Abstract
This study was carried out to assess the effect of methyl jasmonate (MeJA) applied at different concentrations (0.2 mM and 0.4 mM) after harvest on nut quality characteristics of the ‘Erfelek’ chestnut cultivar during cold storage. Chestnuts were kept at 0 ± 0.5 °C and 90 ± 5% relative humidity for 80 days. As a result of cold storage, it was observed that the MeJA treatment had a positive effect on nut quality compared to the control. The effect of 0.2 mM MeJA treatment on weight loss, moisture and firmness values of the chestnuts was more significant than other treatments. While vitamin C, total phenolic and antioxidant activity (DPPH and FRAP assays) were similar between MeJA treatments, they were significantly preserved compared to the control. In addition, at the end of cold storage, the highest protein and the lowest ash content, which is important for nutritional quality, were observed in chestnuts treated with MeJA. On the basis of the results, we can conclude that MeJA treatments can be used as an effective tool to maintain post-harvest quality losses in the ‘Erfelek’ chestnut cultivar and to protect bioactive compounds during cold storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chestnut (Castanea sativa Mill.) is an important and popular type of nut in many European countries. Its high nutritional value has played an important role in human nutrition since ancient times (Mert and Ertürk 2017). In addition to their low fat and salt content, chestnuts, an important source of carbohydrates, are also rich in dietary fibre, minerals such as potassium and calcium, and B‑group vitamins (Mujić et al. 2010). As such, its properties make it suitable for human nutrition and health.
Chestnut is a seasonal fruit that can maintain its optimum commercial quality, turgescence and health for a short time. Preventing deterioration events seen in this product is among the biggest challenges (Jermini et al. 2006). The shelf life of the chestnut is limited and short due to its high metabolic activity, the porous and non-lignified characteristic of the epicarp, and moisture and sugar content (Sacchetti et al. 2005; Correia and Beirão-da-Costa 2010; Mert and Ertürk 2017). In particular, the high water and sugar content affect the emergence of insects and moulds such as Gnomoniopsis castanea and Sclerotinia pseudotuberosa during storage (Vettraino et al. 2020). Moulds similar to these that occur in the post-harvest period in chestnut play a role in the occurrence of diseases and spoilage that reduce the market value of the product (Donis González et al. 2010). To increase the post-harvest storage period of chestnut, techniques such as curing water, underground storage and dehydration by charcoal fires with minimal air have been used (Mignani and Vercesi 2003).
In recent years, different techniques such as methyl jasmonate (MeJA) in kiwifruit (Cao et al. 2009), gibberellic acid (GA3) in sweet cherries (Ozkan et al. 2016), aminoethoxyvinylglycine (AVG) in kiwifruit (Ozturk et al. 2019) and Aloe vera gel (AV) in papaya (Farina et al. 2020) have been used to minimize post-harvest losses in fruits. Of these, MeJA, is known to regulate important aspects of plant physiology, act as a signal in plant cellular responses and modulate the biosynthesis of other phytohormones. At the same time, MeJA is a phytohormone that reduces oxidative stress and secondary metabolite accumulation in plant cells. It is reported that MeJA can be used as a stimulating tool for improving the nutritional content of food products (Wang et al. 2021). In addition, MeJA has effects that increase fruit quality (Serrano et al. 2018), prevent chill injury (Aghdam and Bodbodak 2013; Barman et al. 2018), increase resistance to diseases in the post-harvest period and prolong storage life in fruit. Accordingly, there is no study in the literature investigating the effect of MeJA on the quality preservation of chestnuts. Therefore, the present study aimed to determine the effects of MeJA treatment at different concentrations after harvest on nut quality during cold storage for the ‘Erfelek’ chestnut cultivar.
Materials and Methods
Plant Materials
The plant material of the study consisted of nuts belonging to the ‘Erfelek’ chestnut cultivar grown in Sinop province (Turkey). A total of 15 kg of nuts was hand-harvested for the research. The harvested nuts were brought to the post-harvest laboratory of Ordu University after being separated from the capsules. In addition, care was taken to ensure that the chestnuts were of similar size, had no visual defects and were free from diseases and pests.
Experimental Design and Treatments
Nuts were randomly divided into three groups at the beginning, each weighing 5 kg. Those allocated to the first group were immersed with distilled water (5 min) and then put into 12 packages (plastic net sacks) of 400 g each to be evaluated as a control. Chestnuts in the second group were placed in 12 packages after being immersed in 0.2 mM MeJA solution for 5 min. The chestnuts in the third group were placed in 12 packages after dipping them in 0.4 mM MeJA solution. Chestnuts other than these were used to determine the harvest period measurements.
Measurement periods were planned to be on the 20th, 40th, 60th and 80th day in addition to the harvest. Measurements of the treatments were made in three replications in each period. Also, the net packages prepared for the treatments were placed in 36 plastic boxes, nine to be used for each measurement period. All chestnuts were pre-cooled with cold air at 90 ± 5% relative humidity (RH) until the pulp temperature dropped to 1 °C. They were then stored at 0 ± 0.5 °C and 90 ± 5% RH for up to 80 days.
Weight Loss, Moisture, Respiration Rate and Firmness
The initial weights of each chestnut package (Wi) were determined with a digital precision scale of 0.01 precision (Radwag, Poland). Then, the weight changes occurring on the 20th, 40th, 60th, and 80th day of storage were regularly determined in the final weight (Wf). Accordingly, the weight loss (WL) during storage was determined as a percentage using the equation WL (%) = 100 × (Wi-Wf) / Wi (Faizy et al. 2021).
The moisture content of chestnuts was determined by modifying the AOAC 979.12 method. Accordingly, approximately 40 g of nut (m) from each replication was peeled by hand and placed in Petri dishes that were tared beforehand. This was kept in a vacuum oven at 105 °C for 12 h. At the end of this period, the Petri dishes were taken to the desiccator to cool, and their final weights were measured. Finally, the percent moisture content was calculated (AOAC 1995).
Respiration rate was determined as three replications for each treatment, measuring the amount of CO2 released for 15 fruits in each replication with an infrared CO2 gas analyser (Vernier, OR, USA). The results are shown as mL CO2 kg−1 h−1 (Yarılgaç et al. 2019).
The textural analysis carried out in the Central Research Laboratory of Ordu University determined the firmness of chestnuts. For each replication, measurements were made after eight nuts were manually separated from their skins, and the results are expressed in N.
Crude Protein, Dry Matter and Ash
The protein ratio was determined based on the Kjeldahl method. Percent protein values were calculated by multiplying the obtained percent nitrogen values by 6.25. For the dry matter, the samples dried at 105 °C were then gradually increased (250–300 °C) in the muffle furnace and burned at 550 °C for approximately 10 h, and the percentage was calculated (Venkatachalam and Sathe 2006).
Soluble Solids Content, Titratable Acidity and Vitamin C
In each replication, 25 fruits were manually separated from their shells and ground with a blender (Model No. Promix HR2653, Philips, Turkey). Then, 20 g of fruit samples was weighed and homogenized with 80 g of distilled water. The prepared sample was filtered for soluble solids content (SSC), titratable acidity (TA) and vitamin C measurements. A digital refractometer (Atago PAL‑1, Atago Co., Ltd., Bellevue, WA, USA) was used to measure SSC, which is expressed as %. To measure TA, 10 mL of fruit juice was taken, and 0.1 N NaOH solution was added until the pH value was 8.2. The TA was calculated according to the amount of NaOH consumed in the titration and expressed as g malic acid kg−1. Vitamin C measurements were performed with a reflectometer (RQflex plus 10, Merck, Germany). A total of 0.5 mL of 5% oxalic acid was added to 0.5 mL of juice. Then, measurements were made using ascorbic acid test strips (Catalog no. 116981, Merck, Germany). Results are expressed as mg 100 g−1 (Ozturk et al. 2021).
Total Phenolics and Antioxidant Activity (DPPH and FRAP)
A total of 25 fruit peels were separated by hand and ground in each measurement period with a blender. They were then stored in falcon tubes at −20 °C until analysis. Frozen samples were thawed at room temperature before starting the analysis. Methanol (10 mL) was added to 1 g of fruit sample and left for 2 days. The samples were centrifuged at 12,000 × g at 4 °C for 35 min. The resulting filtrate was used for total phenolics and antioxidant assays. Total phenolics and antioxidant activity assays were performed using a UV-Vis spectrophotometer (Shimadzu, Kyoto, Japan). Total phenolics were determined following the method described by Yılmaz et al. 2019 and are expressed as g GAE (gallic acid equivalent) kg−1. The antioxidant activity of chestnut was determined according to two different procedures: 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH; Blois 1958) and ferric ions (Fe+3) reducing antioxidant power (FRAP; Benzie and Strain 1996), and the results are expressed in mmol Trolox equivalent (TE) kg−1 fw.
Statistical Analysis
The experiment was established in a completely randomized design with three replications for each treatment. Data were analysed by one-way analysis of variance (ANOVA), and the means were separated using Tukey’s test (p < 0.05). Data analyses were performed in JMP (version 16.0 software).
Results
Weight Loss, Moisture, Respiration Rate and Firmness
Weight loss occurred in chestnut fruits during cold storage. However, it was observed that the MeJA treatments delayed weight loss during specific periods of cold storage. On the 20th, 40th, 60th and 80th day of cold storage, weight loss in the control was 12.09%, 21.95%, 26.30% and 28.01%, respectively. Compared to the control, it was determined that 0.4 mM MeJA treatment significantly delayed weight loss at 40 (17.79%) and 60 days (22.51%), and 0.2 mM MeJA treatment at 40 (16.16%), 60 (20.72%), and 80 days (24.33%). The moisture content of chestnuts decreased during cold storage, but MeJA treatments delayed moisture loss in chestnuts. While the moisture content was 50.33% during the harvest period of the control, it was determined as 41.35%, 36.87%, 34.77% and 33.27% on the 20th, 40th, 60th and 80th day of cold storage, respectively. Regarding moisture content, 0.4 mM MeJA treatment was significantly higher at 20 (44.85%), 40 (42.11%), and 60 days (37.61%), and 0.2 mM MeJA treatment at 20 (45.52%), 40 (43.21%), 60 (38.21%), and 80 days (34.88%) compared to the control. The differences between the respiration rate of the chestnut fruits at harvest and on the 20th day were non-significant between all treatments. While the respiration rate of control fruits increased significantly, especially between the 40th (20.58 CO2 kg−1 h−1), 60th (29.99 CO2 kg−1 h−1) and 80th day (48.52 CO2 kg−1 h−1), MeJA treatments significantly delayed the respiration rate on the 40th, 60th, and 80th day. While the respiration rate was 15.27 CO2 kg−1 h−1, 24.37 CO2 kg−1 h−1 and 27.79 CO2 kg−1 h−1 in chestnuts treated with 0.2 mM MeJA on days 40, 60 and 80, respectively, it was determined as 13.38 CO2 kg−1 h−1, 22.33 CO2 kg−1 h−1 and 25.61 CO2 kg−1 h−1, respectively, in the treatment with 0.4 mM MeJA. The firmness of chestnut fruits increased rapidly from the 20th day of cold storage. The firmness measurements of the control were determined as 6.09 N, 8.29 N and 8.64 N, respectively, on the 40th, 60th and 80th day of cold storage. These measurements were determined as 5.33 N, 7.29 N, 8.11 N in chestnuts treated with 0.2 mM MeJA, and 5.20 N, 7.98 N and 8.75 N in chestnuts that were treated with 0.4 mM MeJA, respectively. While the firmness level was significantly lower with all MeJA treatments on the 40th day of cold storage, the fruits treated with 0.2 mM MeJA on the 60th and 80th day preserved the firmness level significantly compared to the other treatments (Fig. 1).
Effect of methyl jasmonate (MeJA) treatment on weight loss (a), moisture (b), respiration rate (c) and firmness (d) of chestnut fruit during cold storage. The differences between the means indicated with lowercase letters are significant (Tukey’s test, p < 0.05). Vertical bars indicate the standard errors
Dry Matter, Crude Protein and Ash Content
The dry matter, crude protein and ash content of chestnut fruits increased during the cold storage period, starting from the harvest period. The difference between the MeJA treatments and the control on the 40th, 60th and 80th day of cold storage was statistically significant in terms of dry matter content. Dry matter content was found to be 65.40%, 67.37% and 67.78% on the 40th, 60th and 80th day of control, respectively. During these measurement periods, the dry matter content of chestnuts treated with 0.2 mM MeJA was determined as 63.10%, 65.78% and 66.10%, respectively, while it was determined as 63.25%, 66.23% and 66.18% in chestnuts treated with 0.4 mM MeJA. Accordingly, the dry matter content of chestnuts treated with MeJA on day 40, 60 and 80 was significantly lower than the control group fruits. In the analyses performed on the 20th, 40th and 60th day of cold storage, no significant differences were observed between the treatments in terms of protein content. While the highest protein content was seen in chestnuts treated with 0.2 mM MeJA with 4.42% on the 80th day measurement, the lowest protein content was found in the control with 4.02%. Ash content was significantly higher in fruits treated with 0.4 mM MeJA on the 20th (1.42%) and 40th day (1.53%) of cold storage than in other treatments. During these periods, the ash content of the control group was determined as 1.29% and 1.37%, while it was determined to be 1.28% and 1.34% in chestnuts treated with 0.2 mM MeJA. The differences between treatments were not significant in the analyses performed on the 60th day of cold storage. In addition, the highest ash content was determined in the control (1.84%) on the 80th day and the lowest in chestnuts treated with 0.4 mM MeJA (Fig. 2).
Soluble Solids Content, Titratable Acidity and Vitamin C
While SSC increased in chestnuts during cold storage from harvest, on the contrary, TA decreased. Regarding SSC, the effect of MeJA treatments was significant on the 20th and 40th day compared to the control, while there was no significant difference between treatments in the other periods. In the measurements made on the 20th and 40th days of cold storage, the SSC was determined as 8.2% and 12.0% in the control, 7.0% and 11.2% in the 0.2 mM MeJA treatment, and 6.4% and 11.0% in the 0.4 mM MeJA treatment. In terms of TA, it was determined that the fruits treated with MeJA had higher values in all measurement periods after harvest compared to the control. The difference between them was statistically significant. Accordingly, the TA content of the control was determined as 0.106%, 0.086%, 0.081% and 0.050%, respectively, on the 20th, 40th, 60th and 80th day of cold storage. In the analyses carried out during the same periods, the TA content of chestnuts treated with 0.2 mM MeJA was determined as 0.113%, 0.105%, 0.100% and 0.086%, while it was determined as 0.115%, 0.102%, 0.094% and 0.089% in chestnuts treated with 0.4 mM MeJA. The vitamin C content of chestnuts increased until the 20th day after harvest and then decreased in all measurement (40th, 60th, and 80th day) periods. It was determined that chestnuts treated with MeJA preserved their vitamin C content significantly compared to the control on the 40th, 60th, and 80th day of cold storage. Accordingly, the vitamin C content of the control was determined as 28.0 mg 100 g−1, 22.8 mg 100 g−1 and 11.4 mg 100 g−1 in the measurements made on the 40th, 60th and 80th day, respectively, while it was 36.0 mg 100 g−1, 26.4 mg 100 g−1, 15.8 mg 100 g−1 in the 0.2 mM MeJA treatment, and 35.4 mg 100 g−1, 26.0 mg 100 g−1 and 17.4 mg 100 g−1 in the 0.4 mM MeJA treatment (Fig. 3).
Effect of methyl jasmonate (MeJA) treatment on soluble solids content (SSC, a), titratable acidity (b) and vitamin C (c) of chestnut fruit during cold storage. The differences between the means indicated with lowercase letters are significant (Tukey’s test, p < 0.05). Vertical bars indicate the standard errors
Total Phenolics, Antioxidant Activity (According to DPPH and FRAP Assays)
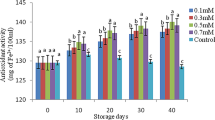
Total phenolics and antioxidant activity of chestnuts decreased during cold storage. Compared to the control, the effect of MeJA treatment on the preservation of total phenolics was significant at the 40th, 60th, and 80th measurement periods. In the analyses performed during these measurement periods, the total phenolics content of the control was determined as 15.2 g GAE kg −1, 14.8 g GAE kg −1 and 11.5 g GAE kg −1, respectively. In addition, while it was determined as 15.9 g GAE kg −1, 15.3 g GAE kg −1 and 13.2 g GAE kg −1 in chestnuts treated with 0.2 mM MeJA, it was determined as 15.9 g GAE kg −1, 15.3 g GAE kg −1 and 12.9 g GAE kg −1 in the 0.4 mM MeJA treatment. Antioxidant activity according to the DPPH assay, in all MeJA treatments, was higher in the 60th and 80th measurement periods compared to the control. In the measurements made during these periods, DPPH content was determined as 4.75 µmol TE kg−1 and 4.64 µmol TE kg−1 in the control, 4.87 µmol TE kg−1 and 4.73 µmol TE kg−1 in the chestnuts treated with 0.2 mM MeJA, and 4.86 µmol TE kg−1 and 4.74 µmol TE kg−1 in the chestnuts treated with 0.4 mM MeJA. Also, according to the FRAP analysis, antioxidant activity was found to be significantly higher in chestnuts treated with MeJA on days 40, 60 and 80 compared to the control. On days 40, 60 and 80, the FRAP content in the control was determined as 8.8 µmol TE kg−1, 8.0 µmol TE kg−1 and 5.6 µmol TE kg−1, respectively. These values were determined as 9.7 µmol TE kg−1, 8.4 µmol TE kg−1 and 6.4 µmol TE kg−1 in chestnuts treated with 0.2 mM MeJA and were determined as 9.6 µmol TE kg−1, 8.5 µmol TE kg−1 and 6.6 µmol TE kg−1 in the 0.4 mM MeJA treatment, respectively (Fig. 4).
Discussion
Post-harvest water loss in fruits and vegetables is an essential factor that negatively affects fruit quality. Fruit quality is mainly affected by weight loss due to relative humidity and respiration events in the storage environment during storage (Vettraino et al. 2020). Weight loss occurs with an increase in the respiration rate of the fruit and a decrease in moisture content. This is undesirable as it causes post-harvest economic losses in the products (Nunes and Emond 2007). With the moisture loss of 15–30% in chestnuts, some of the starch in dried fruits turns into sugar, and the desired characteristic taste is formed. However, since chestnut is a perishable fruit under normal conditions, delaying these losses and keeping the humidity at specific rates are essential for good preservation. Otherwise, the seed will shrink, become firm and quality losses will occur (Özçağıran et al. 2007). Therefore, delaying water loss is crucial in maintaining the chestnut market value. The moisture content of the fruits in the chestnut species is generally around 45–50%. This ratio decreases as the storage period increases. This situation was similarly observed in fruits kept cold during the study. In the study, water loss was significantly delayed in fruits treated with MeJA during storage compared to the control. Also, the respiration rate was slowed down considerably in fruits treated with MeJA. Similarly, Öztürk and Yücedağ (2021) reported that MeJA caused less weight loss by suppressing the respiratory rate in kiwifruit treated with MeJA.
Dry matter content (DMC) in fruits consists of water-soluble and insoluble carbohydrates, protein, minerals, organic acids and other compounds (Suni et al. 2000; Goke et al. 2018). Determining the DMC at harvest is important in revealing the storage potential of the fruit. It is reported that DMC is positively correlated with SSC at harvest and TA after harvest in different fruit species (Vieira et al. 2018). It has been previously reported that DMC can be used as a quality indicator in fruit species such as kiwi (Crisosto et al. 2012), apple (Palmer et al. 2010) and mango (Anderson et al. 2017). Turan and İslam (2016) reported that the decrease in the amount of moisture in hazelnut during storage increases the amount of dry matter; accordingly, the amount of nitrogen and the protein rate increase in the fruit. Similarly, in this study, the DMC of the control group fruits with the highest moisture loss was found to be at the highest level. Therefore, the dry matter remained lower during storage with MeJA treatments. However, the highest values of crude protein content, considered important in determining the nutritional value of foods, were observed in chestnuts treated with MeJA at the end of storage. Accordingly, it is thought that the MeJA treatment can be used as an effective tool for preserving protein and moisture content in chestnuts.
Fruit contains a wide range of phytochemical compounds that exhibit antioxidant activity, which vary widely in chemical structure and function. The most common are phenolics, carotenoids and vitamins, which have protective effects against several chronic diseases (Serrano et al. 2018). Accordingly, consumers especially prefer fruits with high nutritional content, leading producers to products with high quality and nutritional content in this case (Faizy et al. 2021). In the study, the total phenolics, antioxidant activity and vitamin C content, which significantly affect human health, were preserved in the chestnuts treated with MeJA compared to the untreated chestnuts during cold storage. Similarly, the positive effects of MeJA on bioactive compounds have been reported in previous studies in apples (Öztürk et al. 2014), table grapes (García-Pastor et al. 2019), and cherries (Faizy et al. 2021).
Conclusion
In this study, it was determined that methyl jasmonate (MeJA) treatments positively affected the preservation of post-harvest nut quality characteristics such as weight loss, moisture content, dry matter content, firmness and respiration rate in ‘Erfelek’ chestnut during cold storage. In addition, MeJA treatments resulted in better preserved total phenolics, antioxidant activity and vitamin C, which significantly affect consumer preference and are beneficial to human health. A dose of 0.2 mM MeJA is more effective on weight loss, moisture, firmness and total phenolics contents compared with other treatments. Therefore, we suggest that 0.2 mM MeJA can be used as an effective tool to extend the post-harvest storage life of chestnuts and preserve their quality characteristics.
References
Aghdam MS, Bodbodak S (2013) Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Sci Hortic 156:73–85
Anderson NT, Subedi PP, Walsh KB (2017) Manipulation of mango fruit dry matter content to improve eating quality. Sci Hortic 226:316–321
AOAC (1995) Official methods of analysis, 16th edn. Association of official analytical chemists, Washington
Barman K, Sharma S, Asrey R (2018) Postharvest treatments to alleviate chilling injury in fruits and vegetables. Emerging postharvest treatment of fruits and vegetables. Apple Academic, Boca Raton, pp 1–36
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Cao S, Zheng Y, Yang Z, Wang K, Rui H (2009) Effect of methyl jasmonate on quality and antioxidant activity of postharvest loquat fruit. J Sci Food Agric 89(12):2064–2070
Correia P, Beirão-da-Costa ML (2010) Potentialities of chestnut for starch production. Acta Hortic 866:581–586
Crisosto G, Hasey J, Zegbe J, Crisosto C (2012) New quality index based on dry matter and acidity proposed for Hayward kiwifruit. Calif Agric 66(2):70–75
Donis González IR, Fulbright DW, Ryser ET, Guyer D (2010) Shell mold and kernel decay of fresh chestnuts in Michigan. Acta Hortic 866:353–362
Faizy AH, Ozturk B, Aglar E, Yıldız K (2021) Role of methyl jasmonate application regime on fruit quality and bioactive compounds of sweet cherry at harvest and during cold storage. J Food Process Preserv 45(10):e15882
Farina V, Passafiume R, Tinebra I, Scuderi D, Saletta F, Gugliuzza G, Gallotta A, Sortino G (2020) Postharvest application of aloe vera gel-based edible coating to improve the quality and storage stability of fresh-cut papaya. J Food Qual. https://doi.org/10.1155/2020/8303140
García-Pastor ME, Serrano M, Guillén F, Castillo S, Martínez-Romero D, Valero D, Zapata PJ (2019) Methyl jasmonate effects on table grape ripening, vine yield, berry quality and bioactive compounds depend on applied concentration. Sci Hortic 247:380–389
Goke A, Serra S, Musacchi S (2018) Postharvest dry matter and soluble solids content prediction in d’Anjou and Bartlett pear using near-infrared spectroscopy. HortScience 53(5):669–680
Jermini M, Conedera M, Sieber TN, Sassella A, Schärer H, Jelmini G, Höhn E (2006) Influence of fruit treatments on perishability during cold storage of sweet chestnuts. J Sci Food Agric 86(6):877–885
Mert C, Ertürk Ü (2017) Chemical compositions and sugar profiles of consumed chestnut cultivars in the Marmara Region, Turkey. Not Bot Horti Agrobo 45(1):203–207
Mignani I, Vercesi A (2003) Effects of postharvest treatments and storage conditions on chestnut quality. Acta Hortic 600:781–785
Mujić I, Agayn V, Živković J, Velić D, Jokić S, Alibabić V, Rekić A (2010) Chestnuts, a “comfort” healthy food. Acta Hortic 866:659–665
Nunes CN, Emond JP (2007) Relationship between weight loss and visual quality of fruits and vegetables. Proc Fla State Hort Soc 120:235–245
Özçağıran R, Ünal A, Özeker E, İsfendiyaroğlu M (2007) Ilıman İklim Meyve Türleri: Sert Kabuklu Meyveler vol III. EÜ Ziraat Fakültesi Yayınları
Ozkan Y, Ucar M, Yildiz K, Ozturk B (2016) Pre-harvest gibberellic acid (GA3) treatments play an important role on bioactive compounds and fruit quality of sweet cherry cultivars. Sci Hortic 211:358–362
Öztürk B, Yücedağ F (2021) Effects of methyl jasmonate on quality properties and phytochemical compounds of kiwifruit (Actinidia deliciosa cv. ‘Hayward’) during cold storage and shelf life. Turk J Agric For 45(2):154–164
Öztürk B, Özkan Y, Yildiz K (2014) Methyl jasmonate treatments influence bioactive compounds and red peel color development of Braeburn apple. Turk J Agric For 38(5):688–699
Ozturk B, Uzun S, Karakaya O (2019) Combined effects of aminoethoxyvinylglycine and MAP on the fruit quality of kiwifruit during cold storage and shelf life. Sci Hortic 251:209–214
Ozturk B, Yildiz M, Yildiz K, Gun S (2021) Maintaining the postharvest quality and bioactive compounds of jujube (Ziziphus jujuba Mill. Cv.‘Li’) fruit by applying 1‑methylcyclopropene. Sci Hortic 275:109671
Palmer JW, Harker FR, Tustin DS, Johnston J (2010) Fruit dry matter concentration: A new quality metric for apples. J Sci Food Agric 90(15):2586–2594
Sacchetti G, Pittia P, Mastrocola D, Pinnavaia GG (2005) Stability and quality of traditional and innovative chestnut based products. Acta Hortic 693:63–70
Serrano M, Martínez-Esplá A, Zapata P, Castillo S, Martínez-Romero D, Guillén F, Valverde JM, Valero D (2018) Effects of methyl jasmonate treatment on fruit quality properties. In: Emerging Postharvest Treatment of Fruits and Vegetables, pp 85–106
Suni M, Nyman M, Eriksson NA, Björk L, Björck I (2000) Carbohydrate composition and content of organic acids in fresh and stored apples. J Sci Food Agric 80(10):1538–1544
Turan A, İslam A (2016) Çakıldak fındık çeşidinde kurutma ortamları ve muhafaza süresine bağlı olarak meydana gelen değişimler. Ordu Üniversitesi Bilim Ve Teknoloji Dergisi 6(2):272–285
Venkatachalam M, Sathe SK (2006) Chemical composition of selected edible nut seeds. J Agric Food Chem 54(13):4705–4714
Vettraino AM, Vinciguerra V, Pacini G, Forniti R, Goffi V, Botondi R (2020) Gaseous ozone as a suitable solution for postharvest chestnut storage: evaluation of quality parameter trends. Food Bioproc Tech 13(1):187–193
Vieira MJ, Argenta LC, Mattheis JP, Amarante CVTD, Steffens CA (2018) Relationship between dry matter content at harvest and maturity index and post-harvest quality of ‘Fuji’ apples. Rev Bras Frutic. https://doi.org/10.1590/0100-29452018596
Wang SY, Shi XC, Liu FQ, Laborda P (2021) Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem 353:129482
Yarılgaç T, Kadim H, Ozturk B (2019) Role of maturity stages and modified-atmosphere packaging on the quality attributes of cornelian cherry fruits (Cornus mas L.) throughout shelf life. J Sci Food Agric 99(1):421–428
Yılmaz M, Karakaya O, Balta MF, Balta F, Yaman İ (2019) Çakıldak fındık çeşidinde iç meyve iriliğine bağlı olarak biyokimyasal özelliklerin değişimi. Akad Ziraat Dergisi 8(Özel Sayı):61–70
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Uzun declares that he has no competing interests.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Uzun, S. Postharvest Quality Traits of Chestnut (Castanea sativa Mill.) Fruit as Affected by Methyl Jasmonate During Cold Storage. Erwerbs-Obstbau 65, 1453–1462 (2023). https://doi.org/10.1007/s10341-023-00851-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-023-00851-6