Abstract
The present study was carried out in aonla cv. ‘Balwant’ to investigate the biochemical changes occurring in the abscised and non-abscised fruits at three stages of fruit development at monthly intervals, viz. pinhead stage, one month before harvest and at harvest. The fruit weight, dry matter content and antioxidant activity were significantly higher in retained fruits compared to the abscised ones during the different stages of fruit development. In the non-abscised fruits, the pectins, 1‑diphenyl-2-picrylhydrazyl (DPPH) activity, total soluble sugars and proteins were higher, providing mechanical strength and assimilates for strengthening the fruits preventing their abscission. The activity of hydrolytic enzymes polygalacturonase and cellulase were statistically higher in abscised fruits compared to the non-abscised ones. The findings depict the important role of polygalacturonase and cellulase in the phenomenon of abscission. The auxins generally reduce the abscission by reducing the sensitivity of cells of the abscission zone to ethylene. Hence, further studies on understanding the mechanism of abscission through molecular approaches could help to elucidate molecular markers for the management of fruit drop by regulating expression of cell wall degrading enzymes and the use of auxins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aonla, also known as ‘wonder fruit’, is a relatively hardy and nutritious fruit crop with wide adaptability to diverse climates and soil types including marginal and neglected lands (Meena et al. 2022). However, pre-harvest fruit drop during different developmental stages of aonla causes a significant reduction in its fruit yield (Hiwale 2015), inflicting huge economic loss to the growers. The fruit set and fruit growth following successful pollination and fertilization process are dependent upon the endogenous level of IAA. However, any imbalance in auxin synthesis and transportation during fruit development of aonla may cause excessive premature abscission of fruits (Singh et al. 2022; Dal Cin et al. 2005). The fruit abscission occurs at the specific region called abscission zone. The higher activity of polygalacturonase in cell wall leads to the loosening of cells and formation of abscission layer (Patharkar and Walker 2018). Auxin and ethylene interplay tend to initiate the formation of cell wall hydrolases which trigger cell wall breakdown, leading to the detachment of the fruit from the tree branch. These differences in the rate of fruit growth and the concentration of endogenous hormones in both abscised and non-abscised fruits has been studied in several fruit crops, viz. mango, peach, almond and orange (Garner and Lovatt 2016). It was hypothesized that hydrolytic enzymes and the biochemical changes in the cells of abscission zone cause breakdown of cell wall leading to the detachment of the fruit at the abscission zone (Roberts et al. 2002). The biochemical changes in the persisting and abscising fruits during different stages of fruit development may provide the essential information for implementing strategies to check fruit abscission, thus enhancing fruit yield. The physico-chemical, enzymatic and structural changes that occur at different stages of fruit development in both abscised and non-abscised healthy fruits of aonla have been not studied. Hence the aim of the present investigation was to determine the biochemical changes that occur in the abscised and non-abscised fruits of aonla during the course of fruit development for future insight into strategies for efficient management of fruit drop and enhancement in fruit yield. The investigation was planned on cv. ‘Balwant’ which is a chance seedling selection from cv. ‘Banarasi’. It is an early maturing cultivar, maturing in mid-November and has flattened, round medium sized fruits having yellowish green color with pink tinge on the fruits.
Materials and Methods
Experimental Site and Sampling
The present investigation was carried out during 2021–22 at the Fruit Research Farm, Punjab Agricultural University, Ludhiana, India, on 14-year-old healthy aonla trees of cv. ‘Balwant’, located at (30.90°N, 75.79°E). The trees were provided with uniform recommended cultural practices 2 years prior and throughout the course of investigation. The freshly abscised and non-abscised fruits were collected at monthly intervals from mid-September (pinhead stage or stage I), 1 month before harvest (stage II) up to harvest maturity in mid-November (stage III). The abscised fruits were collected by placing horizontal nets underneath the tree canopy 1 day prior to the date of observation, to avoid their direct contact with the soil or falling on the ground. On the following day, the abscised fruits were collected and non-abscised fruits were picked randomly for comparative observations and analysis of physico-chemical and structural attributes. There were four replicates maintained for collecting data on abscised and non-abscised fruits.
Physical Attributes
The observations on fruit weight and specific gravity were recorded from 10 fruits per replication and average was calculated. Fruit dry matter content (%) was calculated by taking the fresh fruit weight, then drying the fruits in the hot air oven for 72 h at 65 °C and the dry matter content was calculated on dry weight basis.
Total Soluble Sugars and Total Soluble Proteins
The estimation of total soluble sugars and total soluble proteins was carried out as per the method given by Singh and Singh (2015). For the determination of total soluble sugars, 0.1 ml of the sugar extract (prepared from 0.5 g of fruit sample) was taken in a test tube and to this 0.9 ml of distilled water was added to make the final volume of 1 ml. Then, 1 ml of 5% phenol was added after which 5 ml of concentrated chilled sulphuric acid was added. These tubes were then cooled under the running tap water. The orange brownish colour appeared in the test tubes and its absorbance was read after 20 min on a spectrophotometer at 490 nm. For preparation of blank, 1 ml of distilled water, 1 ml of 5% phenol and 5 ml of concentrated chilled sulphuric acid was taken. The concentration was determined by the simultaneous running glucose standards (10–100 µg) and expressed in percentage. For extraction of total soluble proteins (Castro et al. 2008), 0.1 g pulp was taken and homogenized in 5 ml of 0.1 N NaOH solution and centrifuged for 10 min at 5000 rpm. The process was repeated using the obtained supernatant and then the final volume was made up to 10 ml. To 1 ml of protein extract was added 1 ml of 15% trichloroacetic acid (TCA), which was refrigerated for 24 h. This solution was then again centrifuged for 20 min at 5000 rpm and the precipitates obtained were dissolved in 0.1 N NaOH solution. Thereafter, 0.1 ml of the protein extract was taken, to which 0.9 ml of the distilled water was added to make the final volume up to 1 ml. This was properly mixed and then to 1 ml of protein extract, 5 ml of mixture of 50 ml of Alkaline Na2CO3 reagent (2% Na2CO3 in 0.1 N NaOH) and 1 ml of 0.5% CuSO4.5H2O in 1% sodium potassium tartrate solution was added. These were mixed thoroughly and kept for 10 min at room temperature. Finally, 0.5 ml of Folin–Ciocalteau reagent diluted with distilled water (1:1) was added and kept at room temperature for 30 min after proper mixing. The absorbance of the blue colour so obtained was read at 540 nm against the blank using spectrophotometer. The total soluble protein concentration was estimated from simultaneous running BSA standards (20–100 µg) and was expressed in mg g−1 FW.
Proline Content, DPPH Radical Scavenging Activity and Pectin
For the estimation of proline content, 0.5 ml supernatant was taken in a test tube (prepared from 0.1 g of fruit sample) to which 2 ml acid ninhydrin and 2 ml glacial acetic acid were added. The reaction mixture was kept for 45 min in a boiling water bath and was then cooled at room temperature, after which 4 ml of benzene was added into it. The mixture was then vortexed. It was then observed in the reaction mixture that the two different separate layers were formed. From these two layers, the chromophore containing pink layer which was formed as the upper layer was collected. Pure benzene was used as blank. Absorbance was taken against the blank at 520 nm. The proline concentration was determined using proline standards (0.02–0.1 µmoles) run simultaneously and it was expressed in mg g−1 FW and calculated using the following equation:
1‑Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was measured as per the method provided by Samant et al. (2020) and was expressed in percentage. Firstly, 0.5 mM solution of DPPH was made in methanol. For the preparation of 0.5 ml of methanol extract, 0.1 g of fruit tissue (pulp) was homogenized in 80% methanol and then 3 ml of DPPH solution was added into it. This solution was allowed to stand at room temperature for 30 min in dark and then the absorbance was read using spectrophotometer at 517 nm. In a similar way, the control was prepared without the use of fruit extract. The aonla pulp (% inhibition) was determined by noting the absorbance at 517 nm DPPH solution. The antioxidant (% inhibition) was determined using the equation given below:
where A0 = absorbance of control, As = absorbance of sample.
The estimation of total pectin content was done by following the method given by Srivastava et al. (2019) and expressed in the form of percent citric acid.
Polygalacturonase and Cellulase Activity
For the determination of polygalacturonase activity, 1 ml of 0.1 M sodium acetate buffer (pH 5.2) and 1 ml of 0.5% pectic acid was added to 1 ml of the enzyme extract. This reaction mixture was then incubated for 1 h at 30 °C. At the end of incubation, for the preparation of blank, the active enzyme was added to the reaction mixture. Then to this reaction mixture 1 ml of DNS reagent was added and it was boiled for 10 min. Test tubes were then kept to cool and the absorbance was taken at 560 nm using spectrophotometer. For the preparation of standard curve D‑galactouronic acid standards (10–100 µg) were used. The function of the enzyme was expressed in mmol kg−1 min−1D-galacturonic acid (Madani et al. 2014). For the determination of cellulase activity, 1 ml of sodium acetate buffer (pH 5.2) and 1 ml of 0.5% carboxymethyl cellulase was added to 1 ml of the enzyme extract. It was then incubated for 1 h at 55 °C. At the end, 1 ml of dinitrosalicylic acid (DNS) was added and was then boiled for 10 min. Test tubes were kept to cool (room temperature) and absorbance was taken at 560 nm. DNS reagent was added to tube for blank before incubation. Activity of the enzyme was expressed as mmol kg−1 min−1D-glucose (Chin et al. 1999), with glucose taken as standard.
Mineral Nutrients
For analysis of mineral nutrients, both abscised and non-abscised fruit samples were washed with distilled water and then dried in hot air oven at 65 °C until the weight became constant. The fruit samples were then ground to make powdered form and were filtered through a 0.5 mm stainless steel sieve. The powder collected was kept in air tight glass vessels (after complete removal of moisture content) with proper labelling till their use for analysis of mineral nutrient content. Nitrogen was analyzed by Kjeldahl’s method using KELPLUS semi-automatic analyzer nitrogen estimation system (M/S Pelican Equipment, Chennai, India). Vanodo-molybdate phosphoric yellow colour method was used to estimate the phosphorus and flame photometer method was followed for the estimation of potassium (Kumar et al. 2020). The estimation of Ca, Fe, Zn, Cu and Mn in the samples was performed using the atomic absorption spectrophotometer (Analyst 200, Perkin Elmer, Shelton, CT, USA).
Microscopy for Abscission Zone Anatomy
At 1 month before harvesting, both abscised and non-abscised fruit stalks of aonla fruits were collected for the anatomical observation (transverse section of fruit stalk). Formalin-acetic acid-ethyl alcohol solution was prepared by mixing 5 ml of glacial acetic acid, 85 ml of 50% ethyl alcohol and 10 ml of 40% formaldehyde. The fruit stalks were dipped into this solution overnight. The transverse section was then sliced from each of the stalks and stained with safranin-fast green combination (Schiff et al. 1967). The prepared sections were initially stained in aqueous solution of 2% safranin for 5 min. These were then washed with distilled water and quickly passed through alcohol. Counter staining was done afterwards by dipping the sections in 2% fast green for a few seconds.
These sections were then passed quickly through alcohol, alcohol:xylene (1:1) and lastly through xylene. The prepared section was then mounted on the slide and was examined using the axiovision software under the light microscope equipped with digital camera and computational imaging system (Microtoma Laica, Model No. RM 2255).
Statistical Analysis
The data obtained were subjected to statistical analysis using statistical package SAS version 9.3. The means where found significant were further subjected to mean comparison for highlighting the treatment effect at different stages of fruit development, using LSD (p ≤ 0.05).
Results and Discussion
Physical Attributes
The weight of aonla fruit increased with fruit development from stage 1 to stage III (Table 1). However, the fruit weight of the abscised fruits, i.e. 7.12, 15.89 and 24.82 g, at stage I, II and harvest, respectively, was significantly lower as compared to non-abscised fruits. The mean specific gravity increased with fruit development. At stage I, II and harvest, it was significantly greater (0.89, 1.13 and 1.13, respectively) than non-abscised fruits. The dry matter content of fruit showed increasing trend with the fruit maturity, and it was significantly lower in abscised fruits as compared to non-abscised fruits at the respective stages. Fruit drop in aonla is preceded by the reduced fruit growth that undergo shedding mainly due to imbalance in free IAA that causes activation of abscission layer in the abscission zone. As the physiological activities of the plants decreased 10–15 days prior to abscission, so the decrease in the fruit weight of abscised fruits in comparison to persisting fruits might be due to reduced cell division, lower cell wall extensibility in the cortical cells of the fruit and increased cell distension as well as lower activity of nuclei in the abscised fruits as compared to the non-abscised fruits. Also the water loss in the tissues just prior to abscission and reduced transport of mineral nutrients and photosynthates from vegetative parts to sinks might have contributed to reduced fruit weight (Abruzesse et al. 1995). The increment in the dry matter in the fruits with maturity may be due to the sigmoidal as well as double sigmoid growth curve in aonla fruits; however the reduced dry matter content of abscised fruits similar to that observed in ‘Golden Delicious’ apple in comparison to non-abscised fruits might be due to the loss of mineral nutrients during abscission that reduced the dry matter content of fruits (Miller and Racsko 2011).
Total Soluble Sugars and Total Soluble Proteins
The total soluble sugars and protein content in both abscised and non-abscised fruits showed an increasing trend during the course of fruit development (Table 1). The total soluble sugars content in the abscised fruits, i.e., at stage I and II, was significantly greater (2.80 and 3.44%, respectively) as compared to non-abscised fruits (2.39 and 2.84%) at the respective stages, whereas no differences in total soluble sugars was observed between abscised and non-abscised fruits at harvest. A similar trend was observed for total soluble proteins. The assimilate accumulation in the fruits which act as sink and the conversion of starch into sugars during the process of fruit development is the main reason for the increase in the sugar content of the fruits with the maturity of fruits (Patel et al. 2014). The higher total soluble sugars in the abscised fruits as compared to the non-abscised fruits might be due to the higher accumulation of sugars in the pericarp of the abscising fruits as compared to the persisting fruits at the point of phloem loading. Also, the higher sugar content in abscised fruits may also be due to their reduced use as the growth of the fruit slows down 1–2 weeks prior to abscission which lowers the rate of respiration that lead to increase in accumulation of total soluble sugars (Yuan and Greene 2000). The accelerated changes during ripening of fruits initiated due to hydrolytic enzymatic activities which might be the reason for the increase in the protein content with fruit maturity (Kulkarni and Aradhya 2005).
Proline Content, DPPH Radical Scavenging Activity and Pectin
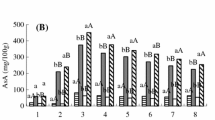
Pectin content and DPPH radical scavenging activity of aonla fruits showed a declining trend during the course of fruit development in aonla cv. ‘Balwant’ (Fig. 1). However, there was no significant variation observed between abscised and non-abscised fruit at stage I, whereas the values at stage II and harvest in abscised fruits, i.e., 5.01 and 2.03%, respectively, were statistically lower than the non-abscised fruits at respective stages. During unripe stages, the pectin together with the cellulase forms insoluble propectin. It is protected from the degradation in this way by the agents such as pectolytic enzymes, acids and alkalis that may be present in the plant tissues. However, with the maturity and ripening of fruit, there is breakdown of propectin and the cellulose withdraws its propectin from the pectin, thus reducing the pectin content (De Assis et al. 2001); however, low pectin content in the abscised fruits in comparison to the persisting fruits might be due to the hydrolysis of both the glycoside bonds and the pectin by the cell wall degrading enzymes that enhances the formation of the abscission layer, stimulating the fruit drop. Significantly lower values of for DPPH radical scavenging activity were observed in abscised fruits (87.03, 86.23 and 83.21%) at stage I, II and harvest, respectively, in comparison to non-abscised fruits (87.03, 77.18 and 74.53%). The results are in concordance with Kondo et al. (2005) who recorded decreased DPPH activity in kumquat fruits approaching maturity and also with Samant et al. (2020) who registered its increased activity by exogenous application of NAA which ultimately retarded the abscission process.
Polygalacturonase and Cellulase Activity
The cell wall degrading enzymes polygalacturonase and cellulase showed increasing trend with the fruit maturity in both the abscised and non-abscised fruits, whereas the enzymatic activity in non-abscised fruits was significantly lower in comparison to abscised fruits (Fig. 1). The activity of enzyme polygalacturonase in the abscised fruits (0.386, 0.453 and 0.558 mmol kg−1) at stage I, II and harvest, respectively, was statistically greater in comparison to non-abscised fruits, i.e., 0.358, 0.398 and 0.503 mmol kg−1 at the respective stages. A similar trend was observed for cellulase in which the value of abscised fruits (0.394, 0.516 and 0.595 at stage I, II and harvest, respectively) was statistically higher in comparison to non-abscised fruits (0.360, 0.400 and 0.480 mmol kg−1) at the respective stages. The enzyme polygalacturonase hydrolyses the glycoside bonds and the important component of pectin, thus degrading the polysaccharides of cell walls, stimulating the formation of abscission layer and enhancing the fruit drop (Khandaker et al. 2015). The behavior of cellulase is similar to that of polygalacturonase, both being cell wall-degrading enzymes, localized in the abscission layer and their activity is enhanced with ethylene that induces abscission as reported by Tang et al. (2019) in citrus.
Mineral Nutrients
The macronutrient (N, P, K and Ca) content of aonla fruits exhibited a declining trend during advancement of fruit development (Table 2). However, there was no significant difference for nitrogen content between abscised and non-abscised fruits at stage I, whereas significantly lower values were observed in abscised fruits, i.e., 1.80 and 1.64% at stage II and harvest, respectively, as compared to non-abscised fruits at the respective stages. The phosphorus content of abscised fruits (0.053, 0.042 and 0.030% at stage I, II and harvest, respectively) was statistically lower in comparison to non-abscised fruits (0.063, 0.050 and 0.048%). While the potassium content in abscised and non-abscised did not differ significantly at stages I and II, whereas significantly lower value was recorded at harvest in the abscised fruits (1.17%) in comparison to non-abscised fruits (1.28%). The calcium content did not vary significantly in abscised and non-abscised fruits at stage I, whereas the values at stage II and harvest in abscised fruits, i.e., 0.40 and 0.28%, respectively, were significantly lower in comparison to non-abscised fruits (0.49 and 0.34% at the respective stages). The decrease in the N, P and K content in the abscised fruits in comparison to persisting fruits might be due to the insufficient supply of nutrients approaching abscission (Singh 2005). Also, the persisting fruits act as strong sink for dry matter content mainly carbohydrates, and mainly mineral nutrients than the abscising fruits (Singh 2005). The decrease in the calcium content with the maturity of the fruits might be due to its reduced content in middle lamellae prior to abscission and changed binding form with maturity that lead to the softening of tissue (Marschner 1995).
The micronutrient (Fe, Zn, Cu and Mn) content of abscised and non-abscised fruits of aonla cv. ‘Balwant’ showed a declining trend during the course of fruit development (Table 2). The iron content of abscised and non-abscised fruit did not differ significantly at stage I and II; however, significantly lower values of abscised fruits (140.96 ppm) were observed at harvest in comparison to non-abscised fruits (155.29 ppm). The zinc content of abscised fruits did not differ significantly from non-abscised fruits at stage I; however, significantly lower values of abscised fruits were observed at stage II and harvest in comparison to non-abscised fruits. Significantly lower values of copper in abscised fruits (26.40, 19.67 and 11.55 ppm), respectively, were recorded as compared to non-abscised fruits (30.31, 24.67 and 19.47 ppm). A similar trend was observed in the manganese content. Our results in the context of Fe, Zn, Cu and Mn content are in accordance with the findings of Gundesli et al. (2021) and Paramasivam et al. (2000) who reported decrease in the content of micronutrients with the fruit maturity which was mainly due to the dilution effect as a result of increase in dry matter content of the fruits. The results of the present study are in accordance with the observations of Lahav and Zamet (1999) who reported a decrease in iron, copper and manganese in the abscised fruits of avocado cv. ‘Fuerte’. The zinc deficiency leads to the abscission as the zinc is essential element for auxin synthesis in the fruits (Addicott and Lynch 1955).
Anatomical Observations
The abscission zone of the fruit stalk of aonla cv. ‘Balwant’ was studied with reference to the structural changes occurring in the cells during the process of abscission (Fig. 2a, b). Sections through the abscission zone of the fruit stalk were studied through light microscopy. The vascular bundles were highly organised in the fruit stalk of non-abscised fruits as compared to the abscised ones (Fig. 2a). The pith diameter was higher in dropped fruit stalks and the parenchymatous cells in the pith region were different in cellular organization in the abscised and non-abscised fruit stalks. These were very well organised in the non-abscised healthy fruit stalks unlike their disorganization in the abscised ones (Fig. 2a, b). During cell wall degradation, the walls appear to swell and become highly flexible and wall of some of the cells under zone of separation invaginated during advanced stage of cell wall degradation which ultimately collapsed, as can be seen in Fig. 2b. The dissolution of middle lamellae and increase in intercellular spaces in the abscission zone cells leads to abscission (Roberts et al. 2002). The data demonstrate the important role of polygalacturonase and cellulase in fruit stalk of abscission. The cell adhesion is destroyed due to degradation of pectin and cellulose through the activity of cell wall degrading enzymes (Deng et al. 2007), chiefly due to polygalacturonase and cellulase activities (Nakano and Ito 2013). The activity of cellulase was found to increase substantially in the fruit stalk abscission zone during fruit drop. The auxin and auxin analogues generally reduce the abscission by reducing the sensitivity of abscission zone to ethylene. Further understanding of the mechanism underlying abscission can help in regulation of fruit production. Hence further molecular research on abscission could help to elucidate new molecular markers for improved genetic breeding programmes.
Transverse sections of abscised and non-abscised fruit stalks of aonla cv. ‘Balwant’. a Transverse Section of non-abscised fruit stalk of aonla (at 10 ×). (VB Uniform vascular bundles, P Uniformly interspersed parenchymatous cells in the pith, C Uniformly interspersed parenchymatous cells in the cortex). b TS of abscised fruit stalk of aonla (at 10 ×). (VB* Coalesced Vascular bundles, P* Non-uniformly parenchymatous cells in the pith, C* Widely spaced and enlarged parenchymatous cells in the cortex)
Conclusion
The investigation on comparing biochemical changes in abscised and non-abscised fruits of aonla revealed more fruit weight, dry matter content and antioxidant activity in retained fruits compared to the abscised ones during the different stages of fruit development. The increased pectin, DPPH activity, total soluble sugars and proteins provided mechanical strength and assimilates to the non-abscised fruits, preventing their abscission. The higher activity of polygalacturonase and cellulase stimulated the formation of abscission layer and enhanced the fruit drop. Further studies in this regard through molecular approaches could help in improved breeding programmes in the future and may be of great significance in the regulation of fruit production by managing fruit drop in aonla.
References
Abruzesse A, Mignani I, Cocucci SM (1995) Nutritional status in apples and June drop. J Amer Soc Hort Sci 120:71–74
Addicott FT, Lynch RS (1955) Physiology of abscission. Annu Rev Plant Physiol 6:211–238
Castro SM, Saraiva JA, Lopes-da-silva JA, Delgadillo I, Loey AV, Smout C, Hendrickx M (2008) Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chem 107(4):1436–1449
Chin LH, Ali ZM, Lazan H (1999) Cell wall modifications, degrading enzymes and softening of carambola fruit during ripening. J Exp Bot 50(335):767–775. https://doi.org/10.1093/jxb/50.335.767
Dal Cin V, Danesin M, Ramina A, Dorigoni A, Boschetti A, Ruperti B (2005) Fruit abscission as related to fruit quality. Acta Hortic 682:781–788
De Assis SA, Lima DC, De Faria OOMM (2001) Activity of pectin methyl esterase, pectin content and vitamin C in acerola fruit at different stages of fruit development. Food Chem 4:133–137
Deng Y, Wu Y, Li Y, Yang M, Shi C, Zheng C (2007) Studies of postharvest berry abscission of “Kyoho” table grapes during cold storage and high oxygen atmospheres. Postharvest Biol Technol 43(1):95–101. https://doi.org/10.1016/j.postharvbio.2006.07.013
Garner LC, Lovatt CJ (2016) Physiological factors affecting flower and fruit abscission of ‘Hass’ avocado. Sci Hortic 199:32–40
Gundesli MA, Kafkas NE, Guney M, Kafkas S (2021) Seasonal changes in the mineral nutrient concentrations of different plant organs of pistachio trees in alternate bearing “on” and “off” years. Erwerbs-Obstbau 63:279–292
Hiwale S (2015) Sustainable horticulture in semi-arid dry lands, 1st edn. Springer
Khandaker MM, Osman N, Hossain AS, Faruq G, Boyce AN (2015) Growth, yield and postharvest quality of wax apple as affected by naphthalene acetic acid application. Rev Bras Frutic 37:410–422
Kondo S, Katayama R, Uchino K (2005) Antioxidant activity in meiwa kumquat as affected by environmental and growing factors. Environ Exp Bot 54(1):60–68
Kulkarni AP, Aradhya SM (2005) Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem 93:319–324
Kumar H, Arora R, Thakur A, Sharma S (2020) Impact assessment of growing media and bioinoculents on growth and bud take of rough lemon. Ind J Hort 77(3):439–449
Lahav E, Zamet D (1999) Mineral losses of avocado tree resulting from abscission of flowers, fruitlets and fruits. Rev Chapingo Ser Hortic 5:101–102
Madani B, Mohamed MTM, Watkins CB, Kadir J, Awang Y, Shojaei TR (2014) Preharvest calcium chloride sprays affect ripening of Eksotika II’papaya fruits during cold storage. Sci Hortic 171(1):6–13. https://doi.org/10.1016/j.scienta.2014.03.032
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, New York
Meena VS, Gora JS, Singh A, Ram C, Meena NKP, Rouphael Y, Basile B, Kumar P (2022) Underutilized fruit crops of Indian arid and semi-arid regions: importance, conservation and utilization strategies. Horticulturae 8(2):171. https://doi.org/10.3390/horticulturae8020171
Miller DD, Racsko J (2011) Rootstock effects on fruit drop and quality of ‘Gala Galaxy’ and ‘Golden delicious reinders’ apples. Acta Hortic 903:397–404
Nakano T, Ito Y (2013) Molecular mechanisms controlling plant organ abscission. Plant Biotechnol 30(3):209–216. https://doi.org/10.5511/plantbiotechnology.13.0318a
Paramasivam S, Alva AK, Hostler KH, Easterwood GW, Southwell JS (2000) Fruit nutrient accumulation of four orange varieties during fruit development. J Plant Nutr 23:313–327
Patel PK, Singh A, Prakash J, Nath A, Deka BC (2014) Physico-chemical changes during fruit growth, development, and maturity in passion fruit genotypes. Ind J Horic 71:486–493
Patharkar OR, Walker JC (2018) Advances in abscission signaling. J Exp Bot 69:733–740
Roberts JA, Elliott KA, Gonzalez-Carranza ZH (2002) Abscission, dehiscence, and other cell separation processes. Annu Rev Plant Biol 53:131–158
Samant D, Kishore K, Acharya GC (2020) Efficacy of some chemicals for crop regulation in Allahabad Safeda guava under coastal Indian conditions of Odisha. Indian J Hort 77:94–100
Schiff JA, Zeldin MH, Rubman J (1967) Chlorophyll formation and photosynthetic competence in euglena during light-induced chloroplast development in the presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl urea (DCMU). Plant Physiol 42(12):1716–1725
Singh Z (2005) Embryo abortion in relation to fruit size, quality and concentrations of nutrients in skin and pulp of mango. J Plant Nutr 28:1723–1737
Singh A, Singh HK (2015) Application of plant growth regulators to improve fruit yield and quality in Indian gooseberry (Emblica officinalis Gaertn.). J Agri Search 2:20–23
Singh S, Mishra DS, Singh AK, Singh AK, Verma S, Mishra P (2022) Plant growth regulators in aonla. In: Ghosh SN, Tarai RK, Ahlawat TR (eds) Plant growth regulators in tropical and subtropical fruit crops Ist edn. CRC press, London, pp 52–63
Srivastava A, Kohli D, Vishnoi S, Badola R (2019) Quality evaluation of prepared guava-orange bar. Int J Chem Stud 7:1574–1581
Tang L, Chhajed S, Vashisth T (2019) Preharvest fruit drop in huanglongbing-affected ‘Valencia’ sweet orange. J Am Soc Hortic Sci 144:107–117
Yuan R, Greene DW (2000) Benzyladenine as a chemical thinner for ‘Mclntosh’ apples. I. Fruit thinning effects and associated relationships with photosynthesis, assimilate translocation, and nonstructural carbohydrates. J Amer Soc Hort Sci 125:169–176
Acknowledgements
The authors are thankful to Punjab Agricultural University Ludhiana for providing the necessary research facilities.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the acquisition conception, lab work, data analysis: Kajal. Drafting the work, revising it critically for important intellectual content: Dr. Rachna Arora, Dr. Nirmaljit Kaur, Dr. Anirudh Thakur. Final approval of the version to be published: Dr. PPS Gill, Dr. Rachna Arora and Dr. Anirudh Thakur. Substantial contribution to design of work and lab analysis work: Dr. Nirmaljit Kaur, Kajal.
Corresponding author
Ethics declarations
Conflict of interest
Kajal, R. Arora, P.P.S. Gill, N. Kaur and A. Thakur declare that they have no competing interests.
Additional information
Data availability statement
The data will be made available on reasonable request.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Kajal, Arora, R., Gill, P.P.S. et al. Biochemical Changes in Abscised and Non-abscised Aonla (Emblica officinalis Gaertn.) During Fruit Development. Applied Fruit Science 66, 239–246 (2024). https://doi.org/10.1007/s10341-023-00971-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-023-00971-z