Sci 353:65–73Philosophical transactions of the Royal Society of London
Bugmann H (1997) Sensitivity of forests in the European Alps to future climatic change. Clim Res 8:35–44
Bunn AG (2008) A dendrochronology program library in R (dplR). Dendrochronologia 26:115–124
Bunn AG, Sharac TJ, Graumlich LJ (2004) Using a Simulation Model to compare methods of Tree-Ring Detrending and to investigate the detectability of low-frequency signals. Tree-Ring Res 60:77–90
Büntgen U, Esper J, Frank DC et al (2005) A 1052-year tree-ring proxy for Alpine summer temperatures. Clim Dyn 25:141–153
Büntgen U, Frank DC, Schmidhalter M et al (2006) Growth/climate response shift in a long subalpine spruce chronology. Trees 20:99–110
Büntgen U, Frank DC, Kaczka RJ et al (2007) Growth responses to climate in a multi-species tree-ring network in the Western Carpathian Tatra Mountains, Poland and Slovakia. Tree Physiol 27:689–702
Campbell EM, Magnussen S, Antos JA et al (2021) Size-, species‐, and site‐specific tree growth responses to climate variability in old‐growth subalpine forests. Ecosphere 12
Carrer Marco (2011) Individualistic and time-varying tree-ring growth to climate sensitivity. PLoS ONE 6:e22813
Carrer Marco, Urbinati C (2004) AGE-DEPENDENT TREE-RING GROWTH RESPONSES TO CLIMATE IN LARIX DECIDUA AND PINUS CEMBRA. Ecology 85:730–740
Chakraborty T, Saha S, Matzarakis A et al (2017) Influence of multiple biotic and abiotic factors on the crown die-back of European beech trees at their drought limit. Flora 229:58–70
Chhin S, Hogg EH, Lieffers VJ et al (2008) Potential effects of climate change on the growth of lodgepole pine across diameter size classes and ecological regions. For Ecol Manag 256:1692–1703
Cienciala E, Russ R, Šantrůčková H et al (2016) Discerning environmental factors affecting current tree growth in Central Europe. Sci Total Environ 573:541–554
Cienciala E, Altman J, Doležal J et al (2018) Increased spruce tree growth in Central Europe since 1960s. Sci Total Environ 619–620:1637–1647
Conedera M, Colombaroli D, Tinner W et al (2017) Insights about past forest dynamics as a tool for present and future forest management in Switzerland. For Ecol Manag 388:100–112
Cook ER (1985) A Time Series Analysis Approach to Tree Ring standardization. PhD, School of Renewable Natural Resources
Cook E, Briffa K, Shiyatov S et al (1990) Data Analysis. Methods of Dendrochronology. Springer, Dordrecht, pp 97–162
Dai Q, Peng X, Wang P et al (2018) Surface erosion and underground leakage of yellow soil on slopes in karst regions of southwest China. Land Degrad Dev 29:2438–2448
de Luis M, Novak K, Čufar K et al (2009) Size mediated climate–growth relationships in Pinus halepensis and Pinus pinea. Trees 23:1065–1073
Delgado-Baquerizo M, Reich PB, Trivedi C et al (2020) Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat Ecol Evol 4:210–220
Duchesne L, Houle D, Ouimet R et al (2019) Large apparent growth increases in boreal forests inferred from tree-rings are an artefact of sampling biases. Sci Rep 9:6832
Ellison SB, Sullivan PF, Cahoon SM et al (2019) Poor nutrition as a potential cause of divergent tree growth near the Arctic treeline in northern Alaska. Ecology 100:e02878
Esper J, Frank D (2009) Divergence pitfalls in tree-ring research. Clim Change 94:261–266
Esper et al (2003) Tests of the RCS method for preserving low-frequency variability in long tree-ring chronologies
Esper J, Niederer R, Bebi P et al (2008) Climate signal age effects—evidence from young and old trees in the Swiss Engadin. For Ecol Manag 255:3783–3789
Esperon-Rodriguez M, Power SA, Tjoelker MG et al (2021) Contrasting heat tolerance of urban trees to extreme temperatures during heatwaves. Urban Forestry Urban Green 66:127387
Fei S, Desprez JM, Potter KM et al (2017) Divergence of species responses to climate change. Sci Adv 3:e1603055
Filotas E, Parrott L, Burton PJ et al (2014) Viewing forests through the lens of complex systems science. Ecosphere 5:1–23
Frank DA, Gehring CA, Machut L et al (2003) Soil community composition and the regulation of grazed temperate grassland. Oecologia 137:603–609
Fraser EC, Lieffers VJ, Landhäusser SM (2005) Age, stand density, and tree size as factors in root and basal grafting of lodgepole pine. Can J Bot 83:983–988
Friedrichs DA, Trouet V, Büntgen U et al (2009) Species-specific climate sensitivity of tree growth in Central-West Germany. Trees 23:729–739
Fritts H (2014) Tree rings and Climate. Elsevier Science, Saint Louis
Gawęda A, Müller A, Stein H et al (2013) Age and origin of the tourmaline-rich hydraulic breccias in the Tatra Granite, Western carpathians. Jour Geosci :133–148
Gerdol R, Marchesini R, Iacumin P (2016) Bedrock geology interacts with altitude in affecting leaf growth and foliar nutrient status of mountain vascular plants. JPECOL:rtw092
Gourlet-Fleury S, Rossi V, Forni E et al (2023) Competition and site weakly explain tree growth variability in undisturbed Central African moist forests. J Ecol 111:1950–1967
Gray SB, Brady SM (2016) Plant developmental responses to climate change. Dev Biol 419:64–77
Hagedorn F, Gavazov K, Alexander JM (2019) Above- and belowground linkages shape responses of mountain vegetation to climate change, vol 365. Science, New York, N.Y., pp 1119–1123
Hahm WJ, Riebe CS, Lukens CE et al (2014) Bedrock composition regulates mountain ecosystems and landscape evolution. Proc Natl Acad Sci USA 111:3338–3343
Harris I, Osborn TJ, Jones P et al (2020) Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci data 7:109
Harsch MA, Hulme PE, McGlone MS et al (2009) Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol Lett 12:1040–1049
Hasenauer H, Nemani RR, Schadauer K et al (1999) Forest growth response to changing climate between 1961 and 1990 in Austria. For Ecol Manag 122:209–219
Hauck M, Zimmermann J, Jacob M et al (2012) Rapid recovery of stem increment in Norway spruce at reduced SO2 levels in the Harz Mountains, Germany. Environmental pollution (Barking, Essex: 1987) 164:132–141
Hentschel R, Rosner S, Kayler ZE et al (2014) Norway spruce physiological and anatomical predisposition to dieback. For Ecol Manag 322:27–36
Ivits E, Horion S, Fensholt R et al (2014a) Drought footprint on European ecosystems between 1999 and 2010 assessed by remotely sensed vegetation phenology and productivity. Glob Change Biol 20:581–593
Ivits E, Horion S, Fensholt R et al (2014b) Drought footprint on European ecosystems between 1999 and 2010 assessed by remotely sensed vegetation phenology and productivity. Glob Change Biol 20:581–593
Jiang Z, Liu H, Wang H et al (2020) Bedrock geochemistry influences vegetation growth by regulating the regolith water holding capacity. Nat Commun 11:2392
Jochner M, Bugmann H, Nötzli M et al (2017) Among-tree variability and feedback effects result in different growth responses to climate change at the upper treeline in the Swiss Alps. Ecol Evol 7:7937–7953
Jones DP, Graham RC (1993) Water-holding characteristics of Weathered Granitic Rock in Chaparral and Forest ecosystems. Soil Sci Soc Am J 57:256–261
Kłapyta P, Kołaczek P (2009) The last millenium slope processes and anthropogenic activity recorded in the sediments from the pyszniañska glade, western Tatra Mountains (Poland). Studia Geomorphologica Carpatho-Balcanica 43:145–163
Konter O, Büntgen U, CARRER M et al (2016) Climate signal age effects in boreal tree-rings: lessons to be learned for paleoclimatic reconstructions. Q Sci Rev 142:164–172
Körner C (1998) A re-assessment of high elevation treeline positions and their explanation. Oecologia 115:445–459
Körner C, Riedl S (2012) Alpine treelines. Functional ecology of the global high elevation tree limits. Springer, Basel, London
Kreuzwieser J, Gessler A (2010) Global climate change and tree nutrition: influence of water availability. Tree Physiol 30:1221–1234
Lebourgeois F, Rathgeber CB, Ulrich E (2010) Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba, Picea abies and Pinus sylvestris). J Veg Sci 21:364–376
Lefcheck JS (2016) piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579
Lévesque M, Saurer M, Siegwolf R et al (2013) Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob Change Biol 19:3184–3199
Lévesque M, Walthert L, Weber P (2016) Soil nutrients influence growth response of temperate tree species to drought. J Ecol 104:377–387
Lüdecke D, Patil I, Ben-Shachar M et al (2021) See: an R Package for visualizing statistical models. JOSS 6:3393
Mage SM, Porder S (2013) Parent Material and Topography Determine Soil Phosphorus Status in the Luquillo Mountains of Puerto Rico. Ecosystems 16:284–294. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s10021-012-9612-5
Mäkinen H, Nöjd P, Kahle H-P et al (2002) Radial growth variation of Norway spruce (Picea abies (L.) Karst.) Across latitudinal and altitudinal gradients in central and northern Europe. For Ecol Manag 171:243–259
Marchand W, Buechling A, Rydval M et al (2022) Accelerated Growth Rates of Norway Spruce and European Beech Saplings from Europe’s Temperate Primary Forests Related to Warmer Conditions. SSRN Journal
Marchin RM, Esperon-Rodriguez M, Tjoelker MG et al (2022) Crown dieback and mortality of urban trees linked to heatwaves during extreme drought. Sci Total Environ 850:157915
Matskovsky V, Venegas-González A, Garreaud R et al (2021) Tree growth decline as a response to projected climate change in the 21st century in Mediterranean mountain forests of Chile. Glob Planet Change 198:103406. https://www.sciencedirect.com/science/article/pii/s0921818120302976
Melo M, Lapin M, Kapolková H et al (2013) Climate trends in the Slovak Part of the carpathians. In: Kozak J, Ostapowicz K, Bytnerowicz A et al (eds) The carpathians: integrating Nature and Society towards sustainability. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 131–150
Mencuccini M, Martínez-Vilalta J, Vanderklein D et al (2005) Size-mediated ageing reduces vigour in trees. Ecol Lett 8:1183–1190
Mérian P, Lebourgeois F (2011) Size-mediated climate–growth relationships in temperate forests: a multi-species analysis. For Ecol Manag 261:1382–1391
Meyer FD, Bräker OU (2001) Climate response in dominant and suppressed spruce trees, Picea abies (L.) Karst., on a subalpine and lower montane site in Switzerland. Écoscience 8:105–114
Milodowski DT, Mudd SM, Mitchard ET (2015) Erosion rates as a potential bottom-up control of forest structural characteristics in the Sierra Nevada Mountains. Ecology 96:31–38
Mitchell A (1976) A field guide to the trees of Britain and Northern Europe. Collins, London
Mitchell FJ (2005) How open were European primeval forests? Hypothesis testing using palaeoecological data. J Ecol 93:168–177
Monger C, Sala OE, Duniway MC et al (2015) Legacy effects in linked ecological–soil–geomorphic systems of drylands. Front Ecol Environ 13:13–19
Morford SL, Houlton BZ, Dahlgren RA (2011) Increased forest ecosystem carbon and nitrogen storage from nitrogen rich bedrock. Nature 477:78–81. https://www.nature.com/articles/nature10415
Muggeo V, Atkins DC, Gallop RJ et al (2014) Segmented mixed models with random changepoints: a maximum likelihood approach with application to treatment for depression study. Stat Modelling 14:293–313
Nehrbass-Ahles C, Babst F, Klesse S et al (2014) The influence of sampling design on tree-ring-based quantification of forest growth. Glob Change Biol 20:2867–2885
Niedzwiedz T (1992) Climate of the Tatra Mountains. Mt Res Dev 12:131
Oberhuber W, Stumböck M, Kofler W (1998) Climate-tree-growth relationships of scots pine stands (Pinus sylvestris L.) exposed to soil dryness. Trees 13:19–27
Oboite FO, Comeau PG (2019) Competition and climate influence growth of black spruce in western boreal forests. For Ecol Manag 443:84–94
Pastore G, Weig AR, Vazquez E et al (2022) Weathering of calcareous bedrocks is strongly affected by the activity of soil microorganisms. Geoderma 405:115408
Peñuelas J (2005) Plant physiology: a big issue for trees. Nature 437:965–966
Peters RL, Groenendijk P, Vlam M et al (2015) Detecting long-term growth trends using tree rings: a critical evaluation of methods. Glob Change Biol 21:2040–2054
Petitcolas V, Rolland C, Michalet R (1997) Croissance De l’épicéa, Du mélèze, Du Pin Cembro Et Du Pin à crochets en limite supérieure de la forêt dans quatre régions des alpes françaises. Ann Sci 54:731–745
Piscová V, Hreško J, Ševčík M et al (2023) Impacts of human activities on the High Mountain Landscape of the Tatras (Example of the Border Area of the high and Belianske Tatras, Slovakia). In: Hufnagel L, El-Esawi A M (eds) Vegetation Dynamics. Changing Ecosystems and Human Responsibility. IntechOpen
Ponocná T, Spyt B, Kaczka R et al (2016) Growth trends and climate responses of Norway spruce along elevational gradients in East-Central Europe. Trees 30:1633–1646
Pretzsch H (2021) Tree growth as affected by stem and crown structure. Trees 35:947–960
Pretzsch H, Biber P, Schütze G et al (2014) Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat Commun 5:4967
R Core Team (2020) R. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Rączkowska Z (2019) Human impact in the Tatra Mountains. CIG 45:219–244
Ramirez JI, Jansen PA, Poorter L (2018) Effects of wild ungulates on the regeneration, structure and functioning of temperate forests: a semi-quantitative review. For Ecol Manag 424:406–419
Rehschuh R, Mette T, Menzel A et al (2017) Soil properties affect the drought susceptibility of Norway spruce. Dendrochronologia 45:81–89
Rempe DM, Dietrich WE (2018a) Direct observations of rock moisture, a hidden component of the hydrologic cycle. Proc Natl Acad Sci USA 115:2664–2669
Rempe DM, Dietrich WE (2018b) Direct observations of rock moisture, a hidden component of the hydrologic cycle. Proc Natl Acad Sci USA 115:2664–2669
Richter Dd, Billings SA (2015a) One physical system’: Tansley’s ecosystem as Earth’s critical zone. New Phytol 206:900–912
Richter Dd, Billings SA (2015b) One physical system’: Tansley’s ecosystem as Earth’s critical zone. New Phytol 206:900–912
Robertson PA (1992) Factors affecting Tree Growth on three Lowland sites in Southern Illinois. Am Midl Nat 128:218
Rose KL, Graham RC, Parker DR (2003) Water source utilization by Pinus jeffreyi and Arctostaphylos patula on thin soils over bedrock. Oecologia 134:46–54
Rossi S, Deslauriers A, Griçar J et al (2008) Critical temperatures for xylogenesis in conifers of cold climates. Glob Ecol Biogeogr 17:696–707
San-Miguel-Ayanz J, de Rigo D, Caudullo G et al (eds) (2016) European atlas of forest tree species. Publication Office of the European Union, Luxembourg
Savva Y, Schweingruber F, Milyutin L et al (2002) Genetic and environmental signals in tree rings from different provenances of Pinus sylvestris L. planted in the southern taiga, central Siberia. Trees 16:313–324
Savva Y, Oleksyn J, Reich PB et al (2006) Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains. Pol Trees 20:735–746
Schmied G, Hilmers T, Uhl E et al (2022) The past matters: previous management Strategies Modulate Current Growth and Drought responses of Norway Spruce (Picea abies H. Karst). Forests 13:243
Schurman JS, Babst F, Björklund J et al (2019) The climatic drivers of primary Picea forest growth along the Carpathian arc are changing under rising temperatures. Glob Change Biol 25:3136–3150
Schweingruber FH, Fritts HC, Bräker OU et al (1978) The X-Ray technique as Applied to Dendroclimatology. 0041–2198. https://repository.arizona.edu/handle/10150/260420
Schwinning S (2008) The water relations of two evergreen tree species in a karst savanna. Oecologia 158:373–383
Seidl R, Thom D, Kautz M et al (2017) Forest disturbances under climate change. Nat Clim Change 7:395–402
Smith KT (2008) An organismal view of dendrochronology. Dendrochronologia 26:185–193
Solár J, Solár V (2020) Land-cover change in the Tatra Mountains, with a particular focus on vegetation. Ecomont 12:15–26
Solberg B, Hofgaard A, Hytteborn H (2002) Shifts in radial growth responses of coastal Picea abies induced by climatic change during the 20 th century, central Norway. Écoscience 9:79–88
Speed JD, Martinsen V, Hester AJ et al (2015) Continuous and discontinuous variation in ecosystem carbon stocks with elevation across a treeline ecotone. Biogeosciences 12:1615–1627. https://bg.copernicus.org/articles/12/1615/2015/
Speer JH (2012) Fundamentals of Tree Ring Research. University of Arizona, Tucson
Splechtna BE, Gratzer G, Black BA (2005) Disturbance history of a European old-growth mixed‐species forest—A spatial dendro‐ecological analysis. J Veg Sci 16:511–522
Szewczyk J, Szwagrzyk J, Muter E (2011) Tree growth and disturbance dynamics in old-growth subalpine spruce forests of the western carpathians. Can J Res 41:938–944
Thornthwaite CW (1948) An Approach toward a rational classification of climate. Geogr Rev 38:55
Treml V, Ponocná T, Büntgen U (2012) Growth trends and temperature responses of treeline Norway spruce in the Czech-Polish Sudetes mountains. Climate Res 55:91–103
Trouillier M, van der Maaten-Theunissen M, Scharnweber T et al (2019) Size matters—a comparison of three methods to assess age- and size-dependent climate sensitivity of trees. Trees 33:183–192
Trouillier M, van der Maaten-Theunissen M, Scharnweber T et al (2020) A Unifying Concept for Growth Trends of Trees and Forests – The Potential Natural Forest. Front. For. Glob. Change 3
Valsecchi V, Carraro G, Conedera M et al (2010) Late-holocene vegetation and land-use dynamics in the Southern Alps (Switzerland) as a basis for nature protection and forest management. Holocene 20:483–495
van der Maaten-Theunissen M, Bümmerstede H, Iwanowski J et al (2016) Drought sensitivity of beech on a shallow chalk soil in northeastern Germany – a comparative study. For. Ecosyst. 3
van der Wal R, Bardgett RD, Harrison KA et al (2004) Vertebrate herbivores and ecosystem control: cascading effects of faeces on tundra ecosystems. Ecography 27:242–252
van Mantgem PJ, Stephenson NL, Byrne JC et al (2009) Widespread increase of tree mortality rates in the western United States. Science (New York, N.Y.) 323:521–524
Vestin JL, Söderberg U, Bylund D et al (2013) The influence of alkaline and non-alkaline parent material on Norway spruce tree chemical composition and growth rate. Plant Soil 370:103–113
Vicente-Serrano SM, Beguería S, López-Moreno JI (2010) A Multiscalar Drought Index sensitive to global warming: the standardized precipitation Evapotranspiration Index. J Clim 23:1696–1718
Vieira J, Campelo F, Nabais C (2009) Age-dependent responses of tree-ring growth and intra-annual density fluctuations of Pinus pinaster to Mediterranean climate. Trees 23:257–265
Wang S-J, Li R-L, Sun C-X et al (2004) How types of carbonate rock assemblages constrain the distribution of karst rocky desertified land in Guizhou Province, PR China: phenomena and mechanisms. Land Degrad Dev 15:123–131
Wang J, Ouyang F, An S et al (2022) Variation, coordination, and trade-offs between needle structures and photosynthetic-related traits across five Picea species: consequences on plant growth. BMC Plant Biol 22:242
Wei L, Hai-Zhou H, Zhi-Nan Z et al (2011) Effects of grazing on the soil properties and C and N storage in relation to biomass allocation in an alpine meadow. J Soil Sci Plant Nutr 11:27–39
Weiskopf SR, Rubenstein MA, Crozier LG et al (2020) Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci Total Environ 733:137782
Wigley TM, Briffa KR, Jones PD (1984) On the average value of correlated Time Series, with applications in Dendroclimatology and hydrometeorology. J Clim Appl Meteor 23:201–213
Wilmking M, van der Maaten-Theunissen M, van der Maaten E et al (2020) Global assessment of relationships between climate and tree growth. Glob Change Biol 26:3212–3220
Wilson R, Elling W (2004) Temporal instability in tree-growth/climate response in the Lower Bavarian Forest region: implications for dendroclimatic reconstruction. Trees 18:19–28
Xu T, Wu X, Tian Y et al (2021) Soil property plays a vital role in Vegetation Drought Recovery in Karst Region of Southwest China. JGR Biogeosciences 126
Yu L, Chen Y, Sun W et al (2019) Effects of grazing exclusion on soil carbon dynamics in alpine grasslands of the Tibetan Plateau. Geoderma 353:133–143
Zang C, Biondi F (2015) Treeclim: an R package for the numerical calibration of proxy-climate relationships. Ecography 38:431–436
Zang C, Pretzsch H, Rothe A (2012) Size-dependent responses to summer drought in scots pine, Norway spruce and common oak. Trees 26:557–569
Zang C, Hartl-Meier C, Dittmar C et al (2014) Patterns of drought tolerance in major European temperate forest trees: climatic drivers and levels of variability. Glob Change Biol 20:3767–3779
Zelina V (1965) Pohyb populácie svišťov v Belianskych Tatrách. Zborník prác o TANAP-e 8:173–189
Zhang Y, Wilmking M (2010) Divergent growth responses and increasing temperature limitation of Qinghai spruce growth along an elevation gradient at the northeast Tibet Plateau. For Ecol Manag 260:1076–1082
Zhang L, Guo S, Wu B (2015) The source, Spatial Distribution and Risk Assessment of Heavy Metals in Soil from the Pearl River Delta Based on the National Multi-purpose Regional Geochemical Survey. PLoS ONE 10:e0132040
Zhang Y, Guo M, Wang X et al (2018) Divergent tree growth response to recent climate warming of Abies faxoniana at alpine treelines in east edge of Tibetan Plateau. Ecol Res 33:303–311
Zielonka T, Malcher P (2009) The dynamics of a mountain mixed forest under wind disturbances in the Tatra Mountains, central Europe — a dendroecological reconstruction. Can J Res 39:2215–2223
Zuur AF, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York, N.Y
Acknowledgements
We especially thank Michal Gazovic (Ph.D.), for his help in organizing and executing parts of the field work throughout the project. We thank Anne Huber and Evelyn von Saucken for their help in preparing samples and data acquisition. Further, we acknowledge Tatranský národný park (TANAP), Slovakia for research permission and their support during tree-ring sampling.
Funding
This research was funded by the German Research Foundation (DFG) within the Research Training Group RESPONSE (DFG RTG 2010).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MW designed the overall study design. SB, AB and FM collected samples with the help of others. SB prepared and analyse the samples and wrote the manuscript with contributions from all authors. All authors revised and refined the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tianjian Cao.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Basnet, S., Burger, A., Homolová, Z. et al. Effect of bedrock, tree size and time on growth and climate sensitivity of Norway spruce in the High Tatras. Eur J Forest Res (2024). https://doi.org/10.1007/s10342-024-01725-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10342-024-01725-6