Abstract
We sampled the small mammal (SM) community in mountain forest ecosystems of the Beskydy Mountains over 5 years in order to study associations with different types of forest habitat. Fourteen species were determined, three being eudominant (yellow-necked mouse—Apodemus flavicollis (45 %), bank vole—Clethrionomys glareolus (23.3 %) and field vole—Microtus agrestis (15.7 %) and one dominant common shrew—Sorex araneus (9.3 %)). Highest abundance was observed in young succession sites (plantations) with dicotyledonous plants dominant (>50 %) in the undergrowth. Highest diversity was observed in plantations and primeval forests. Lowest total abundance and diversity were observed in mature monocultures. Significant differences in diversity were only found between old monocultures and other sites. Using a faunistic similarity index, two basic SM community groups were determined: those inhabiting (1) early (plantation) and late (reserve) successional forest ecosystems with a dense dicotyledonous plant herb layer, and (2) plantations with a dense grass herb layer and forests with a dense canopy closure (fruiting monoculture). Redundancy analysis confirmed separate habitat preferences of the three eudominant species. Generalised linear model indicated increasing preference of field vole for plantations with dominance of grass and <10–15 % admixed dicotyledonous plants while decreasing preference at ratios >10–15 %, and increasing preference of bank vole for plantations with a dicotyledonous plant ratio of >10–15 %. The biotopes monitored proved suitable for long-term survival of the dominant SM species. Early successional plantations and forest reserves also represent important refuges for a number of rarer SM species presently under threat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Western Carpathian mountain range (Czech Republic, Slovakia and Poland), an outer part of the Carpathian sub-province, plays a key role in the protection of biodiversity of mountain forest ecosystem in Central and Eastern Europe. Over recent decades, however, anthropogenic factors have brought about significant changes in forest cover, and a decline in core forests in particular, with increasing disruption, perforation and patchiness resulting in significant impacts on the character of various animal communities, including that of small mammals (SM; Kozak et al. 2007). Most forest stands in the Western Carpathians are characterised by intensive forest management, which results in the formation of even-aged monocultures that, in their present state, provide optimal habitats for only a select spectrum of SMs whose food supply comprises predominantly forest tree seeds and herb layer biomass (Holišová 1971; Hansson 1985; Heroldová 2002; Heroldová et al. 2008). In many cases, however, these monocultures are slowly being converted into mixed, near-natural forests. In addition, a number of monoculture stands were badly damaged by industrial air pollution over the 1970s and 1980s, resulting in vast sections being clear-cut, particularly in the highest parts of the mountains. These sites formerly represented a specific environment for SM communities (Čapek et al. 1998; Bryja et al. 2002).
Small mammal community structure in forest habitats is not accidental, being conditioned by forest area, dietary specialisation of individual species and forest habitat structure and diversity (Gurnell 1985; Flowerdew et al. 1985; Ecke et al. 2001; Schmid-Holmes and Drickamer 2001; Niedziałlkowska et al. 2010). Community structure in biotopes with a high ratio of fruiting trees and high tree seed production, for example, will be characterised by a high dominance of granivorous species, such as the genus Apodemus. On the other hand, habitats with a dense herb layer and low canopy closure will be characterised by a dominance of herbivorous species, such as the bank vole or voles of the genus Microtus. Forest stands are also subject to successional development, and each long-term change in habitat structure is accompanied by changes in SM community structure across the temperate forests sides (Gurnell 1985; Sullivan et al. 2009).
The Western Carpathians belong to a Protected Landscape Area (larger areas with harmoniously formed landscapes, with some close to nature ecosystems) with a number of natural nature reserves (protected areas of importance for wildlife, flora, fauna or geology which is reserved and managed for conservation). These represent important refuges for communities of SM as a whole of which some species are regionally endangered and protected (Alpine shrew—S. alpinus, Hazel dormouse—Muscardinus avellanarius, Northern birch mouse—Sicista betulina, Edible dormouse—Glis glis Plesník et al. 2003).
A detailed knowledge of SM communities in a given forest habitat allows one to predict changes in the community and the response of the forest ecosystem, and thence undertake appropriate conservation measures.
The aim of this paper was to analyse five years of SM community abundance records from mountain forest ecosystems subject to differing degrees of management and to describe SM species composition and diversity in relation to their habitat preferences in particular biotope structure.
Materials and methods
Study area and experimental plots
The study was conducted in the Moravian–Silesian Beskid (Beskydy) Mountains (Czech Republic) on the edge of the outer Western Carpathians. The vast majority of local forest stands are of anthropogenic origin and comprise primarily species-poor Norway spruce (Picea abies) monocultures (75 %) that have been affected by industrial air pollution in the past. As broad-leaved tree species form only about 25 % of the forests, present tendency is to convert these monocultures into near-natural forests with a high ratio of broadleaf tree species, predominantly European beech (Fagus sylvatica) (Bryja et al. 2002). Twenty-five forest sites covering a minimum of 0.25 ha were selected for the study. The plots differed in stand management character and ranged from early successional forest developmental stages (plantations) to production monocultures and forest reserves. All biotopes were surrounded by other forest habitats, regardless of their respective successional stages. Each plot was defined according to selected environmental variables [electronic supplement (ES; Tab. 1)]. As granivorous SM species dynamics in the study area are also influenced by food supply (primarily beech nuts), mast harvests were also monitored in fruiting beech monocultures with average 42 g/m2 of beech nuts in 2007, 2009 and 2011 mast years. As the trapping period was in autumn, European blueberry (Vaccinium myrtillus), blackberry (Rubus sp.) and European mountain ash (Sorbus aucuparia) fruit contributed to the food supply of rodents.
The 25 forest sites differed in tree species composition, exposure, successional stage, character and composition of herb layer (for details see ES; Tab. 1), potential food supply and management system applied. Plot definitions were based on current cultivated tree species composition and on forest typology according to Randuška et al. 1986 The following brief descriptions outline the basic divisions of the experimental plots into groups (for details see ES; Tab. 1). In individual plots, besides the dicotyledons (dicots) as a whole, forest weeds were of special importance to forest regeneration. Forest weeds were defined as a bulky dicots which may slow the growth of young trees, especially during the first years after implantation (for example: blackberry, stinging nettle—Urtica dioica, bracken—Pteridium sp.) Stand age refers to 2007. Each grouping contains information on the number of trapping nights (TN; number of traps multiplied by the number of nights in which the traps were active; number of traps in each biotope depends on size of plot) used for SM collection over the 5-year period.
-
1.
Plantations with dicots dominant (>50 %) in the undergrowth—six plots.
The plots were mainly located around the Smrk mountain complex (49°30′N, 18°22′E). Young plantations of 3–7 years (plots 5, 6, 15, 18) and plantations of 9–13 years (plots 10, 11), with European beech and European silver fir (Abies alba), and European blueberry dominant in the undergrowth.
TN: 510 per plot
-
2.
Plantations with grasses dominant (>50 %) in the undergrowth—twelve plots.
Young plantations of 1–9 years (plots 7, 8, 9, 12, 13) located around the Kněhyně mountain complex (49°29′N, 18°18′E) and plantations of 6–10 years (plots 1–4) located around the Smrk mountain complex (49°30′N, 18°22′E). Plantations of European beech and European silver fir, with grasses (e.g. reed grass—Calamagrostis sp., hair grass—Deschampsia sp.) dominant in the undergrowth.
TN: 510 per plot
-
3.
Natural forest reserves—four plots.
The reserves are located within the supra-regional Kněhyně-Čertův Mlýn bio-centre (49°29′N, 18°18′E) and include plots 19–21 and 23. These non-intervention forest stands have a primeval forest character and are at the disintegration stage. They are situated mainly at elevations of between 1,120–1,200 m and are comprised predominantly of European mountain ash or Norway spruce. The undergrowth is dominated by ferns (e.g. broad buckler fern—Dryopteris dilatata and Alpine lady-fern—Athyrium distentifolium), feathered reed grass (Calamagrostis arundinacea) and European blueberry.
TN: 1,500 per plot
-
4.
Fruiting tree monocultures—three plots.
The plots are located on two mountains in the Kněhyně mountain complex (49°30′N, 18°18′E), plot 22 on the southern slope of Malá Stolová (TN 1,500) and plot 24 on Velká Stolová (TN 750). The stands are comprised of mature European beech forests with feathered reed grass dominant in the undergrowth. Plot 25, also on Velká Stolová, is a Norway spruces monoculture, an old production stand (>100 years) at felling age with reduced undergrowth (TN 750).
Small mammal collection
Small mammals were collected once in the autumn (September) of 2007–2011 in snap traps placed in lines approximately 3 m apart. Trapping intensity at each site varied depending upon how the research plots had been used under two previous projects. In plots 1–18, 34 traps were placed per line, 100 traps per line in plots 19–23 and 50 traps per line in plots 24 and 25. One trapping session took 4 days, the traps being exposed for three consecutive nights and checked the following day. They were baited with a candle wick fried in flour and animal fat and smeared with peanut butter. For evaluation, we specified community structure as a number of species in SM community and number of its individuals. Occurrence of species in particular biotopes under study within the area was specified as a species distribution. All aspects of trapping complied with EU Council Directive 86/609/EEC on experimental use of animals.
Data preparation and analysis
The relative abundance of a population was determined as the number of individuals collected per 100 traps and nights. The abundance index (I) was calculated according to the equation I = 100 × n/TN, where n is the number of individuals collected in the given period, and TN is number of traps multiplied by trapping nights. All species were classified according to Tischler’s scale for species dominance (i.e. eudominant = dominance D > 10 %, dominant = 10 % > D > 5 %, subdominant = 5 % > D > 2 %, recedent = 2 % > D > 1 % and sub-recedent = 1 % > D > 0 %; )Tischler 1949.
Community similarity within individual habitats was tested using the quantitative Renkonen dominance similarity index R = Σd min, where d min is the lower dominance threshold in the samples compared. The resulting values are expressed by numbers ranging from 0 to 1, where 0 indicates lowest similarity and 1 the highest. The values obtained were subsequently analysed by cluster analysis using Ward’s fusing method.
As species richness and abundance are expected to differ in individual habitat types, we applied standard indicators, such as Shannon’s Index (H), using the natural logarithm; the Reciprocal Simpson Index (1/D); and Shannon’s evenness (E) and Simpson’s evenness (E1/D) scores, each of which differ in their focus on a sample’s species richness and/or abundance/dominance of the most abundant species in a sample (see Magurran ,Magurran 1988 Magurran 2004for definitions) to assess diversity and heterogeneity.
One-way ANOVA was used to test for significant differences in relative abundance and species dominance, and for differences in habitat characteristics. In cases where differences were statistically significant, a post hoc test (Tukey’s honest significant difference method) was applied for multiple comparisons. Linear regression analysis (StatSoft Inc. ) 2012 was used to verify whether environmental variables changed in relation to altitude.
The influence of environmental variables on species spectrum and distribution of individual species was tested using redundancy analysis (RDA) and partial RDA, applying the split-plot design and the Monte Carlo permutation test (999 permutations). Data were log(y + 1) transformed, and, prior to RDA analysis, detrended correspondence analysis (DCA) was undertaken to check for unimodal distribution of the data (Ter Braak and Šmilauer 2002; Lepš and Šmilauer 2003). The length of the longest gradient was >3. Thus, RDA was considered suitable for these data sets. Following Blanchet et al. 2008, we then ran a global RDA with the set of all explanatory variables to model the response variable for matrix. As the global test was significant for a given set of variables, we then used partial RDA to test each variable in order to reveal its marginal effect on SM species. Subsequently, we used stepwise selection, following Lepš and Šmilauer 2003, to select an appropriate subset of predictors. The relationship between environmental factors and selected SM species was assessed using species response curves and by applying a generalised linear model (GLM) (Ter Braak and Šmilauer 2002; Lepš and Šmilauer 2003).
All calculations were undertaken using the Statistica CZ 10.0, CANOCO 4.5 for Windows and CanoDraw 4.1 for Windows software packages.
Results
A total of 14 SM species were collected, including nine rodent species (Rodentia) and five insectivores (Eulipotyphla). Three species were eudominant, the yellow-necked mouse at 45 %, bank vole at 23.3 % and the field vole at 15.7 %, the three together representing 84 % of all individuals collected (ES; Tab. 2b). The remaining species included the dominant common shrew at 9.3 %, subdominant wood mouse (A. sylvaticus) at 2.7 %, two recedent species and seven sub-recedent species.
Yellow-necked mouse was dominant at 12 of the 25 sites evaluated (ES; Tab. 2a–c) and also in some European beech plantations (plots 1–4; D = 60–90.5 %, I = 3.5–5.7), which are considered unsuitable habitats owing to the presence of a thick herb layer and an absence of fruiting trees. Yellow-necked mouse was most abundant in mature European beech monocultures with fruiting trees (plots 22 and 24; D = 71–75 %, I = 2.8–4.0) and showed very high dominance at plot 25, a P. abies monoculture, though abundance was significantly lower, due to a limited food supply (D = 77.8 %, I = 0.9). Bank vole was dominant at nine plots, abundance being highest in plantations with dicotyledonous plants dominant in the herb layer (D = 41.7–63 %, I = 1.8–3.3), and especially in plots with European blueberry (plots 5, 6, 11, 14, 16 and 18; Tab. 1). Highest dominance and abundance were observed in primeval forests reserves (plots 19, 21, 23), also characterised by a species-rich herb layer (D = 38–54.8 %, I = 1.3–2.3). Field voles were dominant at four plantations (plots 7, 8, 12, 13) with a dense cover of grasses (D = 50–55.9 %, I = 2.7-3.1; ES; Tab. 2a–c). Common shrew was the only insectivorous species to reach eudominance, doing so at ten plots (ES; Tab. 2b). Highest abundance (D > 20 %, I = 1.0–1.4) was achieved in plantations with a dense dicots herb layer (mostly European blueberry), though it was also eudominant in primeval forest reserves characterised by a species-rich herb layer (ES; Tab. 1). Abundance in these plots, however, was lower than in the more open plots (D = 10.9–18.0 %, I = 0.5-0.6).
The highest abundance of any SM community was observed at plot 15, a European beech plantation with European blueberry dominant in the herb layer (I = 9.8), though high abundance levels were also observed in other plantations (I = 4.1–8.0). The abundance in mature forests was lower (I = 1.2–5.3), reaching lowest values in a mature Norway spruce monoculture (plot 25; I = 1.2). Significant differences in abundance and dominance between individual plots are presented as a comparison of mean values in Tab. 2a–c (ES).
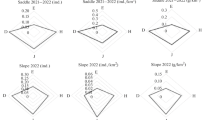
Highest diversity (H index; ES; Tab. 3) was observed in primeval forest reserves, with highest number of species (n = 9), and in plantations dominated by dicots. Plantations dominated by grasses had a lower diversity, while lowest diversity of all was determined in mature European beech and Norway spruce monocultures. Simpson’s evenness index (ES; Tab. 3) was highest in early successional forests with a high ratio of dicots, followed by reserves and plantations with grasses dominant. Mature monocultures showed lowest diversity (ANOVA with Tukey’s post hoc test), with significant differences only between monoculture and other habitats (Fig. 1).
a, b Comparison of mean results for Shannon’s diversity index (left) and Simpson’s index of evenness (right) using ANOVA with Tukey’s HSD post hoc test. Different letters denote statistically significant differences. R = nature reserves; D = sites with dicots dominant (>50 %); G = sites with grasses dominant (>50%); M = monocultures
Despite the dynamic development of habitats, cluster analysis (ES; Fig. 2) identified two basic biotope groups (both containing a number of sub-groups) with similar SM communities. The characteristics of their respective herb layers proved to be of particular importance.
Group 1—Early- and late-stage successional forest ecosystems with extensive herb cover and a high ratio of dicotyledonous plants. Two sub-groups were further defined: (1a) primeval forest reserves with a diverse herb layer, natural habitat structure and low canopy closure (20–50 %; plots 20, 21, 23), and (1b) plantations with dicots dominant (particularly European blueberry).
Group 2—Plantations with extensive herb cover dominated by grasses, and forests with a dense canopy closure. Again, two sub-groups were determined: (2a) plantations with a thick herb layer dominated by grasses, and (2b) mature forests (90–100 % canopy closure) encompassing both monocultures (plots 22, 24, 25) and natural habitats (plot 19) with a low herb layer.
Redundancy analysis (Table 1; Fig. 2) confirmed that environmental variables, which had a significant impact on both community structure and species distribution, were related primarily to herb layer character (occurrence of grasses and dicots, forest weeds and grass litter) and occurrence of a tree layer. Elevation had no effect on site characteristics (ES; Tab. 4b). Exposure (especially northern) had a significant effect (RDA: F = 3.201, p < 0.001) on both community structure and species distribution (ES; Fig. 2). Field vole showed a clear preference for open habitats with grasses dominant, but this refers only to sites with dicots admixed up to approx. 10 % (plots 7, 8, 12, 13, 17; ES; Tab.1). Grassy sites with dicots at levels of 10–45 % (plots 1–4, 14, 16; ES; Tab. 1) tended to be avoided (Fig. 2). This was confirmed by field vole abundance, which fluctuated between 0.2 and 3.7 in more grassy habitat type (<10 % of dicots) and showed low fluctuations between 0 and 1.0 in more dicots habitat (>10 %), the difference being significant (p < 0.05; Fig. 3). This preference was also confirmed by the GLM (Fig. 4). The same test confirmed a response in bank vole abundance in habitats with ratio of dicots exceeding 10–15 %. Even a relatively low ratio of dicots appears to play a key role in the habitat preferences of this species. This was confirmed by a comparison of abundances in grassy habitats with dicots >10 %, where bank vole abundance was higher (I = 0.2–2.9) than that of field vole (I = 0–1.0), despite grasses dominance [though not significant (Fig. 3)]. Even removing the blackberry from the dicot plants in the GLM analyse the results were still highly significant, with predicted onset of preference corresponding to approximately 10 % of dicots in the herb layer.
Redundancy analysis indicating preference of small mammals for individual habitats (numbers). Only significant variables are shown (for further details see Table 2). The model was significant (F = 14.397; p = 0.016), with the first axis explaining 13.3 % and both the first and second axes explaining 22.9 % of variation in the species data. The full model, with both significant and non-significant variables, was also significant (for further details see Table 2). Filled squares = sites with grasses dominant (dicots ratio not exceeding 10 %); filled down-triangles = sites with dicots dominant (grass ratio not exceeding 10 %); filled circles = sites with grasses dominant (>50 %) but with a dicots ratio of over 10 %; empty up-triangles = monocultures; black stars = nature reserves. Species legend: AA = Apodemus agrarius; AF = A. flavicollis; AS = A. sylvaticus; CG = Clethrionomys glareolus; GG = Glis glis; MA = Muscardinus avellanarius; Mag = Microtus agrestis; Mar = M. arvalis; MS = M. subterraneus; NA = Neomys anomalus; Sal = Sorex alpinus; SA = S. araneus, SMin = S. minutus; TE = Talpa europea. Environmental variables: E3 cover = total tree cover; Calamagrostis = Calamagrostis sp.; grass = grass cover; dicotyl = dicots cover; dic+rub = dicots and Rubus sp. cover combined; Rubus = Rubus sp. cover; weeds = forest weeds cover, grass litter = its mightiness
Comparison of mean relative abundance of Microtus agrestis (Magr) and Clethrionomys glareolus (CG). Capital letters indicate differences in relative abundance of individual species among various site types. Small letters indicate differences in relative abundance between the species within a single site. T = sites with grasses dominant (dicots ratio <10 %); D = sites with dicots dominant (>50 %); T+D = sites with grasses dominant (>50 %), but with a dicots ratio of >10 %; M = monocultures; and R = nature reserves
Response curves of four dominant small mammal species based on the ratio [G/(D+R)]; fitted in CanoDraw using generalised linear models. [G/(D+R)] expresses the ratio between grass cover (Grass) and dicots cover (Dicots+Rubus). Values ranged between 0.05 and 27.5, with lowest values indicating highest dicots ratio and highest values indicating its lowest ratio within total herb cover. The model was significant for Clethrionomys glareolus (F=5.05; p=0.027), Microtus agrestis (F=18.03; p=0.000) and Sorex araneus (F=5.53; p=0.021), but not for Apodemus flavicollis. ‘T” indicates the theoretical threshold of probable change in dominance and habitat preference in C. glareolus and M. agrestis. Its value oscillates around 6 and corresponds to approx. 10–15% dicots ratio in the herb cover
Redundancy analysis demonstrated a clear preference of common shrew and bank vole for plantations with dicots dominant and for primeval forest reserves. The preference of yellow-necked mouse overlapped with various habitat types, confirming the broad adaptability of the species and, unlike the other dominant species, the absence of any specific preference. Other SM species tended to favour habitats with a diverse herb layer (i.e. grasses, dicots, forest weeds), though their preferences could only be determined to a limited degree due to the small number of individuals collected (Fig. 2).
Results of GLM, showing dependence of species abundance on site characteristics (Table 2), confirmed positive influence of Rubus sp. on presence of yellow-necked mouse. Bank vole was the only species with abundance to be significantly positively influenced by elevation. This may be due to a clear trend for an increased ratio of dicots (particularly European blueberry) at higher elevations as also these significantly influenced its presence. Field vole abundance was positively influenced by Calamagrostis sp. (the dominant grass sp.) but negatively influenced by forest weeds (mostly dicots). Common shrew abundance positively depended on Rubus sp. and the dicots combination on the plots.
Discussion
Small mammal community of the Carpathian mountain forests comprise of 14 species of which three were regionally endangered (Plesník et al. 2003). Their abundance and dominance were highly uneven. Yellow-necked mouse was the most abundant species, occurring in all habitats and showing highest dominance and abundance in some early successional habitats with a rich herb layer (European beech plantations with a high ratio of grasses and blackberry) and in old forests with high tree seed production (beech monoculture) (Pucek et al. 1993). This is due to the size of its home range (i.e. 1,300 m2 for females and 3,200 m2 for males in lowland forests; Zejda and Pelikán 1969). Open clearings surrounded by fruiting stands are usually included within this range. Mice are capable of tracking tree seeds in the forest faster than any other rodent (Perea et al. 2012), which is an advantage even when they occur concurrently in tree plantations. This combined with the considerable migratory abilities of mice (Montgomery and Gurnell 1985) and the relatively small distances between habitats in the study area, means that yellow-necked mouse may be found looking for food in virtually any type of stand, particularly at times of high population density (Juchiewicz et al. 1986; Nesvadbová and Gaisler 2000). By Castien and Gosalbes 1994, yellow-necked mouse, in years without mast production, in a low density inhabits patches with brambles or other fruiting plants.
Also bank vole occurred in all forest habitats, showing highest abundance and dominance in early successional habitats with extensive herb cover (80–100 %) and a high ratio of dicots, particularly European blueberry (52–77 %), though also finding a significant refuge in forest reserves. Bank voles’ somewhat limited abundance in other biotopes is related to its dietary requirements and competition from the more adaptable and aggressive yellow-necked mouse (Montgomery and Gurnell 1985). Field vole, the third most abundant species, proved to be more specialised than the previous two species and reached highest dominance and abundance at a number of plantations with grasses in dominance (approximately 82–90 %) and dense herb layer (96–99 %). As most of these plots had northern exposures (ES; Tab. 1; Fig. 2), this may be linked to the fact that field vole is a predominantly cryophilic species and, as such, prefers cooler habitats with higher humidity (Niethammer and Krapp 1978). In total, these three species represented 84 % of the SM community. This corresponds with the results of Schröpfer 1990, who also observed that over 75 % of individuals in European SM communities consisted of just three species.
Compared to rodents, insectivore dominance and population density (9.9 %) was low. The only species to be a dominant was common shrew (9.3 %), whose key habitats are early successional stands with dicots dominating and forest reserves. Most other shrew species (e.g. southern water shrew—Neomys anomalus, pygmy shrew—S. minutus, Alpine shrew—S. alpinus) were also found in these environments, indicating the general importance of such habitats for insectivores. Despite the low ratio of insectivores in the community, mountain forests have come to represent an important Central European refuge for these mammals as their numbers decline due to changes in the surrounding lowland landscape (Ryszkowski et al. 1973; Kozakiewicz and Kozakiewicz 2008). For example, insectivores represent just 1.23 % of total dominance in lowland forests within the intensively managed Moravian cultural landscape (Suchomel et al. 2012), and only 0.51 % in the agricultural landscape (Heroldová et al. 2007).
Cluster analysis divided the forest biotopes within our study area into two basic groups: (1) early and late successional stage forests with extensive herb cover and a high ratio of dicots, and (2) early successional stage forests (plantations) with extensive herb cover and grasses dominant, and mature even-aged monoculture forests with a dense canopy closure and low herb layer. In other mountain ranges of the Czech Republic, SM diversity depended on ratio of herbs and grasses in herb layer (Bejček et al. 1999; Nesvadbová and Gaisler 2000).
Site character and structure has a significant influence on SM community structure and diversity (Ecke et al. 2002; Sullivan et al. 2009), as shown by the difference in community composition in the two groups identified by cluster analysis. Three species, namely yellow-necked mouse, bank vole and common shrew, dominated in forest reserves and plantations with dicots dominant in the herb layer, together representing approx. 70–88 % of species. Field vole occurred in group with high dominance of grass (approx. 10–20 %). Depending on their herb layer characteristics, plantations may serve as refuges for those species dominant within them, providing protection to species such as common shrew or, alternatively, acting as population reservoirs for species that prevent forest regeneration through bark gnawing (field vole, bank vole) or tree seed consumption (yellow-necked mouse, bank vole) (Drożdż 1966; Heroldová et al. 2012). Only yellow-necked mouse and bank vole reached dominance in fruiting monocultures, representing approx. 90 % of species. Strong influence of the good beech mast harvest on above mentioned species densities is known (Jensen 1985; Pucek et al. 1993).
In general, insectivores represent a negligible part of the SM community; however, their abundance was significantly higher in group (1) habitats (5.9–25 %; I = 0.4–1.4) compared to plantations with grass cover (2.9–14.3 %; I = 0.2–0.8) and fruiting monocultures (2.5–3.4 %; I = 0.1–0.3). The dominance and abundance figures for group (1) habitats refer almost exclusively to common shrew, suggesting that plantations with dicots and nature reserves represent an important refuge for this species. These habitats with more diverse flora and fauna can offer a better food supply for shrew. This is in accordance with Thomas and Marshall 1999 finding positive correlation between faunal (arthropod) and floral diversity in their experimental fields.
Habitat preferences in our study plots were influenced by a number of factors, with character of herb layer being one of the key factors (Nesvadbová and Gaisler 2000; Suchomel et al. 2012), e.g. the significant association of field vole with grass and bank vole with dicots (Miklós and Žiak 2002). The close associations observed (with field vole decreasing and bank vole increasing their habitat preference) even at a very low dicots ratios of 10–15 % may mean that even small changes to the herb layer could result in changes in abundance and distribution of these species, with consequent changes in SM community character and structure. This has been identified in a number of previous studies (Gębczyńska 1976; Hansson 1985; Suchomel et al. 2009).
Studies of SM habitat preference under Central European conditions are relatively rare. Miklós and Žiak 2002 focused their research on an oak-elm (Quercus-Ulmus sp.) stand in Slovakia. These authors confirmed preference of the common vole (M. arvalis) for open habitats with minimum canopy closure and bank vole preference for a rich herb layer. As in our study, Jensen 1984 noted that bank vole was collected mainly in the thick undergrowth of an ecotone between a forest stand and an open area in a plantation, taking advantage of both forest and open habitats with a rich herb layer undergrowth. Field voles were only collected in plantations.
Conclusions
The mountain forests of the Western Carpathians represent an important refuge for populations of SM forest species thanks to their habitat diversity, with differing levels of successional development and management (ranging from plantations to clear-cut areas induced by air pollution and forest monocultures to primeval forest reserves). Some dominant species (e.g. yellow-necked mouse, bank vole, common shrew) form constant populations with long-term survival in all habitat types under different types of management, while a number of more specialised species are associated with specific habitats of natural (primeval forest reserves) or anthropogenic character. The presence of early successional developmental stages, which represent refuges for abundant populations of certain species (e.g. field vole, bank vole, common shrew), represents irreplaceable habitats in areas subject to strong human impact. Knowledge of the structure and diversity of SM communities in different forest habitats will facilitate decision-making concerning measures related to species diversity, as well as regarding damage to forest stands through seed consumption or bark gnawing. Within this part of protected area conservation, attention should be concentrated on protection of species communities as well as their habitats and ecosystems. Mountain forests represent an important Central European refuge for rare SM as their numbers, due to changes in the surrounding lowland landscape decline.
References
Bejček V, Sedláček F, Šťastný K, Zima J (1999) Small mammals in the emission area of the Krušné hory Mts.: the environmental state monitoring and the damages to the substitute tree plantations. In: Conference proceedings problems of preservation of substitute tree plantations in an emission area of the Krušné hory Mts., Ministerstvo Zemědělství, Praha, pp 83–88 (in Czech)
Blanchet FG, Legendre P, Borcard D (2008) Forward selection of explanatory variables. Ecology 89:2623–2632
Bryja J, Heroldová M, Zejda J (2002) Effects of deforestation on structure and diversity of small mammal communities in the Moravskoslezské Beskydy Mts (Czech Republic). Acta Theriol 47:295–306
Čapek M, Heroldová M, Zejda J (1998) Bird and small mammal communities in a clearing caused by air pollution. Folia Zool 47:21–28
Castien E, Gosalbes J (1994) Habitat selection of Apodemus flavicollis in a Fagus sylvatica forest in the western Pyrenees. Folia Zool 43:219–224
Drożdż A (1966) Food habits and food supply of rodents in the beech forest. Acta Theriol 11:363–384
Ecke F, Lögfren O, Hörnfeldt B, Eklund U, Ericsson P, Sörlin D (2001) Abundance and diversity of small mammals in relation to structural habitat factors. Ecol Bull 49:165–171
Ecke F, Lögfren O, Sörlin D (2002) Population dynamics of small mammals in relation to forest age and structural habitat factors in northern Sweden. J Appl Ecol 39:781–792
Flowerdew JR Gurnell J, Gipps JHW (eds.) (1985) The Ecology of woodland rodents: bank voles and wood mice. In: Proceedings of a symposium held at the Zoological Society of London on 23rd and 24th of November 1984, Oxford University Press, pp 411
Gębczyńska Z (1976) Food habits of the bank vole and phonological phases of plants in an oak hornbeam forest. Acta Theriol 21:223–236
Gurnell J (1985) Woodland rodent communities. Symp Zool Soc Lond 55:377–411
Hansson L (1985) The food of bank voles, wood mice and yellow-necked mice. Symp Zool Soc Lond 55:141–168
Heroldová M (2002) Food selection of Microtus agrestis in air-pollution affected clearings in the Beskydy Mts, Czech Republic. Folia Zool 51(Suppl. 1):83–91
Heroldová M, Bryja J, Zejda J, Tkadlec E (2007) Structure and diversity of small mammal communities in agriculture landscape. Agric Ecosyst Environ 120:206–210
Heroldová M, Suchomel J, Purchart L, Homolka M (2008) The role of granivorous forest rodents in beech regeneration in the Beskidy Mts. (Czech Republic). Beskydy 1:131–134
Heroldová M, Bryja J, Jánová E, Suchomel J, Homolka M (2012) Rodent damage to natural and replanted mountain forest regeneration. Sci World J. doi:10.1100/2012/872536
Jensen TS (1984) Habitat distribution, home range and movements of rodents in mature forest and reforestations. Acta Zool Fenn 171:305–307
Jensen TS (1985) Seed predator interactions of European beech Fagus sylvatica and forest rodents Clethrionomys glareolus and Apodemus flavicollis. Oikos 44:49–156
Juchiewicz M, Zemanek M, Bieniek M, Suita E (1986) Small rodent communities in the Tatra mountain forests. Acta Theriol 31:433–447
Kozak J, Estreguil C, Vogt P (2007) Forest cover and pattern changes in the Carpathians over the last decades. Eur J For Res 126:77–90
Kozakiewicz M, Kozakiewicz A (2008) Long-term dynamics and biodiversity changes in small mammal communities in a mosaic of agricultural and forest habitats. Ann Zool Fenn 45:263–269
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, London
Magurran AE (1988) Ecological diversity and its measurement. Chapman and Hall, London
Magurran AE (2004) Measuring biological diversity. Blackwell Science, Oxford
Miklós P, Žiak D (2002) Microhabitat selection by three small mammal species in oak-elm forest. Folia Zool 51:275–288
Montgomery WI, Gurnell J (1985) The behaviour of Apodemus. In: Flowerdew SR, Gurnell J, Gipps JMW (eds) The ecology of woodland rodents bank voles and wood mice. Oxford University Press, Oxford, pp 89–115
Nesvadbová J, Gaisler J (2000) Communities of terrestrial small mammals in two mountain ecosystems influenced by air pollution. Folia Zool 49:295–304
Niedziałlkowska M, Kończak J, Czarnomska S, Jędrzejewska B (2010) Species diversity and abundance of small mammals in relation to forest productivity in Northeast Poland. Ecoscience 17:109–119
Niethammer J, Krapp F (1978) Handbuch der Saugetiere Europas, vol 1. Rodentia, Akademische Verlagsgesellschaft, Wiesbaden (in German)
Perea R, San Miguel A, Martínez-Jauregui M, Valbuena-Carabaňa M, Gil L (2012) Effects of seed quality and seed location on the removal of acorns and beechnuts. Eur J For Res 131:623–631
Plesník J, Hanzal V, Brejšková L (eds) (2003) Red list of endangered species in the Czech Republic. Vertebrates. Příroda, Praha, 22:1–184 (in Czech)
Pucek Z, Jędrzejewski W, Jędrzejewska B, Pucek M (1993) Rodent population dynamics in a primeval deciduous forest (Białewieża National Park) in relation to weather, seed crop, and predation. Acta Theriol 38:199–232
Randuška D, Vorel J, Plíva K (1986) Phytocenology and forest typology. Príroda, Bratislava (in Slovak)
Ryszkowski L, Goszczynski J, Truszkowski J (1973) Trophic relationships of the common voles in cultivated fields. Acta Theriol 18:125–165
Schmid-Holmes S, Drickamer LC (2001) Impact of forest patch characteristics on small mammal communities: a multivariate approach. Biol Conserv 99:293–305
Schröpfer R (1990) The structure of European small mammal communities. Zool Jahrb Abt Syst 117:355–367
StatSoft Inc. (2012) Electronic Statistics Textbook, Tulsa, OK: StatSoft. http://www.statsoft.com/textbook/stathome.html. Accessed 20 Dec 2012
Suchomel J, Krojerová J, Heroldová M, Purchart L, Barančeková M, Homolka M (2009) Habitat preferences of small terrestrial mammals in the mountain forest clearings. Beskydy 2:195–200
Suchomel J, Purchart L, Čepelka L (2012) Structure and diversity of small-mammal communities of lowland forests in the rural central European landscape. Eur J For Res 131:1933–1941
Sullivan TP, Sullivan DS, Lindgren PMF, Ransome DB (2009) Stand structure and the abundance and diversity of plants and small mammals in natural and intensively managed forests. For Ecol Manag 258:127–141
Ter Braak CJF, Šmilauer P (2002) CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for canonical Community ordination (version 4.5) microcomputer power, Ithaca NY
Thomas CFG, Marshall EJP (1999) Arthropod abundance and diversity in differently vegetated margins of arable fields. Agric Ecosyst Environ 72:131–144
Tischler W (1949) Grundzüge der terrestrischen Tierökologie. Friedrich Vieweg und Sohn, Braunschweig (in German)
Zejda J (1973) Small mammals in certain forest types groups in southern Moravia. Zool Listy 22:1–13
Zejda J, Pelikán J (1969) Movements and home ranges of some rodents in lowland forests. Zool Listy 18:143–162
Acknowledgments
The authors are very much obliged to Kevin Roche for language correction and the anonymous reviewers for improving of the manuscript. This study was supported by the Ministry of Education, Youth and Sports of the Czech Republic, Project No. 6215648902, and the Ministry of Agriculture of the Czech Republic, Project No. QH72075.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Pretzsch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suchomel, J., Purchart, L., Čepelka, L. et al. Structure and diversity of small mammal communities of mountain forests in Western Carpathians. Eur J Forest Res 133, 481–490 (2014). https://doi.org/10.1007/s10342-013-0778-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-013-0778-y