Abstract
Fruit drop is a major physiological disorder limiting the productivity of plum fruit. The present study was conducted to test the efficacy of foliar application of different concentrations of plant growth regulators (NAA, Salicylic acid, 2,4-D) and CaNO3 on management of fruit drop with improvement of fruit quality parameters of Plum (Prunus salicina) cv. Satluj Purple. The foliar spray application was done twice, first ten days after the pit hardening stage, and the second ten days after the first application. Among the different treatments, 0.15 mM SA reduced fruit drop significantly and translated into highest fruit yield, higher content of ascorbic acid, total and reducing sugar content. The reduced activity of cell wall degrading enzymes cellulase, polygalactouranase and glucanase in the fruit pedicels registered with 0.15 mM SA suggest their significant role in reducing fruit drop. Hence, two foliar applications of 0.15 mM SA are beneficial for reducing fruit drop and improvement of fruit quality in plum cv. Satluj purple.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plum occupies a distinguished position amongst the stone fruit crops cultivated in the temperate zones across the globe. Plum falls under the genus Prunus, family Rosaceae and is a climacteric fruit. In India, plums are grown on a commercial scale in Jammu and Kashmir, Himachal Pradesh, Uttarakhand and plains of Punjab and Haryana. The mature plum fruits may have a dusty-white coating that gives them a glaucous appearance. The abundance of biomolecules such as phenolic acids, anthocyanins, carotenoids, minerals, pectins and organic acids in the plum fruits make it popular among the nutritionists (Birwal et al., 2017).
In Punjab, the Japanese plum cultivars which require low chilling are cultivated successfully and extensively. Among these, Satluj Purple (open-pollinated seedlings from cross of Prunus safictna L. from Taiwan x Methyley and four other plum cultivars grown in USA), an exotic cultivar is superior than the other cultivars grown in the state. This cultivar is gaining popularity amongst the farmers because of its medium vigour, quality fruits, lush and plentiful bearing habit. It is an early ripening cultivar and enters the market when other fruits are scarce ensuring better returns. However, a major constraint in increasing the area under cultivation of the Japanese plums is the fruit drop as the fruits drop erratically throughout the period of its development (Rajput et al., 2017). Although the flowering and fruiting occurs, the fruit drop reduces the ultimate yield, rendering the farmer with an unexpected economic loss. The preharvest fruit drop yields fruits with deteriorated quality as the fruit get detached before attaining its maximum size and thus reduces the final output. In an agricultural perspective, abscission results in great loss for productivity (Zhao & Li, 2020).
It has been reported that the fruit drop in plum cv. Satluj purple occurs in two waves, the first wave of maximum fruit drop initiates 7 days after pea stage; and the second between 49 and 55 days after the pea stage (Hazra, 2004). The fruit retention and development of the fruits is influenced by a wide range of factors including growth hormones, mineral nutrients, irrigation and environmental factors which affect the assimilation of photosynthates and may act as inducer of fruit abscission. A crosstalk between plant growth regulators in response to environmental signals mediates the biosynthesis of cellulase and polygalacturonase (Taylor & Whitelaw, 2001). These enzymes cause cell wall distortion in the cells of the abscission layer of the abscission zone present in the point of attachment of the pedicel and the fruit, this leads to a reduction in the pedicel strength resulting in a structural fracture which eventually leads to fruit drop. In the abscission zone, the growth hormones involved primarily are auxins and ethylene (Beyer, 1973). Abscisic acid and ethylene are reported to have abscission accelerating properties while gibberellins, auxin and polyamines play a role in inhibition of process of abscission (Aziz, 2003; Taylor & Whitelaw, 2001). The hormonal regulation depends upon their endogenous content, affinity of the receptors, transport and homeostasis for ececuting any physiological function.
In 2005, Blanusa and co-workers examined the efficacy of different concentrations of exogenous IAA on the pedicels of sweet cherry and reported that the indicators of fruitlet abscission were about forty per cent less on the fruit pedicels treated with auxin. Similar findings with respect to abscission in Kinnow in response to salicylic acid and 2, 4-D were reported by Ashraf et al. (2013). Among mineral nutrient supplement studies, Dar et al. (2016) evaluated the effect of CaNO3 in Santa Rosa plum and observed that while acting as a source of nitrogen, CaNO3 exerted a positive influence on fruit set and fruit growth. Keeping in view the serious consequences of preharvest fruit drop in plum cv. Satluj purple and possibility of its reduction with plant growth regulators and CaNO3, the present investigation was performed to devise a management strategy for management of physiological disorder.
Materials and method
The present investigation on fruit drop in plum cv. Satluj purple was performed at the Research Farm of Department of Fruit Science and Plant Physiology Laboratory, Punjab Agricultural University, Ludhiana in 2018 and 2019. Ten year old plum trees of cv. Satluj Purple uniform in vigor were marked for the studies, these were grown as per standard cultivation practices recommended by Package of practices for cultivation of fruit crops, PAU, Ludhiana.
The experiment comprised of seven treatments and one untreated control. Each treatment consisted of three replications arranged in a Randomized Block Design. The treatments were NAA (10 ppm), Salicylic Acid (0.1 mM and 0.15 mM), 2, 4-D (10 ppm and 20 ppm) and CaNO3 (0.5% and 1%). The dose of NAA was kept as 10 ppm only as it is the dose recommended for control of fruit drop in plum by Package of Practices for Cultivation of Fruits, PAU, Ludhiana. The standardized volume of treatment application (5L per tree) was conducted as foliar spray using a pneumatic back sprayer so as to ensure proper saturation of the foliage. The treatments were given twice, the first foliar spray was given ten days after pit hardening stage and the second followed ten days after the first application.
Fruit physical and quality parameters
Five fruits from each experimental unit were selected at ripening stage i.e. during first week of May. The length & breadth of fruits was measured by putting the fruits end to end using a meter rod for computing their average length & breadth and represented in centimeters. The weight of fruits was measured using a weighing balance and presented in grams. Fruit firmness was measured using a penetrometer and expressed in Newtons. The Pulp:Stone ratio was calculated by recording the average pulp and stone weight and working out their ratio.
Standard procedures were followed for the determination of total soluble solids, titrable acidity, ascorbic acid and TSS: Acid ratio in the plum fruits (AOAC, 1990). The total soluble sugar and reducing sugar content of the fruits was determined using standard methods given by (Dubois et al., 1956).
Fruit yield and fruit drop percent
Experimental trees of plum cv. Satluj purple were marked and four branches were tagged in each direction on each tree. The number of fruits on each branch were counted in the first week of March. The dropped fruits were counted periodically at weekly interval starting from 5th March till the date of harvesting i.e. 7th May. The percentage of fruit drop was evaluated on the basis of fruit number initially present on the tree by using the formula:
The fruit yield/tree was noted at the end of the harvest period and represented as Kg/tree.
Enzymatic activity
A uniform sample of fruit pedicels of fruits was picked at the fruit ripening stage in the first week of May from each unit and analysed to determine the activity of cellulase, polygalacturonase and β 1, 4- Glucanase. Cellulase activity (EC 3.2.1.4) was determined by measuring the amount of reducing sugar released in the reaction, using Carboxyl methyl cellulose (CMC) as substrate by the technique described by Malik and Singh (1980). The reaction mixture contained 1 ml of 0.5% (w/v) CMC in 0.1 M citrate phosphate buffer (pH 5.2). The reaction mixture for cellulase assay consisted of 1 ml 0.1 M acetate buffer (pH 5.2), 1 ml 0.5% CMC and 1 ml of enzyme extract. This reaction mixture was further incubated for 1 h at a temperature of 550C and then 1 ml of DNS (Dinitrosalicyclic acid) reagent was added to it. For DNS reagent preparation, 4 g of NaOH was dissolved in of distilled water (50 ml) followed by addition of 2.5 g Dinitrosalicyclic acid. Then sodium potassium tartarate (75 g) was added and final volume was made to 250 ml by adding distilled water. For blank, DNS reagent was added before incubation. The reaction mixture containing tubes were then boiled for 10 min. These tubes were cooled to room temperature and then the absorbance was recorded at 560 nm. The enzymatic activity was expressed as µg D-glucose released g−1 fresh weight min−1.
Polygalacturonase (EC 3.2.1.15) activity was estimated by following the method of Malik and Singh (1980). The reaction mixture for assaying polygalactouronase activity comprised of 1 ml 0.1 M sodium acetate buffer (pH 5.2), 1 ml of pectic acid (0.5%) and 1 ml of enzyme extract. The reaction mixture was incubated for 1 h at 370C. The active enzyme was added to it at the end of incubation period which served as blank. The reaction mixture was terminated by adding 1 ml of DNS reagent. The contents in the tubes were boiled for 10 min and then the tubes were brought to room temperature & absorbance was recorded at 560 nm. The enzymatic activity was expressed as µg D-galactouronic acid g−1 fresh weight min−1.
For the extraction of enzyme β-glucanase (EC 3.2.1.6) the method of Fink et al. (1988) was used. The pedicel tissue (0.2 g) was extracted with 2 ml of 100 mM of sodium phosphate buffer (pH 6). The supernatant was taken after centrifugation at 10,000 rpm for 10 min. To 100 µl of extract, 100 µl of β-glucan (prepared in 50 mM sodium acetate buffer with pH 5) was added and then the volume was made up to 500 µl with 50 mM sodium acetate buffer. The test tubes were incubated for one and half hour at 370C. Then 500 µl of copper reagent (Nelson A (alkaline copper reagent) and Nelson B (copper sulfate reagent) in the ratio of 25:1) was added and incubated at 1000C for 10 min. After cooling the tubes, 500 µl of arsenomolybdate reagent was added. Then the greenish blue colour developed and the absorbance was read at 500 nm. The enzyme was expressed as µg D-glucose released g−1 fresh weight min−1.
Statistical analysis
The replicated healthy and dropped fruit samples were analyzed statistically using HSD Tukey’s test. Differences were considered statistically significant at the levels (p < 0.05) using statistical analysis software SAS (Version 9.3 for Windows).
Results
Fruit drop percent and fruit yield
The foliar application of the plant growth regulators and CaNO3 caused significant reduction in fruit drop as compared to control (Fig. 1). A significant reduction in fruit drop as compared to the treatments and control was observed in trees sprayed with 0.15 mM salicylic acid (19.59%), followed by 0.1 mM salicylic acid (20.64%) which was however, closely followed by the recommended dose 10 ppm NAA (21.54%). The presentation of fruit yield given in Fig. 1 depicts that foliar application of different plant growth regulators and CaNO3 led to a significant rise in total yield per tree as compared to control. The maximum fruit yield was observed in 0.15 mM salicylic acid (14.26 kg) followed by 0.1 mM SA (13.55 kg) it being minimum in control (8.97 kg). The increase in yield in treated units may be due to reduced fruit drop and increased fruit weight.
Physical attributes and fruit quality defining parameters
The outcome of the study represented in Table 1 reveals that the foliar application of NAA, SA, 2.4-D and CaNO3 affected the fruit weight, length, diameter and fruit firmness significantly. The fruit weight was maximum with the application of 0.15 mM salicylic acid (34.02 g) which was significantly higher than control (28.73 g) and rest of the treatments. The fruit length (4.58 cm) and diameter (4.24 cm) were also significantly high with the foliar supply of 0.1 mM SA. It was observed that application of NAA (10 ppm) and CaNO3 (1%) also exerted a positive influence on fruit weight, length and diameter.
Fruit firmness is an important quality parameter as it is a suitable predictor of potential shelf life of plums. The fruit firmness was positively influenced by the application of different plant growth regulators and CaNO3 as depicted in Table 1. The maximum fruit firmness was observed in fruits treated with 1% CaNO3 (14.8 N) as compared to control (12.69 N). However, there was no significant variation observed in firmness of fruits treated with NAA, SA or 2, 4-D.
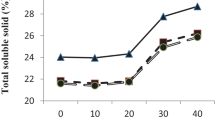
The content of total soluble solids, titrable acidity, amino acids, total soluble sugars and reducing sugars in the fruit juice were determined at ripening (Table 2). The maximum TSS content was observed in the fruits treated with 0.15 mM SA (14.32 Brix) followed by 10 ppm 2, 4-D (13.51
Brix) followed by 10 ppm 2, 4-D (13.51 Brix) which was significantly higher than the TSS content in control (9.85
Brix) which was significantly higher than the TSS content in control (9.85 Brix). Titrable acidity significantly reduced following foliar application of the plant growth regulators. It was minimum in fruits treated with 0.15 mM SA (0.993 percent) which was followed by 0.1 mM SA and 10 ppm NAA (1.03%) as compared to 1.81 percent in control. The TSS: acidity ratio (Fig. 2) was significantly high in treated fruits as compared to control, maximum being in 0.15 mM SA (14.56).
Brix). Titrable acidity significantly reduced following foliar application of the plant growth regulators. It was minimum in fruits treated with 0.15 mM SA (0.993 percent) which was followed by 0.1 mM SA and 10 ppm NAA (1.03%) as compared to 1.81 percent in control. The TSS: acidity ratio (Fig. 2) was significantly high in treated fruits as compared to control, maximum being in 0.15 mM SA (14.56).
The ascorbic acid, total soluble sugar and reducing sugar content of fruit juice was significantly influenced by the foliar application of plant growth regulators and mineral nutrient (Table 2). Maximum ascorbic acid content (3.69 mg/100 g pulp) was recorded with foliar application of NAA (10 ppm) which was statistically at par with 10 ppm 2,4 D (3.54 mg/100 g pulp) and it was registered as minimum (3.40 mg/100 g pulp) in untreated control. Maximum total soluble sugar content (11.37%) was recorded with the foliar application of 20 ppm 2,4–D (11.37%) being statistically at par with 10 ppm NAA (11.21%), it being minimum in fruits treated with 1% CaNO3 (10.73%). A similar trend was observed in reducing sugar content, where the maximum concentration was observed in 10 ppm NAA (4.02%) followed by 20 ppm 2,4-D (3.97%).
Enzymatic analysis
The activity of cell wall hydrolyzing enzymes cellulase, polygalacturonase and β 1, 4- glucanase was determined in the fruit pedicels of the treated and untreated fruits (Fig. 3). The activity of cellulase in the fruit pedicel was minimum in 0.15 mM SA (0.783 µg D-glucose released/g FW/min) and maximum was registered in control (1.88 µg D-glucose released/g FW/min). A similar trend was observed in activities of enzymes polygalacturonase and β-glucanase. A reduction in the enzymatic activity was observed in all the treated fruit pedicels. The minimum activity of PGA (0.506 µg D-galacturonic acid released/g FW/min) and β-glucanase (0.844 µg D-glucose released/g FW/min) was observed in 0.15 mM SA. The maximum PGA activity (0.881 µg D-galacturonic acid released/g FW/min) and β-glucanase activity (2.14 µg D-glucose released/g FW/min) was recorded in the pedicels of fruits picked from unsprayed trees.
Discussion
Fruit drop is preceded by a reduced growth of fruits undergoing shedding possibly due to the activation of specific abscission zones (Roberts et al., 2002). The onset of the process of abscission exerts a transitionary influence on the plant metabolic processes and several compounds enhance the cell separation process. Plant growth regulators have been reported to influence the process of abscission and the regulators of abscission are auxin and ethylene. Ethylene stimulates or induces abscission, whereas auxin inhibits the onset of abscission (Abdia et al., 1997). In the present study, the foliar application of 0.15 mM salicylic acid reduced fruit drop significantly when compared to the other treatments which was followed by 0.1 mM SA, it being statistically at par with the recommended dose of 10 ppm NAA. The reduction in the fruit drop with the exogenous application of salicylic acid may be attributed to its negative impact on biosynthesis of ethylene as it acts as a signaling molecule for ethylene biosynthesis (Molassiotis et al., 2005).
The application of synthetic auxin compounds NAA and 2,4-D have been reported to play an important role in reducing fruit drop in many crops. An increase in the concentration of auxins probably protects the cells of abscission zone (AZ) from the inducers of abscission. A low concentration of auxin is a prerequisite for the events which constitute cell separation to take place (Gonzales-Carranza et al. 2007). Anand et al. (2003) reported that four sprays of NAA at 15 or 30 ppm recorded the lowest fruit drop in litchi cv. Dehradun. Rahemi and Moghaddas (2003) studied the effect of 2,4-dichlorophenoxy acetic acid isopropyl ester and naphthaleneacetic acid on fruit drop, size and quality of local tangerine and suggested that spray of NAA (30 ppm) reduced pre-harvest fruit drop.
The increase in fruit yield with different treatments may be attributed to increase in fruit retention due to a reduction in abscission and increase in fruit size. Maibangsa and Ahmed (2000) described pineapple fruits if treated with auxin were heavier, which might be because of accelerated cell division with subsequent cell elongation and enlargement. A study conducted by Gill and Bal (2009) revealed that the application of NAA to ber fruits at pea size stage improved the inherent physiology of the leaves causing improved translocation of the essential components required in the development of fruits. Hence, the increased assimilation and utilization of photosynthates in developing fruits results in improving quality of fresh fruits. With the foliar application of NAA, an increase in pulp TSS, total acid and ascorbic acid of ber fruit content was reported by Gill and Bal (2009).
Plum fruit is prone to texture softening due to breakdown of pectic substances, hemi-cellulose and cellulose in the region of middle lamella, which weakens the cell wall and reduces the cohesive forces that binds the cells together (Wills et al., 1998). Loss of fruit firmness gets initiated by the activity of pectolytic and cellulolytic enzymes that results in subsequent changes to its texture (Elhassan, 2016). The application of calcium nitrate resulted in improved fruit firmness as an increased calcium concentration of the fruit delays the fruit ripening by reducing ethylene emission and slightly retarding the climacteric rise (Hansford, 1994). It also causes strengthening of the structure of cells by maintaining the micro fibrillar packaging in the cell walls, reinforcing the cell to cell contact which is necessary for the deposition of calcium pectate and counter acts the pectin methylesterase activity as seen in the calcium treated fruits of pear (Alandes et al., 2009).
The fruitlet abscission in plum followed a definite sequence of events that commenced with the upregulation in ethylene biosynthesis which leads to the synthesis of the cell wall hydrolytic enzymes. The increased activity of these enzymes leads to the distortion of the cell walls of the cortical cells and formation of patches of disintegrated cells resulting in the reduction of the mechanical strength of the pedicel and eventually fruit drop. The foliar application of salicylic acid (0.15 mM) was observed to be the most effective in reducing the preharvest fruit drop by reducing the activity of cellulase, polygalacturonase and β-1,4glucanase as well as enhancing the fruit quality and yield.
The process causing loss of adhesion between the cells of an abscission zone is the production of cell wall-degrading enzymes such as polygalacturonases and cellulase. The activity of these enzymes along with that of endoglucanases and pectin methylesterase tend to destruct the middle lamella of the cell wall thus leading to cell separation (Roberts et al., 2002, Khandakar et al. 2016). In the present study, the activity of cell wall degradative enzymes in the pedicel showed a directly proportional relationship with fruit drop. It suggests that the genes coding for the synthesis of these enzymes may get unregulated when the cells become sensitive to ethylene. Once induced, these genes stimulate a multifactorial response, including cell wall degradation leading to reduction in pedicel strength and hence fruit drop.
It may thus be concluded that two foliar applications of 0.15 mM SA, first ten days after pit hardening stage and second ten days after the first application reduced the fruit drop in plum cv. Satluj purple concomitant with improvement of fruit quality by reducing the activity of cell wall hydrolyzing enzymes viz., cellulose, polygalacturonase and glucanase in the fruit pedicels.
Data Availability
Original Data Available (can provide as and when required).
References
Abdia, N., Holforda, P., Mcglassona, W. B., & Mizrahib, Y. (1997). Ripening behaviour and responses to propylene in four cultivars of Japanese type plums. Postharvest Biology and Technology, 12, 21–34.
Alandes, L., Perez, I., Llarca, E., Quiles, A., & Hernondo, I. (2009). Use of calcium lactate to improve structure of ‘Flor de Invierno’ fresh cut pears. Postharvest Biology and Technology, 53, 145–151.
Anand, M., Kahlon, P., & Mahajan, V. C. (2003). Effect of exogenous application of growth regulators on fruit drop, cracking and quality of litchi {Litchi chinensis Sonn.) cv Dehradun. Agricultural Science Digest, 23, 191–194.
AOAC. (1990). Official methods of analysis (pp 58–61). Washington DC, USA: Association of Analytical Chemists.
Ashraf, Y. M., Ashraf, M., Akhtar, M., Mahmood, K., & Saleem, M. (2013). Improvement in yield, quality and reduction in fruit drop in kinnow (Citrus reticulata) by exogenous application of plant growth regulators, potassium and zinc. Pakistan Journal of Botany, 45, 433–440.
Aziz, A. (2003). Spermidine and related-metabolic inhibitors modulate sugar and amino acid levels in Vitis vinifera L: Possible relationships with initial fruitlet abscission. Journal of Experimental Botany, 54, 355–363.
Beyer, E. M. (1973). Abscission: Support for a role of ethylene modification of auxin transport. Plant Physiology, 52, 1–5.
Birwal, P., Deshmukh, G., Saurabh, S. P., & Pragati, S. (2017). Plums: A brief introduction. Journal of Food Nutrition and Popular Health, 1, 1–8.
Dar, G. A., Misger, F. A., & Rather, J. A. (2016). Growth, fruit set and yield of Santa Rosa plum as affected by nitrogen and boron under rainfed conditions of Kashmir Valley. Journal of Applied and Natural Science, 8, 2081–2086.
Dubois, M., Giles, K. A., Hamilton, J. K., Reters, P. A., & Smith, F. (1956). Calorimetric method for the determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Elhassan, S. Y. M. (2016). Role of cellulase enzyme in fruit softening during muskmelon fruit ripening. American Journal of Scientific and Industrial Research, 7, 98–105.
Fink, W., Liefland, M., & Mendgen, K. (1988). Chitinase and β Glucanase in the apoplastic compartment of oat leaves. Plant Physiology, 88, 270–275.
Gill, P. P. S., & Bal, J. S. (2009). Effect of growth regulator and nutrients spray on control of fruit drop, fruit size and quality of ber under sub-montane zone of Punjab. Journal of Horticultural Sciences, 4, 161–163.
González-Carranza, Z. H., Elliott, K. A., & Roberts, J. A. (2007). Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. Journal of Experimental Botany, 58, 3719–3730.
Hansford, R. (1994). Role of calcium in respiratory control. Medical Science in Sports and Exercise, 26, 44–51.
Hazra, K. S. (2004). Studies on fruit development and fruit drop in plum cv. Satluj Purple under high density planting. M.Sc. Thesis. Punjab Agricultural University. Ludhiana, India.
Khandaker, M. M., Idris, N. S., Ismail, S. Z., Majrashi, A., Alebedi, A., & Mat, N. (2016). Causes and prevention of fruit drop of Syzygium Samarangense (Wax Apple): A review. Advances in Environmental Biology, 10, 112–123.
Maibangsa, S., & Ahmed, F. (2000). Effect of post flowering spray with NAA and GA3 on ratoon pineapple. Annals of Agricultural Research, 21, 133–134.
Malik, C. P., & Singh, M. B. (1980). Plant enzymology and histo-enzymology: A text manual. Kalyani publishers.
Molassiotis, A., Therios, I., Dimassi, K., Diamantidis, G., & Chatzissavvidis, C. (2005). Induction of Fe(III)-chelate reductase activity by ethylene and salicylic acid in iron-deficient peach rootstock plants. Journal of Plant Nutrition, 28, 669–682.
Rahemi, M., & Moghaddas, M. (2003). Effects of 2,4-D(chlorophenoxy) acetic acid isopropyl ester and naphthalene-acetic acid on fruit drop, size and quality of local tangerine. Iranian Journal of Agricultural Science, 34, 439–445.
Rajput, V., Sehrawat, S. K., & Bhatia, S. K. (2017). Growth regulators and nutrient application reduces fruit drop and improves fruit quality in Prunus salicina Lindl. Cv. Kala amritsari. International Journal of Pure and Applied Bioscience, 5, 735–743.
Roberts, J. A., Elliott, K. A., & Gonzalez-Carranza, Z. H. (2002). Abscission, dehiscence and other cell separation processes. Annual Reviews of Plant Biology, 53, 131–158.
Taylor, J. E., & Whitelaw, C. A. (2001). Signals in abscission. New Phytologist, 151, 323–329.
Wills, R. H. H., McGlasson, B., Graham, D., & Joyce, D. (1998). Postharvest. An introduction to the physiology and handling of fruit, vegetables and ornamentals. 4th ed. 262 p. Adelaide, Hyde Park Press.
Zhao, M., & Li, J. (2020). Molecular events involved in fruitlet abscission in litchi. Plants, 9, 1–11.
Funding
The Authors did not receive any funding for research work.
Author information
Authors and Affiliations
Contributions
Planning of study: Dr. NK and Dr. HS. Experimenting and data collection: AK and SM. Writing Manuscript: AK, Dr. NK, SM, Dr. SKJ and Dr. HS
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, A., Kaur, N., Singh, H. et al. Efficacy of plant growth regulators and mineral nutrients on fruit drop and quality attributes of plum cv. Satluj purple. Plant Physiol. Rep. 26, 541–547 (2021). https://doi.org/10.1007/s40502-021-00609-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-021-00609-w